Successful combat of an outbreak due to Clostridium difficile PCR ribotype 027 and recognition of specific risk factors
Abstract
Clin Microbiol Infect 2009; 00: 000–000
In the period April–September 2005, an outbreak of Clostridium difficile infection (CDI) due to PCR ribotype 027 occurred among 50 patients in a 341-bed community hospital in Harderwijk, The Netherlands. A retrospective case–control study was performed to identify risk factors specific for CDI, using a group of patients with CDI (n = 45), a group of randomly selected control patients without diarrhoea (n = 90), and a group of patients with non-infectious diarrhoea (n = 109). Risk factors for CDI and for non-CDI diarrhoea were identified using multiple logistic regression analysis. Independent risk factors for CDI were: age above 65 years (OR 2.6; 95% CI 1.0–5.7), duration of hospitalization (OR 1.04 per additional day; 95% CI 1.0–1.1), and antibiotic use (OR 12.5; 95% CI 3.2–48.1). Of the antibiotics used, cephalosporins and fluoroquinolones were identified as the major risk factors for development of CDI. The risk of developing CDI was particularly high in people receiving a combination of a cephalosporin and a fluoroquinolone (OR 57.5; 95% CI 6.8–483.6). The main factors affecting the risk of non-CDI diarrhoea were proton-pump inhibitors, immunosuppressive drugs, underlying digestive system disease, previous surgery, and gastric tube feeding. The outbreak ended only after implementation of restricted use of cephalosporins and a complete ban on fluoroquinolones, in addition to general hygienic measures, cohorting of patients in a separate ward, education of staff, and intensified environmental cleaning. The results of this study support the importance of appropriate antimicrobial stewardship in the control of hospital outbreaks with C. difficile PCR ribotype 027.
Introduction
Clostridium difficile infection (CDI) is one of the most common hospital-acquired infections, and is a frequent cause of morbidity and mortality among elderly hospitalized patients [1]. Recent reports indicate an increasing occurrence and severity of CDI [2–5]. This change in epidemiology and clinical presentation can, to a certain extent, be explained by the spread of a new, potentially more virulent isolate, referred to as PCR ribotype 027/toxinotype III/pulsed-field gel electrophoresis type NAP1/REA group BI (027/III/NAP1/BI), which has caused outbreaks in North America and Europe [6–12].
The most important risk factor for CDI is prior antibiotic use. Other risk factors are: increasing age, severe underlying disease, prolonged duration of hospitalization, CDI pressure (defined as the sum of a patient’s daily exposure to patients with CDI who share the same unit or ward divided by the length of stay of the patient at risk [13,14]), gastrointestinal surgery, and enteral tube feeding [15–19]. During the recent outbreaks caused by C. difficile PCR ribotype 027, several new putative risk factors have been reported, e.g. the use of proton-pump inhibitors [20–22], of non-steroidal anti-inflammatory drugs [22], and of fluoroquinolones [23–25].
Given the high a priori chance of non-infectious diarrhoea developing in hospitalized patients, it is often difficult to distinguish between risk factors specific for CDI and risk factors for diarrhoea due to other causes in the setting of an epidemic of CDI.
To unravel the risk factors specific for CDI, we performed a case–control study using a group of patients with CDI and a group of patients with non-infectious diarrhoea, both diagnosed during an outbreak of C. difficile PCR ribotype 027 in a community hospital.
Materials and Methods
Study population and definition of CDI cases
This study was conducted during an epidemic of CDI caused by C. difficile PCR-ribotype 027 in St Jansdal Hospital, a 341-bed community hospital in Harderwijk, The Netherlands. CDI was defined by the presence of diarrhoea (two or more loose bowel movements per day) and a positive C. difficile toxin assay result from a stool sample. All faecal samples were tested within 1–18 h after arrival at the laboratory, using a rapid enzyme immunoassay (ImmunoCard Toxin A and B (ICTAB); Meridian, Boxtel, The Netherlands). In patients with diarrhoea and a negative rapid immunoassay result, a second faecal sample was tested after 24–48 h. When two tests gave negative results, CDI was considered to be unlikely.
Characterization of C. difficile isolates
Toxin-positive faecal samples were cultured for the presence of C. difficile, using non-selective and selective agar supplemented with cefoxitin, amphotericin B, and cycloserin (CLO-medium; Biomérieux), with and without ethanol shock pretreatment. After incubation in an anaerobic environment at 37°C for 48 h, colonies of Gram-positive rods with subterminal spores were tested for the production of l-proline aminopeptidase and for the hydrolysis of esculine. All culture-positive strains isolated from faecal samples were identified as C. difficile using a PCR for the presence of the gluD gene encoding the glutamate dehydrogenase specific for C. difficile [9]. C. difficile isolates were tested for the presence of the tcdA and tcdB binary toxin genes and deletions in tcdC, as described previously [9]. PCR ribotyping and toxinotyping were performed as described previously [26,27]. For all isolates, susceptibility to erythromycin, ciprofloxacin, clindamycin and metronidazole was determined by Etest (AB Biodisk, Solna, Sweden).
Case–control study
To identify risk factors specific for CDI, patients were assigned to three different study groups during the peak of the outbreak in St Jansdal Hospital (Table 1). Study group I consisted of 45 patients diagnosed with CDI as described above. Study group II consisted of 109 patients diagnosed with non-CDI diarrhoea, i.e. patients with diarrhoea who tested negative in the C. difficile toxin assay of two faecal samples collected at least 24 h apart.
| Characteristic | CDI | Non-CDI | Controls |
|---|---|---|---|
| n | 45 | 109 | 90 |
| Gender | |||
| Male | 19 (42.2) | 36 (33.0) | 42 (46.7) |
| Female | 26 (57.8) | 73 (67.0) | 48 (53.3) |
| Age, years | |||
| 18–64 | 8 (17.8) | 41 (37.7) | 30 (33.3) |
| ≥65 | 37 (82.2)* | 68 (62.3) | 60 (66.7) |
| Main comorbidity (ICD-10 classification) | |||
| Neoplasm | 12 (26.7) | 28 (25.7) | 23 (25.6) |
| Endocrine disease | 16 (35.6) | 30 (27.5) | 18 (20.0) |
| Cardiovascular disease | 28 (62.2)** | 52 (47.7) | 34 (37.8) |
| Respiratory system disease | 16 (35.6)* | 14 (12.8) | 17 (18.9) |
| Digestive system disease | 11 (24.4) | 32 (29.4)* | 14 (15.6) |
| Musculoskeletal disease | 6 (13.3) | 14 (12.8) | 10 (11.1) |
| Genitourinary disease | 13 (28.9) | 22 (20.2) | 20 (22.2) |
| Duration of stay in hospital (prior to diarrhoea), in days: median (range) | 7 (0−77)* | 4 (0−97) | 4 (0−63) |
| Level of care | |||
| Intensive-care unit stay | 9 (20.0) | 19 (17.4) | 8 (8.9) |
| Surgery | 7 (15.6) | 42 (38.5)** | 20 (22.2) |
| Endoscopy prior to CDI | 6 (13.3) | 9 (8.3) | 11 (12.2) |
| Nasogastric tube | 10 (23.3)** | 24 (22.9)** | 7 (7.8) |
| Antibiotics received in the preceding 3 months | |||
| Any antibiotic | 42 (93.3)*** | 53 (50.5) | 42 (46.7) |
| Penicillins | 10 (22.2) | 22 (20.2) | 21 (23.3) |
| Cephalosporins | 33 (73.3)*** | 18 (16.5)* | 25 (27.8) |
| Tetracycline | 3 (6.7) | 0 | 0 |
| Aminoglycosides | 2 (4.4) | 4 (3.7) | 2 (2.2) |
| Macrolides | 16 (35.6)*** | 4 (3.7) | 9 (10.0) |
| Clindamycin | 1 (2.2) | 5 (4.6) | 5 (5.6) |
| Quinolones | 13 (28.9)*** | 7 (6.4) | 3 (3.3) |
| Other | 12 (26.7) | 13 (11.9) | 14 (15.6) |
| Other drugs received in the preceding 3 months | |||
| Proton-pump inhibitors | 21 (46.7) | 27 (24.8) | 31 (34.4) |
| H2 blockers | 2 (4.4) | 0 | 2 (2.2) |
| Drugs used in diabetes | 7 (15.6) | 10 (9.2) | 11 (12.2) |
| Antithrombotic agents | 30 (66.7)** | 55 (50.5) | 40 (44.4) |
| Cardiovascular system, all agents | |||
| Digoxin | 31 (68.9)* | 37 (33.9)* | 43 (47.8) |
| Diuretics | 11 (24.4)*** | 2 (1.8) | 5 (5.6) |
| β-Blocking agents | 18 (40.0) | 21 (19.3) | 26 (28.9) |
| Calcium channel | 8 (17.8) | 17 (15.6) | 15 (16.7) |
| Blockers | 8 (17.8) | 9 (8.3) | 10 (11.1) |
| Renin–angiotensin | 17 (37.8)** | 17 (15.6) | 17 (18.9) |
| Modifying agents | 7 (15.6) | 8 (7.3) | 10 (11.1) |
| Lipid-modifying agents | 28 (62.2)** | 17 (15.6)** | 30 (33.3) |
| Respiratory medication | 17 (37.8) | 7 (6.4)*** | 22 (24.4) |
| Immunosuppressive agents, NSAIDs | 24 (53.3) | 48 (44.0) | 43 (47.8) |
- Data are no. (%) of patients, unless otherwise indicated.
- n, number of patients; CDI, Clostridium difficile infection; non-CDI, diarrhoea due to another cause; NSAIDs, non-steroidal anti-inflammatory drugs.
- Significantly different from control group (*p <0.05, **p <0.01, ***p <0.001).
Study group III consisted of 90 randomly selected control patients without diarrhoea. Patients with non-CDI diarrhoea (study group II) and control patients (study group III) were randomly selected from among all patients residing at the same time and in the same ward as the patient newly diagnosed with CDI.
A standardized questionnaire was used to collect clinical and demographic data from hospital records. Data were collected concerning each participant’s age and gender, time of onset and duration of diarrhoea, duration of hospital stay, previous hospitalization, co-morbidity, and level of care prior to the development of diarrhoea. Comorbidity was defined according to the International Classification of Disease, version 10 (ICD-10). For study groups I and II, the duration of hospital stay was defined as the number of days from admission to the development of diarrhoea; for study group III, it was defined as the number of days from admission to discharge. Information on the use of antibiotics or other medication within the preceding 3 months was extracted from an electronic pharmacy database. This database contained information on all medications prescribed both within and outside the hospital for every participant in this study. All medications used were categorized according to the latest international ATC code [28]. The defined daily dose of antibiotics was established according to the WHO Collaborating Centre for Drug Statistics Methodology guidelines for ATC classification and defined daily dose assignment [28].
For each patient diagnosed with CDI, additional information was collected concerning severity of disease, treatment regimen, disease recurrence, and 30-day mortality. Recurrent disease was defined as a second episode of diarrhoea within 30 days of diagnosis of CDI following initial clinical improvement, combined with a positive C. difficile toxin assay result from a stool sample.
Statistical analysis
The distributions of risk factors in study group I and study group II were compared to the distribution in the control group (study group III). Continuous data were compared among groups using analyses of variance. A Yates-corrected chi-square test was used for the analysis of proportions. If a cell value was less than five in the two-by-two table, Fisher’s exact test was used. A multiple logistic regression model was used to study the association of putative risk factors with CDI and non-CDI diarrhoea. Relative risks were estimated as ORs and presented with a 95% CI. Both crude ORs and ORs after adjustment for the possible confounder’s age, duration of hospital stay, comorbidity (ICD-10 category), level of care and comedication are presented in Table 2. All p-values were two-sided. Finally, for both cephalosporin therapy and fluoroquinolone therapy, the population-attributable risk percentage (PAR%) was calculated as previously described [29]. All analyses were performed using SPSS for Windows, version 13.0.
| CDI | Non-CDI | |||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI)a | Crude OR (95% CI) | Adjusted OR (95% CI)a | |
| Age, years | ||||
| 18–64 | 1 (reference) | 1 | 1 | 1 |
| ≥65 | 2.6 (1.0–5.7)* | 2.6 (1.0–5.7)* | 0.8 (0.5–1.5) | 0.8 (0.5–1.5) |
| Duration of stay in hospital | 1.03 (1.0–1.1)* | 1.04 (1.0–1.1)* | 1.0 (0.9–1.0) | 1.0 (0.9–1.0) |
| Cardiovascular disease | 2.7 (1.3–5.6)** | 1.9 (0.8–5.2) | 1.5 (0.8–2.6) | 1.9 (0.9–3.8) |
| Respiratory system disease | 2.3 (1.0–5.2)* | 2.7 (0.9–7.9) | 0.6 (0.3–1.4) | 1.3 (0.5–3.7) |
| Digestive system disease | 1.7 (0.7–4.2) | 2.7 (0.9–8.9) | 2.2 (1.1–4.5)* | 3.1 (1.2–7.8)* |
| Surgery | 0.6 (0.3–1.6) | 0.7 (0.2–2.4) | 2.2 (1.2–4.1)* | 2.1 (1.0–4.3)* |
| Nasogastric tube | 3.4 (1.2–9.5)* | 3.6 (1.0–14.3)* | 3.3 (1.4–8.1)** | 4.8 (1.7–13.6)** |
| Antibiotics | ||||
| Any antibiotic | 15.3 (4.4–53.2)*** | 12.5 (3.2–48.1)*** | 1.1 (0.6–2.0) | 1.8 (0.9–3.7) |
| Cephalosporins | 7.0 (3.1–15.7)*** | 5.7 (1.8–18.6)b,*** | 0.5 (0.3–1.0) | 0.9 (0.3–2.1) |
| Macrolides | 4.9 (2.0–12.3)*** | 2.4 (0.7–8.7)c | 0.3 (0.1–1.2) | 0.3 (0.1–1.6) |
| Quinolones | 11.6 (3.1–43.6)*** | 15.3 (2.7–84.6)d,** | 2.0 (0.5–7.8) | 2.2 (0.5–10.6) |
| Other drugs | ||||
| Proton-pump inhibitors | 1.6 (0.8–3.4) | 1.1 (0.5–2.6) | 0.6 (0.3–1.0)* | 0.9 (0.5–1.7) |
| Antithrombotic agents | 2.5 (1.2–5.2)* | 1.2 (0.5–2.9) | 1.1 (0.7–2.2) | 1.7 (0.8–3.6) |
| Cardiovascular agents, all | ||||
| Digoxin | 2.4 (1.1–5.0)* | 1.3 (0.4–4.9) | 0.6 (0.3–1.0)* | 0.7 (0.4–1.5) |
| Renin–angiotensin | 5.4 (1.8–16.8)** | 2.3 (0.6–12.1) | 0.3 (0.1–1.7) | 0.5 (0.1–3.2) |
| Modifying agents | 2.6 (1.2–5.7)* | 2.2 (0.7–7.0) | 0.8 (0.4–1.6) | 0.9 (0.4–2.2) |
| Respiratory medication | 3.2 (1.5–6.8)** | 1.1 (0.4–3.2) | 0.4 (0.2–0.7)** | 0.6 (0.2–1.2) |
| Immunosuppressive agents | 1.8 (0.9–4.0) | 0.9 (0.3–2.5) | 0.2 (0.1–0.5)** | 0.4 (0.1–1.0)* |
| NSAIDs | 1.2 (0.6–2.5) | 1.0 (0.4–2.4) | 0.8 (0.5–1.5) | 1.0 (0.5–1.9) |
- CDI, Clostridium difficile infection; non-CDI, diarrhoea due to another cause.
- aAdjusted for differences in age, duration of hospital stay, comorbidity (ICD-10), level of care, and comedication.
- bAdditional adjustment for concomitant use of macrolides and quinolones.
- cAdditional adjustment for concomitant use of cephalosporins and quinolones.
- dAdditional adjustment for concomitant use of cephalosporins and macrolides.
- *p <0.05; **p <0.01; ***p <0.001.
Results
Description of the outbreak
The background incidence of CDI in St Jansdal Hospital was 3.8 patients per 10 000 admissions in 2004. In 2005, a more than ten-fold increase in the incidence of CDI was observed (Fig. 1). In this study, we included the first 45 patients diagnosed with CDI in 2005. In total, 50 patients with CDI were diagnosed during the outbreak. Faeces were cultured, and C. difficile isolates were identified as toxinotype III and PCR ribotype 027. In addition, the strain had the binary toxin genes and contained an 18-bp deletion in the toxin regulator gene tcdC. The isolates were resistant to erythromycin (MIC >256 mg/L) and ciprofloxacin (MIC >32 mg/L), and susceptible to clindamycin (MIC 2 ml/L) and metronidazole (MIC 0.19 mg/L).
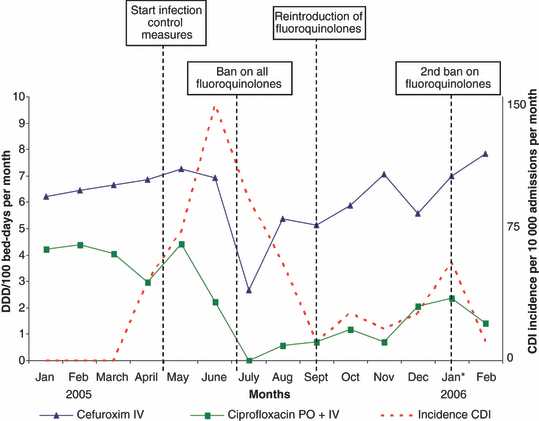
Course of the epidemic and dynamics of antibiotic use in St Jansdal Hospital. DDD, defined daily dose; PO, oral administration; IV, intravenous administration.
A multidisciplinary hospital outbreak management team (OMT) was formed to coordinate measures to control the epidemic. Special folders informed medical personnel in the hospital. In addition, all clinicians were informed personally. The medical microbiologist and infection control practitioner organized special meetings on the involved wards with the nursing staff. The cleaning team received special instructions for intensified cleaning procedures from the infection control practitioner. All measures were described in a CDI hospital guideline by the OMT.
Measures taken by the OMT to control the epidemic (from 1 May 2005 onwards) included isolation of all patients with diarrhoea (until two tests, 24 h apart, gave negative results for C. difficile toxin), hand washing with water and soap, use of chlorine-containing disinfectant (0.1% sodium hypochlorite), and cohorting of all C. difficile-infected patients on a separate ward. In addition, from 7 July 2005 until 14 September 2005, a complete ban on all fluoroquinolones was established, and the use of cephalosporins and clindamycin was limited.
The course of the epidemic, including the time-scheme of all infection control measures taken and the use of antibiotics in the hospital, are depicted in Fig. 1. The outbreak came to an end in September 2005. After the re-introduction of fluoroquinolones, however, a temporary increase in CDI was noticed.
Description of C. difficile-associated disease cases
From 1 April 2005 until the end of August 2005, a total of 45 patients met the case definition of CDI. Clinical characteristics of the CDI cases are given in Table 1. Thirty-five patients developed diarrhoea during their stay in the hospital (mean duration of hospital stay prior to development of symptoms was 13 days). Of the ten patients admitted with diarrhoea, nine patients had healthcare-associated CDI, as they had been hospitalized in the same hospital within the preceding 3 months. The only patient who had not been hospitalized before was suffering from ulcerative colitis and was known to have frequent periods of diarrhoea.
The symptoms and signs most frequently observed within the first 2 weeks following onset of diarrhoea were fever (53.3%), abdominal pain (20%), high white blood cell count in the first 2 weeks after onset of diarrhoea (mean 1.6 × 1010 cells/mL; >2.0 × 1010 cells/mL in 23.7% of cases), high erythrocyte sedimentation rate (mean, 48.2 mm/h), high serum creatinine level (mean, 0.149 mmol/L; >0.200 mmol/L in 17.5% of cases), and low serum albumin level (mean, 28.6 g/L). Bloody stools were noticed in only three patients (6.7%). All but two patients were treated with vancomycin or metronidazole or a combination of both. Recurrence of diarrhoea following initial improvement was observed in ten patients (22%). In nine of these patients, a positive C. difficile toxin assay result was obtained from a stool sample. Recurrence of CDI was more often seen in patients with a peak white blood cell count >2.0 × 1010 cells/mL (p 0.002; OR 16, and 95% CI 2.8–90.4) or a peak serum creatinine level >0.200 mmol/L (p 0.03; OR 7.1, and 95% CI 1.3–40.2). Nine patients (20%) with CDI died within 30 days after diagnosis, three (7%) as a direct result of CDI. A peak white blood cell count >2.0 × 1010 cells/mL within the first 2 weeks following onset of diarrhoea was a strong predictor of mortality (p 0.01; OR 7.8, and 95% CI 1.5–39.1).
Case–control study
Table 1 presents the characteristics of the participants in the case–control study. Table 2 shows the risk of CDI and non-CDI diarrhoea. Both crude ORs (univariate analysis) and adjusted ORs (multivariate analysis) are given (only characteristics that were significantly different among study groups in the univariate analysis are shown). After adjustment for differences in comorbidity, level of care, and comedication, the independent risk factors for CDI were age above 65 years (OR 2.6), duration of hospitalization (OR 1.04 per additional day), and antibiotic use (OR 12.5). Independent risk factors for non-CDI diarrhoea were underlying digestive system disease (OR 3.1) and previous surgery (OR 2.1). Although immunosuppressive agents and proton-pump inhibitors were not associated with CDI, patients with non-CDI diarrhoea were less often treated with these. Finally, nasogastric tube feeding appeared to be a general risk factor for diarrhoea, being associated both with CDI (OR 3.6) and with non-CDI diarrhoea (OR 4.8).
Antibiotic use was exclusively associated with CDI. Of all antibiotics, cephalosporins, macrolides and fluoroquinolones were associated with CDI in the univariate analysis (Table 2). After correction for differences in comorbidity, level of care, co-medication, and the use of multiple antibiotics, the association of CDI with macrolides was no longer significant. Even with the small numbers in our study, we could demonstrate a statistically significant interaction between cephalosporin and fluoroquinolone use in the multivariate analysis (OR for the interaction factor, 13.6; p 0.006). To study this interaction in more detail, we analysed the risk of CDI for different treatment schemes (Fig. 2). In this analysis, cephalosporin monotherapy (OR 7.8, 95% CI 2.9–20.9) and fluoroquinolone monotherapy (OR 28.8, 95% CI 2.6–319.2) were shown to be independent risk factors for CDI. Patients who used a combination of both antibiotics in the preceding 3 months had the highest risk of developing CDI (OR 57.5, 95% CI 6.8–483.6). The PAR%, i.e. the proportion of CDI cases in the study population that was attributable to the use of cephalosporin or fluoroquinolone therapy, was calculated as 56% and 33%, respectively.
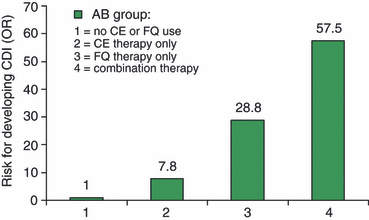
Risk for development of Clostridium difficile infection (CDI), stratified by cephalosporin (CE) and fluoroquinolone (FQ) therapy within the preceding 3 months.
Discussion
In 2005, the first outbreak of CDI due to C. difficile 027/III/NAP1/BI occurred in a medium-size hospital in The Netherlands. As was also observed during the recent epidemics in Europe and North America, the outbreak was very difficult to control, and came to an end only after implementation of measures in addition to general measures of hygiene, i.e. cohorting of all C. difficile-infected patients on a separate ward, education of staff, intensified cleaning of the environment, and strong limitations on antibiotic use. These measures have also been described as an effective comprehensive ‘bundle’ approach to combat CDI oubreaks in the USA [30,31].
The use of cephalosporins is a well-documented risk factor for the development of CDI [15–17]. In this study, fluoroquinolone therapy, especially in combination with cephalosporin therapy, was identified as another major risk factor for the development of CDI. Ciprofloxacin is still the main fluoroquinolone used in The Netherlands. In our study population, 22 patients used ciprofloxacin and only one patient used moxifloxacin. Although fluoroquinolones account only for a small proportion of all antibiotics used in St Jansdal Hospital (9.5% of all antibiotics prescribed, in contrast to 31% for cephalosporins), the proportion of CDI cases in the study population that was attributable to the use of fluoroquinolones was as high as 33%. This finding is in line with results reported by Pepin et al. [23], who calculated a PAR% of 35.9% for fluoroquinolones during a large outbreak of nosocomial CDI in Canada.
Associations between CDI and fluoroquinolones, including ciprofloxacin, have been described previously [23–25,32–36]. A recent study, which included ‘CDAD pressure’ as a risk factor for the development of CDI, found ciprofloxacin to be an independent factor [14]. In The Netherlands, ciprofloxacin has been recognized as a risk factor for acquisition of CDI, particularly infection due to PCR ribotype type 027 [12]. However, the exact role of fluoroquinolones in the aetiology of CDI is still unclear. An important factor might be the increasing fluoroquinolone resistance of C. difficile, which has been observed worldwide [37,38], coupled with an increasing use of fluoroquinolones, leading to more efficient proliferation of resistant clones following disruption of colonic flora. Until 2000, no relationship between CDI and the use of ciprofloxacin and ofloxacin had been reported. Interestingly, two historical isolates of C. difficile from 1987, which were also typed as 027/III/NAP1/BI, were susceptible to fluoroquinolones [6]. Therefore, we consider it very likely that the acquisition of fluoroquinolone resistance contributed to the increased spread of this hypervirulent strain. Recently, several authors have underlined the importance of the improved anti-anaerobe spectrum of the newer fluoroquinolones in the aetiology of CDI [25,37]. However, this does not apply to ciprofloxacin, which possesses poor in vitro activity against anaerobic bacteria.
As correctly stated by Wilcox et al. [1], the duration of treatment and antibiotic polypharmacy affect the incidence of CDI, and may confound risk analyses for antimicrobial agents. Pepin et al. [23] suggested that long duration of fluoroquinolone therapy, in particular, enhances the risk of CDI. Unfortunately, we did not have sufficient data to assess the possible effect of duration of treatment on the risk of CDI in our study. With respect to polypharmacy, it must be noted that, in The Netherlands, fluoroquinolones are often administered together with cephalosporins, e.g. in empirical therapy of severe community-acquired pneumonia. In a separate analysis, after correcting for differences in comedication and the use of multiple antibiotics, we could demonstrate that patients who had received fluoroquinolone monotherapy within the preceding 3 months were also at very high risk of developing CDI. This clearly demonstrates that fluoroquinolones represent an independent risk factor for C. difficile-associated disease in our population. Surprisingly, the risk of developing CDI was extremely high in people receiving a combination of cephalosporins and fluoroquinolones. The fact that the OR in these subjects was much higher (57.5) than could be explained by simply summing the ORs for the separate antibiotics (7.8 and 28.8, respectively) could suggest a synergistic effect of cephalosporins and fluoroquinolones in the aetiology of CDI.
In addition to antibiotic use, several other risk factors have been associated with the development of CDI [15–25]. Analysing three different study populations, we were able to demonstrate that underlying digestive system disease, previous surgery and gastric tube feeding are not specifically associated with CDI, but are general risk factors for (non-infectious) diarrhoea. In addition, we demonstrated that although proton-pump inhibitors and immunosuppressive medication were not associated with CDI, subjects with non-infectious diarrhoea less frequently used these drugs. This observation indicates that differences in selection of control subjects may underlie the inconsistency among studies regarding the role of proton-pump inhibitors and immunosuppressive medication in the aetiology of CDI. Unfortunately, we were unable to determine the role of ‘CDI pressure’ as a risk factor [13,14].
Most experts emphasize that antimicrobial intervention alone is unlikely to result in successful control of all CDI outbreaks. Issues related to the environment, education and infection control should also be addressed [30] A recently published ECDC-supported guideline emphasizes the importance of antimicrobial stewardship in conjunction with proper environmental disinfection, hand hygiene compliance, protective clothing, education of staff, and single-room isolation or cohorting of CDI patients [39]. The outbreak described here ended only after the formation of a multidisciplinary hospital OMT to coordinate measures to control the epidemic, the enhancement of case-finding and compliance by continuous education, isolation of all patients with diarrhoea until CDI was excluded, increasing the rapidity of microbiological diagnosis by using repeated stool ICTAB testing, the implementation of specific hygiene measures (including hand washing with water and soap and intensified environmental cleaning procedures), the cohorting of all CDI patients on a separate ward, and the implementation of an antimicrobial stewardship programme. The value of implementation of a CDI control ‘bundle’, including early identification, coupled with appropriate control measures, in reducing the rate of CDI and the frequency of adverse events in a university hospital was shown recently by Muto et al. [31]. The importance of appropriate antimicrobial stewardship has recently been illustrated by a report from Canada. Valiquette et al. [40] reported that no change in CDI incidence was noted after strengthening of infection control procedures, but that implementation of the antimicrobial stewardship programme was followed by a marked reduction in incidence. These observations are very similar to those made in this study, as an effective outbreak control was only obtained after strong restrictions on the use of cephalosporins and a complete ban on the use of ciprofloxacin. The decline in CDI cases following restriction of cephalosporin use and a complete ban on the use of fluoroquinolones in our hospital, followed by an increase in CDI cases following the reintroduction of fluoroquinolones, underline the importance of these antibiotics in the development of CDI.
In conclusion, cephalosporin therapy and fluoroquinolone therapy were identified as important risk factors for the development of CDI during an outbreak of C. difficile PCR ribotype 027 in The Netherlands. The risk of developing CDI was particularly high in people receiving a combination of cephalosporins and fluoroquinolones. Our data indicate the importance of good antimicrobial stewardship, in relation with other measures, to control outbreaks of C. difficile PCR ribotype 027.
Transparency Declaration
This study was not sponsored commercially. St Jansdal Hospital provided a grant for a 6-month fellowship to A. Choudry. The authors did not have any dual or conflicting interests.




