Healthcare-associated infective endocarditis: an undesirable effect of healthcare universalization
Abstract
Clin Microbiol Infect 2010; 16: 1683–1690
Invasive medical technology has led to an increase in the incidence of healthcare-associated infective endocarditis (HAIE). A prospective multicentre cohort study was conducted at seven hospitals in Andalusia, Spain, to establish the characteristics of HAIE and to compare them with those of community-acquired infective endocarditis (CAIE). HAIE was defined as either infective endocarditis (IE) manifesting >48 h after admission to hospital, or IE associated with a significant invasive procedure performed in the 6 months before diagnosis. Seven hundred and ninety-three cases of IE were investigated, and HAIE accounted for 127 (16%). As compared with patients with CAIE, patients with HAIE were older (60.1 ± 14.4 years vs. 53.6 ± 17.5 years) and had more comorbidities (Charlson index 3.3 ± 2.3 vs. 1.8 ± 2.3) and staphylococcal infections (58.3% vs. 24.8%). Vascular manipulation was the main cause of bacteraemia responsible for HAIE (63%). Peripheral vein catheter-associated bacteraemia accounted for 32.8% of the catheter-related bacteraemias. In-hospital mortality (44.9% vs. 24.2%) was higher in the HAIE group. Septic shock (OR 2.2, 95% CI 2.9–30.2) and surgery not performed because of high surgical risk (OR 1.6, 95% CI 1.2–20) were independent predictors of mortality in HAIE. The present study demonstrates that HAIE is a growing health problem associated with high mortality. Careful management of vascular devices is essential to minimize the risk of bacteraemias leading to HAIE.
Introduction
Healthcare-associated infective endocarditis (HAIE) is a clinical entity that has been known for decades, and it is associated with high morbidity and mortality rates [1,2]. The incidence of HAIE continues to increase because of increasing invasive therapeutic and diagnostic procedures in healthcare settings, and increasing degenerative valve pathology and replacement in older patients. These situations create greater risks that microorganisms will attach to valves during nosocomial bacteraemias [3,4].
Reported incidence rates of HAIE range from 7% to 34% [1,5–10]. The few published studies are based on small numbers of cases, reported from single hospitals and with different criteria for defining HAIE. We therefore performed a large, prospective, multicentre cohort study of infective endocarditis (IE) in order to better define the characteristics and extent of this problem, and to compare HAIE with community-acquired infective endocarditis (CAIE).
Patients and Methods
Patients and settings
This prospective analysis was carried out by the Grupo Andaluz para el Estudio de las Infecciones Cardiovasculares (Andalusian Group for the Study of Cardiovascular Infections) at the Sociedad Andaluza de Enfermedades Infecciosas (SAEI—The Infectious Diseases Society of Andalusia), and performed in seven hospitals of the Public Health Service of Andalusia (south of Spain). Five hospitals were referral and teaching centres (1.000–1.850 beds) with cardiac surgery, and two were community hospitals (550–600 beds). All consecutive cases of definite or possible left-sided IE in adult patients (≥18 years of age) recorded between January 1984 and December 2007 were included in the study. Patients were identified from the infectious diseases units, the microbiology departments’ blood culture registries, the echocardiography laboratories and the autopsy departments. The cases were classified according to modified Duke criteria [11].
Data collection
Data on predisposing IE factors, comorbidities, events during hospitalization, invasive procedures, therapeutic options and patient evolution were collected by the same principal investigators in each centre during the entire study period. We use a standardized reporting form, and episodes were periodically entered into a common database.
Definitions
We considered HAIE as either IE manifesting >48 h after admission to hospital or IE acquired in association with a significant invasive procedure performed in the 6 months before diagnosis [12] in the following situations: (i) during a stay and/or manipulation in a hospital setting (nosocomial healthcare-associated IE); or (ii) in patients with extensive out-of-hospital contact with healthcare interventions (non-nosocomial healthcare-associated IE). IE was considered to be CAIE in patients without hospitalization or healthcare contact within the previous 6 months. Vascular catheter-associated bacteraemia (CAB) was diagnosed according to established criteria [13]. When catheter tip culture and/or simultaneous quantitative blood cultures were not available, the diagnosis of CAB required the presence of phlebitis and exclusion of an alternative explanation for bacteraemia.
Prosthetic valve endocarditis (PVE) was classified as early PVE within 12 months after valve implantation and late PVE after 12 months [14,15]. Early PVE is nosocomial by definition, and because of its special characteristics it was excluded from our study, as were right-sided IE and patients with pacemaker infection.
We considered comorbidity as the simultaneous presence of two medical conditions. We used the age-adjusted Charlson index [16] to stratify patients according to overall comorbidity. Patients were classified as having severe heart failure if they needed inotropic agents and/or mechanical ventilation. Renal failure was defined as a creatinine level >1.5 mg/dL in patients with prior normal renal function or when there was a deterioration of >25% of the prior creatinine clearance in patients with chronic renal insufficiency.
Echocardiography
Transoesophageal echocardiography (TOE) has been available in our centre since 1995. We performed TOE in the following situations: (i) when there was a strong clinical suspicion after non-diagnostic transthoracic echocardiography; or (ii) to obtain a better evaluation of the valvular complications.
Microbiology
Microbiological information was obtained from blood cultures, intra-operative heart tissue sample cultures and serological studies. Blood cultures were always performed serially (a minimum of two separate samples), by semi-automated methods. Patients with culture-negative IE was defined as those with negative blood and/or intra-operative cultures. Episodes of IE due to Coxiella burnetii were analysed separately.
Surgery
All information was collected by the attending physician, who established the indication for cardiac surgery according to American Heart Association guidelines [17]. Five of the hospitals offered cardiac surgery, whereas the other two referred their patients to other centres in the group when necessary. Those patients not offered surgery and the reasons for this were recorded. The additive European System for Cardiac Operative Risk Evaluation (EuroSCORE) [18] was calculated for all patients.
Mortality
In-hospital mortality was defined as mortality from any cause during hospitalization.
Follow-up
Following discharge, control blood cultures were performed 2 months later to ensure definite microbiological cure. Clinical follow-up continued for at least 6 months, and at least one echocardiographic examination was performed during this period to evaluate the need for deferred surgery.
Statistical analysis
The chi-squared test was used for comparison of qualitative variables (or the Fisher test when appropriate), and the Mann–Whitney U-test was used for quantitative variables. For the multivariate analysis, risk factors for in-hospital mortality and variables related to non-performance of cardiac surgery that were statistically significant in a univariate analysis (p <0.1) were included in a multivariate logistic regression model, where p <0.05 was considered to be significant.
Results
A total of 877 cases of IE were collected during the study period. Once early PVE cases (N = 84) had been excluded, there were 793 left-sided IE episodes in 788 patients in the analysis. There were 666 (84%) CAIE episodes and 127 (16%) HAIE episodes. Of these 127 HAIE cases, 117 (92.1%) were considered to be ‘definite’ and ten (7.9%) to be ‘possible’. Nosocomial infections accounted for 79.5% of the HAIE episodes. Non-nosocomial HAIE cases were mainly haemodialysis-associated (Fig. 1). The incidence of HAIE increased from 13.3% (26/195) during 1984–1995, to 15% (28/186) during 1996–2000, and to 17.7% (73/412) during 2001–2007.
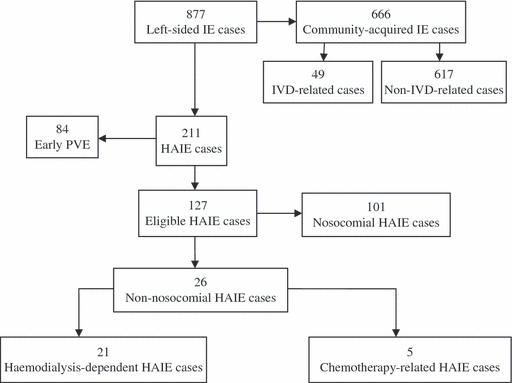
Classification of infective endocarditis cases. IE, infective endocarditis; IVD, intravenous drug; PVE, prosthetic valve endocarditis; HAIE, healthcare-associated infective endocarditis.
Patient characteristics
The clinical characteristics of patients with HAIE and CAIE are shown in Table 1. Patients in the HAIE group had a higher Charlson index than those with CAIE. The most frequent pathologies among HAIE patients were chronic renal insufficiency (14.1%), chronic liver disease (9.1%) and malignancies (8.1%) (data not shown). In 112 of the HAIE patients, the infection was on a native valve, underlying native valve pathology being present in 47.2% of these.
| HAIE Variables | Patients with | All patients with IE | ||||
|---|---|---|---|---|---|---|
| NHAIE (n = 101) | p | NNHAIE (n = 26) | HAIE (n = 127) | p | CAIE (n = 666) | |
| Age (mean years ± SD) | 61.3 ± 14.6 | 0.04 | 55 ± 12.6 | 60.1 ± 14.4 | <0.001 | 53.6 ± 17.5 |
| Males, n (%) | 80 (79.2) | <0.01 | 13 (50) | 93 (73.2) | 0.3 | 457 (68.6) |
| Native valve pathology, n (%) | 50 (49) | 0.3 | 10 (38.5) | 60 (47.2) | 0.6 | 334 (50.2) |
| Valve affected, n (%) | ||||||
| Aortic | 47 (46.5) | 0.2 | 8 (30.8) | 55 (43.3) | 0.9 | 285 (42.8) |
| Mitral | 42 (41.6) | 0.9 | 10 (38.5) | 52 (40.9) | 0.8 | 267 (40.1) |
| Multivalve | 13 (12.9) | 0.1 | 6 (23.1) | 19 (15) | 0.9 | 97 (14.6) |
| PVE, n (%) | 14 (13.9) | 0.3 | 1 (3.8) | 15 (11.8) | 0.1 | 116 (17.4) |
| Comorbidity, n (%) | 91 (90) | 0.4 | 24 (92) | 115 (90.6) | <0.001 | 378 (56.8) |
| Charlson index (average ± SD) | 3.1 ± 2.2 | 0.02 | 4.2 ± 2.4 | 3.3 ± 2.3 | <0.001 | 1.8 ± 2.3 |
| In-hospital surgery, n (%) | 44 (43.5) | 0.1 | 7 (27) | 51 (40.2) | 0.2 | 228 (34.2) |
| Surgical treatment, n (%) | 49 (48.5) | 0.07 | 7 (27) | 56 (44.1) | 0.9 | 293 (44) |
| EuroSCORE (average ± SD) | 0.4 | 11.1 ± 3.1 | 10.5 ± 4 | <0.01 | 9 ± 5.3 | 10.4 ± 4.2 |
| Rejected surgerya, n (%) | 0.1 | 6 (23.1) | 18 (23.7) | <0.001 | 44 (6.6) | 12 (11.9) |
| Intrahospital mortality, n (%) | 0.2 | 15 (58) | 57 (44.9) | <0.001 | 161 (24.2) | 42 (41.6) |
- NHAEI, nosocomial healthcare-associated IE; NNHAIE, non-nosocomial healthcare-associated IE; HAIE, healthcare-associated IE; CAIE, community-acquired IE; PVE, prosthetic valve endocarditis; SD, standard deviation..
- aPatients with an indication for surgery and poor clinical status.
Origin of infection
HAIE was related to a vascular manipulation in 63% of the cases, most commonly catheter-related procedures (61 cases; 48%). This group was led by vascular manipulations associated with haemodialysis (34.4%), and followed by peripheral catheter-related bacteraemia (32.8%), where Staphylococcus aureus accounted for 75% (n = 15) of the cases. Other sources of infection were genitourinary and digestive tract instrumentation (Table 2).
| Medical procedures | n (%) |
|---|---|
| Vascular | |
| Catheter-related | 61 (48) |
| Haemodialysis | 21 |
| Peripheral vein catheter | 20 |
| Several procedures | 12 |
| Central vein catheter | 8 |
| Implanted cardiac devices | 6 (4.7) |
| Coronary arteriography | 7 (5.5) |
| Cardiovascular surgery | 4 (3.1) |
| Carotid angioplasty | 1 (0.8) |
| Pulmonary stent | 1 (0.8) |
| Total | 80 (63) |
| Others | |
| Digestive | 19 (15) |
| Urinary | 18 (14.2) |
| Skin and soft tissue | 5 (3.9) |
| Several procedures | 2 (1.6) |
| Maxillofacial | 1 (0.8) |
| Osteoarticular | 1 (0.8) |
| Non-identified | 1 (0.8) |
| Total | 47 (37) |
Microbiology
HAIE was associated with positive blood cultures in 106 of the episodes (83.4%), positive intra-operative valve cultures in two (1.6%) cases, and both positive blood and intra-operative valve cultures in another nine (7.1%). The main microorganisms responsible for HAIE and CAIE are shown in Table 3.
| HAIEVariables, n (%) | Patients with | All patients with IE | ||||
|---|---|---|---|---|---|---|
| NHAIE (n = 101) | p | NNHAIE (n = 26) | HAIE (n = 127) | p | CAIE (n = 666) | |
| Staphylococcus aureus | 33 (32.6) | 0.4 | 6 (23) | 39 (30.7) | <0.001 | 113 (17) |
| MSSA | 21 (20.8) | 0.7 | 6 (23) | 27 (21.3) | 0.2 | 112 (16.8) |
| MRSA | 12 (11.9) | 0.7 | – | 12 (9.4) | <0.001 | 1 (0.2) |
| CoNS | 23 (22.7) | 0.01 | 12 (46) | 35 (27.6) | <0.001 | 52 (7.8) |
| Streptococcus viridans | 3 (3) | 1 | – | 3 (2.4) | <0.001 | 181(27.2) |
| Enterococcus faecalis | 21 (20.8) | 0.07 | 1 (3.8) | 22 (17.3) | 0.1 | 68 (10.2) |
| Streptococcus bovis | 1 (1) | 1 | – | 1 (0.8) | 0.03 | 33 (5) |
| Streptococcus agalactiae | 3 (3) | 1 | – | 3 (2.4) | 0.9 | 17 (2.6) |
| Other Streptococcus species | – | NA | – | – | 0.02 | 24 (3.6) |
| Culture-negative IE | 6 (5.9) | 0.3 | 3 (11.5) | 9 (7.1) | 0.1 | 73 (11) |
| EGNB | 5 (5) | >0.05 | 1 (4) | 6 (4.7) | 0.1 | 17 (2.6) |
| Fungi | 3 (3) | 1 | 1 (4) | 4 (3.1) | <0.01 | 3 (0.4) |
| Polymicrobial | 4 (4) | 1 | 1 (4) | 5 (3.9)a | 0.3 | 16 (2.4)b |
| Coxiella burnetii | – | NA | – | – | 0.05 | 19 (2.9) |
| Brucella species | – | NA | – | – | 0.1 | 12 (1.8) |
| HACEK group infections | – | NA | – | – | 0.1 | 11 (1.7) |
- IE, infective endocarditis; NHAEI, nosocomial healthcare-associated IE; NNHAIE, non-nosocomial healthcare-associated IE; HAIE, healthcare-associated IE; CAIE, community-acquired IE; NA, not applicable; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; CoNS, coagulase-negative Staphylococcus; EGNB, enteric Gram-negative bacilli.
- aTwo cases for S. aureus and Candida albicans, one case for S. aureus and E. faecalis, one case for Staphylococcus lugdunensis and Staphylococcus epidermidis, and one case for Enterobacter aerogenes and Staphylococcus hominis.
- bOne case each of the following: E. faecalis and Streptococcus bovis; Streptococcus agalactiae and S. aureus; Streptococcus mitis and S. epidermidis; Streptococcus viridans and Rothia dentocariosa; E. faecalis and C. albicans; E. faecalis and Candida glabrata; Arcanobacter species and Streptococcus mutans; E. faecalis, Pseudomonas aeruginosa and C. albicans; E. faecalis and S. epidermidis; S. aureus and E. faecalis; Streptococcus pyogenes and S. epidermidis; Streptococcus agalactiae and Enterobacter cloacae; Candida lusitaniae and E. faecalis; Haemophilus influenzae and E. faecalis; S. aureus and Streptococcus pyogenes; and S. epidermidis and Streptococcus viridans.
HAIE caused by Staphylococcus species was usually related to a vascular procedure (62/74 cases; 83.8%). In our series, the percentage of methicillin-resistant S. aureus (MRSA) has been increasing. The first MRSA isolate was collected in 2001, and by 2007 MRSA accounted for 50% of all S. aureus HAIE episodes. Considering only the last 5 years, the percentage of S. aureus isolates causing HAIE that were MRSA was 43.4% (10/23 cases). Staphylococcus epidermidis was the most prevalent species of coagulase-negative Staphylococcus (CoNS) (27 cases; 77.1%). CoNS caused native valve HAIE in 85.7% of the cases. HAIE caused by Enterococcus faecalis was usually related to a genitourinary procedure (13 cases; 59.1%).
Complications
Forty-four patients (34.6%) experienced some embolic manifestation. Among these, the central nervous system was the principal target (28 cases; 22%), followed by the spleen or large blood vessels (18 cases; 14.1%) and the bone and joints (arthritis or osteomyelitis) (six cases; 4.7%). On 11 occasions (8.7%), more than one embolic site was involved. The percentage of severe heart failure was higher in the HAIE group than in the CAIE group. The two groups had similar rates of neurological complications, but the HAIE group had more cases of septic shock and renal failure (Table 4).
| HAIEVariables, n (%) | Patients with | All patients with IE | ||||
|---|---|---|---|---|---|---|
| NHAIE (n = 101) | p | NNHAIE (n = 26) | HAIE (n = 127) | p | CAIE (n = 666) | |
| Severe HF | 26 (25.7) | 0.8 | 6 (23) | 32 (25.2) | <0.01 | 96 (14.4) |
| Embolic manifestations | 30 (29.7) | 0.01 | 14 (54) | 44 (34.6) | 0.1 | 276 (41.4) |
| Septic shock | 20 (19.8) | 0.1 | 8 (30.7) | 28 (22) | <0.001 | 71 (10.7) |
| Renal failurea | 51 (50.5) | 0.4 | 3 (60) | 54 (50.5) | <0.001 | 161 (24.2) |
| CNS events | 29 (28.7) | 0.06 | 12 (46) | 41 (32.3)b | 0.1 | 194 (29.1) |
| Intrahospital mortality | 42 (41.5) | 0.2 | 15 (57.7) | 57 (44.9) | <0.001 | 161 (24.2) |
- IE, infective endocarditis; NHAEI, nosocomial healthcare-associated IE; NNHAIE, non-nosocomial healthcare-associated IE; HAIE, healthcare-associated IE; CAIE, community-acquired IE; HF, heart failure; CNS, central nervous system.
- aExcluding haemodialysis patients.
- bEmbolic stroke, 22 (17.3%); encephalopathy, 16 (12.6%); mycotic aneurism, two (1.7%); acute meningitis, one (0.8%).
Outcome
Fifty-six (44.1%) patients underwent heart surgery, 51 (40.2%) during the acute phase and five during follow-up. The main indications for surgery were heart failure (60.8%), valve dysfunction (11.8%) and sepsis (7.8%). However, severe heart failure was the only significant difference between patients who underwent surgery and those who did not (Table 5). Eighteen patients in whom surgery was indicated were not operated on because of poor clinical status. As compared with patients who were operated on, those who were rejected for surgery had more S. aureus infections, a higher median additive EuroSCORE, a higher Charlson index, and more acute complications, such as severe heart failure, septic shock, central nervous system events and embolic manifestations (Table 5). When these factors were examined in a multivariate analysis, only septic shock (OR 7.7, 95% CI 2.1–27.5, p 0.001) and S. aureus (OR 3.8, 95% CI 1.3–13.3, p 0.03) aetiology maintained their statistical significance. The frequency of intrahospital mortality was higher in the HAIE group than in the CAIE group (44.9% vs. 24.2%) The in-hospital mortality of patients rejected for surgery was 83.3%. The median follow-up was 12 months (interquartile range 8–125); 70 patients (55.1%) were cured, and there were no relapses.
| Variable | Medical therapy (n = 53) | pa | Surgery performedb (n = 56) | pc | Surgery not performedd |
|---|---|---|---|---|---|
| Age (mean years ± SD) | 61.2 ± 14.5 | 0.3 | 58.3 ± 14.9 | 0.2 | 62.6 ± 12.4 |
| Charlson index (average ± SD) | 3.2 ± 1.9 | 0.3 | 2.4 ± 2.3 | 0.03 | 3.8 ± 2.2 |
| EuroSCORE (average ± SD) | 10.1 ± 2.7 | 0.8 | 10.1 ± 4.4 | 0.02 | 12.5 ± 3.5 |
| Staphylococcus aureus, n (%) | 17 (32.1) | 0.2 | 12 (21.4) | <0.01 | 10 (55.6) |
| MRSA | 5 (9.4) | 0.6 | 4 (7.1) | 0.2 | 3 (16.7) |
| MSSA | 12 (22.6) | 0.2 | 8 (14.3) | 0.02 | 7 (38.9) |
| CoNS | 15 (28.3) | 0.8 | 17 (30.4) | 0.2 | 3 (16.7) |
| Enterococcus species | 10 (18.9) | 0.9 | 11 (19.6) | 0.2 | 1 (5.6) |
| Embolic manifestations, n (%) | 21 (39.6) | 0.06 | 13 (23.2) | <0.01 | 10 (55.6) |
| Septic shock, n (%) | 11 (20.8) | 0.2 | 7 (12.5) | <0.001 | 10 (55.6) |
| Severe heart failure, n (%) | 6 (11.3) | 0.02 | 16 (28.6) | 0.03 | 10 (55.6) |
| Renal failuree, n (%) | 16 (30.2) | 0.05 | 27 (48.2) | 0.3 | 11 (61.1) |
| CNS event, n (%) | 18 (34) | 0.2 | 13 (23.2) | <0.01 | 10 (55.6) |
| Intrahospital mortality, n (%) | 19 (35.8) | 0.5 | 23 (41.1) | 0.001 | 15 (83.3) |
- CNS; central nervous system; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus; CoNS, coagulase-negative staphylococci; SD, standard deviation.
- aStatistical difference between ‘medical therapy’ and ‘surgery performed’.
- bSurgery indicated and performed.
- cStatistical difference between ‘surgery performed’ and ‘ surgery not performed’.
- dSurgery indicated but not performed.
- eExcluding haemodialysis patients.
Mortality risk factors
In the univariate analysis, intrahospital mortality in patients with HAIE was significantly associated with S. aureus infection, severe heart failure, septic shock and surgery not being performed because of high surgical risk. However, only septic shock and ‘surgery not performed’ remained independently associated with intrahospital mortality in the multivariate analysis (Table 6). Hospital-associated origin was associated with intrahospital mortality in the multivariate analysis of the whole series (OR 1.7, 95% CI 1.1–2.9, p 0.03).
| Variable | Death (n = 57) | Survival (n = 70) | Univariate analysis p | Multivariate analysis OR (95% CI) |
|---|---|---|---|---|
| Age (average ± SD) | 62.7 ± 12.5 | 58 ± 15.5 | 0.06 | |
| Charlson index | 3.6 ± 2.2 | 3.1 ± 2.3 | 0.2 | |
| Staphylococcus aureus, n (%) | 24 (42.1) | 15 (21.4) | <0.01 | |
| MRSA, n (%) | 9 (15.8) | 3 (4.3) | 0.02 | |
| Severe heart failure, n (%) | 22 (38.6) | 10 (14.3) | <0.01 | |
| Septic shock, n (%) | 24 (42.1) | 4 (5.7) | <0.001 | 2.2 (2.94–30.28) |
| Renal failurea, n (%) | 29 (50.9) | 25 (35.7) | 0.08 | |
| CNS event, n (%) | 23 (40.4) | 18 (25.7) | 0.07 | |
| Embolic manifestations, n (%) | 22 (38.6) | 22 (31.4) | 0.3 | |
| In-hospital surgery, n (%) | 22 (38.6) | 29 (41.4) | 0.7 | |
| Surgery not performedb, n (%) | 15 (26.3) | 3 (4.3) | <0.001 | 1.6 (1.23–20.09) |
- CNS, central nervous system; MRSA, methicillin-resistant S. aureus; SD, standard deviation.
- aExcluding haemodialysis patients.
- bSurgery indicated but not performed.
Haemodialysis-associated IE
There were 21 cases of haemodialysis-associated IE (24.1%). The mean age of these patients was 58.3 years, and they had a great number of comorbidities (Charlson index number, 4.4 ± 2.2). Haemodialysis-associated cases had a significantly greater number of multi-valve infections (23.8% vs. 4.5%; p 0.01) than cases of IE related to other vascular procedures. CoNS accounted for 52.4% of the episodes, followed by S. aureus (28.6%). Intrahospital mortality of these cases was 61.9%.
Discussion
In recent years, there has been an increasing number of reports of HAIE [1,8–10]. This has been associated with increasing numbers of bacteraemias due to invasive diagnostic and therapeutic procedures [19]. Other reasons for the growing numbers of reported HAIE cases are: (i) the greater diagnostic yield of TOE [20,21]; (ii) broadening of the definition of nosocomial infection to include non-hospitalized patients with healthcare contact [21]; and (iii) extension of incubation periods to culture a greater range of causal organisms [22,23].
The present study is one of the largest series of HAIE to be reported, with prospective recruitment over a long period and a broadened definition of the disease [12]. Our definition of HAIE encompasses a long clinical history because: (i) once a patient has been colonized or infected by a healthcare-related pathogen, colonization persists for a long time [22]; and (ii) some microorganisms involved in this kind of infection, such as CoNS, have a long incubation period [23]. Nevertheless, with our definition of HAIE, the microorganisms reported in our study are those classically associated with healthcare-related infections.
We have observed a continuous increase in the incidence of HAIE over the period of the study. Overall, we found a higher incidence than has previously been reported from Spanish studies that did not use our extended definition [8,24] but a lower incidence than has been reported from more recent studies [9,10] that included early PVE cases, right-sided IE and pacemaker infections. With our exclusion criteria and definitions, HAIE accounted for 16% of all IE cases in the present study.
In recent reports, vascular manipulation was the leading source of bacteraemia causing HAIE [1,8–10], and we found this in 63% of our cases. This contrasts sharply with initial historic figures from the 1960s, where this risk factor occurred in fewer than 20% of cases [20]. It is notable that peripheral venous catheters (PVCs) were the source of HAIE in 15.7% of our cases, and accounted for one-third of the catheter-related manipulations. Central venous catheters carry a greater risk of CAB than PVCs, but the far greater number of PVC insertions increases their significance as a risk factor. A recent publication observed that PVC-related bacteraemias were mainly caused by S. aureus [21]. In our series, S. aureus accounted for 75% of the PVC-related cases of HAIE, and Staphylococcus species accounted for 58.3% of all causative organisms, reflecting the vascular origin of bacteraemia in these patients [24,25].
The overall incidence of MRSA in this study reflects the lower prevalence of this microorganism in Europe than in the USA [26]. However, this series started in 1984, and MRSA has become more common in recent years, so this situation may change in the future. The percentage of HAIE cases caused by CoNS (27.6%) confirms the rising trend described in other publications [4,14]. Although the presence of CoNS is associated with infections of prosthetic valves, in our series, which excluded early PVE, 86.7% of the HAIE cases caused by CoNS were found on native valves. CoNS causing IE on native valves has traditionally been considered to be a relatively benign entity, with a subacute course [27,28]. However, two recently published studies in Spain [29,30] show that the mortality of this entity occupies an intermediate position between S. aureus and the viridans group streptococci. The importance of Enterococcus species in HAIE our series (17.3%) parallels recently published studies, in which it was present in 5–20% of HAIE cases [31–33]. Our study study confirms other reports that genitourinary tract instrumentation is the main source of enterococcal bacteraemia leading to HAIE [34].
HAIE is associated with a higher mortality rate and increased length of hospital than CAIE, and is often potentially avoidable. The higher mortality rate is explained by a number of risk factors, including infections in elderly people in poor general health and with frequent comorbidities, frequent complications (septic shock, and renal and heart failure) and infections with more virulent or antibiotic-resistant organisms (S. aureus, MRSA and CoNS). In the multivariate analysis of the whole series, HAIE was an independent risk factor for in-hospital mortality, as has been described before [1,5,10]. Our in-hospital mortality rate (44.9%) differs from that in another a recently published report from a multicentre international group [10]. However, that study included patients with right-sided IE and pacemaker infections. Furthermore, their percentage of surgical treatment was higher than ours (51% vs. 44.1%), and surgery has been related to a better outcome. However, our in-hospital mortality rates are very similar to those published by other groups from areas close to ours [1,9].
In this study, surgery itself was not associated with a different outcome. However, patients who were recommended for surgery but could not have it because of poor clinical condition had a very high intrahospital mortality rate (83.3%). The EuroSCORE was designed to predict 30-day mortality of patients undergoing cardiac surgery, including coronary artery bypass grafting [18], but it may be useful when making decisions on patients with IE [35]. Further data are needed to assess the exact value of this scale in predicting mortality in patients with IE who are operated on.
In conclusion, this study shows progressive increase in the number of HAIE cases. The underlying pathology, comorbidities, complications and pathogens associated with this condition lead to a high mortality rate. In this study, the initial bacteraemia that caused HAIE had a vascular origin in two-thirds of cases. Physicians must be aware of the risk of endocarditis with invasive procedures in patients with predisposing factors, and maintain proper aseptic measures during the manipulation of vascular lines. Prompt diagnosis and treatment of HAIE may prevent irreversible valvular damage, so this condition should always be thought of in patients with prolonged bacteraemia and previous vascular manipulation.
Acknowledgements
This study was presented in part as an oral presentation at the 48th Annual ICAAC/IDSA 46th Annual Meeting, 28 October 2008, Washington, DC.
Transparency Declaration
The study was supported by the Ministerio de Sanidad y Consumo, Instituto de Salud Carlos III, Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), Madrid, Spain. The authors declare that they have no conflicts of interest.