A technique for dating toxoplasmosis in pregnancy and comparison with the Vidas anti-toxoplasma IgG avidity test
Abstract
A comparative evaluation of 384 selected sera was performed using the Beckman Coulter Access and Abbott Axsym Toxo-IgG assays. The Axsym assay yields positive early results following infection, while the Access assay gives higher titres during chronic infection. The ratio between the two complementary tests, Axsym Toxo-IgG/Access Toxo-IgG (Ax/Ac), was compared with the Vidas anti-Toxoplasma IgG avidity index (AI). The Ax/Ac ratio decreased progressively as the time between infection and sampling increased. The mean Ax/Ac values (±SE) were 2.50 (±0.26), 2.14 (±0.13), 2.33 (±0.22), 1.34 (±0.09), 1.32 (±0.10), 0.92 (±0.08) and 0.74 (±0.07) for groups of sera sampled at 1, 2, 3, 4–5, 6–8, 9–12 and 13–24 months, respectively, after infection in pregnant women. These values were much smaller for cases with chronic infection (>24 months), i.e., 0.56 (±0.03), 0.44 (±0.04) and 0.53 (±0.04), respectively, for pregnant women and immunodepressed patients with and without reactivation. Taking a ratio of 1 as a threshold for recent infection, the patients in the groups sampled at 1, 2 and 3 months had Ax/Ac ratios >1 in 49/50 (98%), 53/55 (96.4%) and 36/36 (100%) cases, respectively. Thus, an Ax/Ac ratio of <1 in serum from a pregnant woman allows a recent infection (<3 months) to be excluded. This technique has the advantage of yielding positive results that develop much more rapidly than the AI, thereby helping to reassure large numbers of pregnant women and avoiding costly and unnecessary prophylactic treatment and follow-up.
Introduction
The follow-up of obstetrical toxoplasmosis depends mainly on the detection of anti-Toxoplasma-specific IgM and IgG [1–3]. When an individual is positive for both IgM and IgG in the absence of a previously diagnosed infection, complementary tests are required to differentiate between a recently acquired infection (<3 months) and a more chronic infection. Such tests include the detection of specific IgA and IgE antibodies [4,5], but the avidity index (AI), a technique developed about 15 years ago [6], is the test used most widely [1–3]. An elevated AI in the first trimester of pregnancy excludes a recent infection and helps to prevent unnecessary follow-up and treatment. Different techniques to measure the AI in pregnancy have been developed, evaluated and compared in a number of studies [5,7–16]. Although the AI has been universally accepted as a reliable test, recent studies [10,11,16] have shown that the evolution of a positive reaction is slow, so that its use for excluding infections that have occurred only a few months before pregnancy is limited. Using 271 sera, it was recently demonstrated that an avidity threshold of 0.2 was reached 6–24 months after infection (mean 11.49 ± 3.9 months), and that the avidity threshold of 0.3 was reached 7 - >24 months after infection (mean 14.20 ± 4.8 months) [10]. This slow evolution of positive results, which varies according to the technique used for detection, is the principal limitation of this test and justifies the use of complementary assays [16].
Another dating technique that compares the results of two serological tests with different antigenic targets (i.e., predominantly membranous antigen and predominantly cytoplasmic antigen) has also been studied with a view to differentiating recent and chronic infection [17,18]. One approach involves comparing the kinetics of two commercially available assays in parallel, e.g., the Axsym Toxo-IgG assay (Abbot Diagnostic, Rungis, France) and the Assess Toxo-IgG assay (Beckman Coulter, Roissy, France). The principles and the techniques of these two automated tests are very similar. Axsym Toxo-IgG uses a microparticle enzyme immunoassay, while Access Toxo-IgG uses a chemiluminescence immunoassay with microparticles, and both use a two-step indirect antibody test. Axsym Toxo-IgG seems to become IgG-positive earlier, and gives significantly elevated IgG titres, compared with Access Toxo-IgG, with sera taken in the first 3 months after infection. The present study used 384 selected sera to compare these two commercial immunoassays in terms of their sensitivity and speed in detecting acute and chronic infection, and also investigated the use of the Axsym Toxo-IgG/Access Toxo-IgG (Ax/Ac) ratio as a new technique for dating toxoplasmosis infection. The kinetics of this ratio and its correlation with the time after infection were evaluated and compared with the kinetics of the AI.
Materials and Methods
Follow-up of pregnant women and immunodepressed patients
The majority of sera (304/384) were from pregnant women with known dates of infection (part 1), and the remainder (80/384) were from cases of chronic toxoplasmosis with known seroconversions >24 months previously (part 2), during the period July 1998 to July 2005. All samples were stored at −20°C before investigation.
Part 1 of the study included 79 pregnant women with seroconversion. The mean age at the time of seroconversion was 28.8 ± 4.9 years (range 16–39 years). Clinical follow-up of the pregnant women and their neonates was continued for up to 1 year for 65 of these women. This group included 23 infants who were born with congenital toxoplasmosis, and 43 infants (including one pair of twins) who did not acquire the disease (Table 1). The 304 sera were obtained from the 79 pregnant women at 1–24 months after infection, and were divided into seven equal sample groups on the basis of the interval between sampling and infection (1, 2, 3, 4–5, 6–8, 9–12 and 13–24 months (±15 days).
| Time of maternal infection (WG) | Mothers | Infants | |||
|---|---|---|---|---|---|
| No. of seroconversions | No. treated during pregnancy | No. lost to follow-up before 1 year of age | No. with proven maternofetal transmission | Fetal and paediatric disease | |
| −6–4 | 10 | 10 (100%) | 2/10 (20%) | 0/8 (0%) | No cases |
| 5–14 | 24 | 23 (96%) | 6/25 (24%) (one pair of twins) | 2/19 (11%) | 1 spontaneous abortion1 clinical infection |
| 15–27 | 24 | 24 (100%) | 4/24 (17%) | 11/20 (55%) | 3 terminations of pregnancy2 clinical infectionsa6 subclinical infections |
| 28–40 | 21 | 9 (45%) | 2/21 (10%) | 10/19 (53%) | 10 subclinical infections |
| Total | 79 | 66 (84%) | 14/80 (17.5%) | 23/66 (35%) | 1 spontaneous abortion3 terminations of pregnancy3 clinical infectionsa16 subclinical infections |
- WG, week of gestation.
- aCerebral calcifications and/or chorioretinal scar without severe visual impairment at an age of 1 year.
It was possible to date infections because of the obligatory monthly serological screening policy in France. Dating was based on the following serological kinetic criteria. The first IgM-positive (or doubtful) serum according to the ISAgA assay (bioMérieux, Durham, NC, USA) or the Toxo-IgM Access assay (Beckman Coulter), with or without IgG, was dated as 1 month (±15 days) after infection. If IgG was absent from the first serum (IgM-positive, IgG-negative), its presence (according to the Access and Axsym Toxo-IgG) during the follow-up period was obligatory for confirming infection. It was necessary for the interval between the first positive serum (IgM with or without IgG) and the preceding negative serum to be >2 months for inclusion in the study. Using these dating criteria, the decision to start treatment was made as follows: (i) no treatment was given when diagnosis was made at delivery or following spontaneous abortion (13/79 cases); or (ii) treatment with spiramycin (9 MIU/mL) was continued for a minimum of 15 days (66/79 cases), with changes made as necessary (pyrimethamine + sulfadiazine for 9/66 cases), as described previously by Flori et al. [6].
In part 2 of the study, samples from chronic cases of toxoplasmosis with seroconversions >24 months previously were investigated. These included 30 sera from 30 pregnant women known to be immunised during their previous pregnancy, 20 sera from 20 immunodepressed patients (infected with human immunodeficiency virus or with organ transplants) who had serological reactivation (two or more increasing antibody titres), and 30 sera from 30 immunodepressed human immunodeficiency virus and organ transplant patients without reactivation.
Serological tests
Toxoplasma-specific IgG was detected using two commercial enzyme immunoassays (Access Toxo-IgG and Axsym Toxo-IgG) and an in-house immunofluorescent assay (IFA). For the latter, a positive cut-off of 8 IU/mL was determined using international standards [19]. An intermediate zone of 4–8 IU/mL was included because of the inter-reader subjectivity of the IFA. For the two commercial assays, the following values were considered negative, equivocal and positive: Access Toxo-IgG, <4.0, 4.0–6.0 and >6.0 IU/mL, respectively, and Axsym Toxo-IgG, <2.0, 2.0–3.0 and >3.0 IU/mL, respectively, according to the manufacturers’ recommendations.
Toxoplasma-specific IgM was detected by enzyme immunoassay (Access Toxo-IgMII; Beckman Coulter), by an in-house IFA [19] and by an immunosorbent agglutination assay (IgM ISAgA; bioMérieux). The results obtained were quantitative and were expressed in arbitrary units. The following titres were considered negative, equivocal and positive: Access Toxo-IgM, <0.80, 0.80–1.00 and >1.00 signal/cut-off, respectively; IFA, <1/20, 1/20 and ≥1/40 inverse of dilution, respectively; and ISAgA for obstetrical samples, ≤4+, 5+-8+, and 9+ -12+, respectively.
The IgG avidity test was performed as described previously by Pelloux et al. [14], using a commercial immunoenzymatic kit (Vidas Toxo IgG Avidity Kit; bioMérieux) according to the manufacturer’s instructions. This test was performed using the fully automated Vidas machine, which also calculates and interprets the results. The AI is the ratio of the signal in the test sample washed with 6 M urea (which disrupts low-avidity complexes) to that of a sample washed without 6 M urea, with <0.20 indicating low avidity, 0.20–0.30 indicating borderline avidity and >0.300 indicating high avidity. According to the manufacturer, high avidity excludes the possibility of an infection acquired during the 4-month period before the sample was taken.
Ax/Ac IgG ratios were calculated only when the titres were equal to or higher than the positivity threshold for each test, i.e., ≥3 IU/mL for Axsym Toxo-IgG and ≥6 IU/mL for Access Toxo-IgG. When the titres were higher than the last calibration point (>300 IU/mL for Axsym Toxo-IgG; >500 IU/mL for Access Toxo-IgG), a 1:10 dilution was made using serum from negative cord blood in order to obtain quantifiable titres for determining the ratio. The values after dilution were found to be reproducible for the two tests after testing 20 diluted sera in duplicate (range after dilution for Axsym Toxo-IgG was 16–252 IU/mL; range after dilution for Access Toxo-IgG was: 26–493 IU/mL). The mean coefficient of variation of the Ax/Ac IgG ratio was 6.2% (0–13.0%); the range of the 20 Ax/Ac IgG ratios was 0.43–3.70.
Statistical analysis
Paired Student’s t-tests were used for comparing the mean Axsym Toxo-IgG and Access Toxo-IgG values for each group of patients (p <0.05 was considered significant). Spearman’s rank correlation test was used to determine the association between the AI, the Ax/Ac ratio, and the time elapsed between infection and sampling. Statistical assessment of differences in the mean number of months before reaching the threshold level for the avidity test or for the Ax/Ac ratio in the different treatment groups was estimated by ANOVA (Fisher–Snedecor test, with p <0.05 considered significant).
Results
Kinetics of Axsym and Access Toxo-IgG assays
For the cases in part 1 of the study, the kinetics of IgG development were different for the two techniques (Table 2); in the first 3 months after infection (acute phase), the IgG titres were almost always higher with Axsym Toxo-IgG than with Access Toxo-IgG (one exception with three successive sera) (paired Student’s t-test, p <0.001). Axsym Toxo-IgG thus gave higher titres, and titres that were more often positive, with 41 of the 50 sera in the 1-month group being positive, compared with only 24 according to Access Toxo-IgG (chi-square test, p <0.001). This early detection of IgG by Axsym Toxo-IgG allowed rapid confirmation of the specificity of the IgM titres. The tendency towards higher titres according to Axsym Toxo-IgG levelled off at 4–5 months after infection, and was then progressively inverted (i.e., Access Toxo-IgG titres were greater than Axsym Toxo-IgG titres) for the 9–12-month and the 13–24-month groups (paired Student’s t-test, p 0.02) (Table 2). Moreover, in part 2 of the study, involving cases of chronic infection (>24 months), the IgG titres according to Access Toxo-IgG were twice as high for both pregnant women and immunodepressed patients. This was even more so for 20 immunodepressed cases with serological reactivation. Among the 60 sera from individuals with chronic infection and without reactivation (pregnant women and immunodepressed patients), Axsym Toxo-IgG was negative for two sera (<2 IU/mL) and borderline for two others (2–3 IU/mL), whereas, with one exception (5.3 IU/mL), all had titres >6 IU/mL according to Access Toxo-IgG.
| Time after infection (months ± 15 days) | No. of sera | Access | Axsym | Paired Student’s t-test | ||||
|---|---|---|---|---|---|---|---|---|
| No. positive | Mean IgG, IU/mL (±SE) | No. positive | Mean IgG, IU/mL (±SE) | Mean (Axsym– Access) (±SE) | t | p value | ||
| Part 1 | ||||||||
| 1 | 50 | 24a (48%) | 10.6 (±1.7) | 41a (82%) | 29.0 (±5.5) | −18.4 (±4.1) | −4.45 | <0.001 |
| 2 | 55 | 52 (95%) | 95.6 (±17.1) | 54 (98%) | 183 (±28.9) | −87.9 (±14.1) | −6.25 | <0.001 |
| 3 | 36 | 36 (100%) | 211 (±36.8) | 36 (100%) | 422 (±67.1) | −211 (±47.0) | −4.49 | <0.001 |
| 4–5 | 45 | 45 (100%) | 295 (±67.5) | 45 (100%) | 299 (±56.5) | −4.05 (±40.3) | −0.1 | 0.92 |
| 6–8 | 41 | 36 (88%) | 228 (±82.9) | 41 (100%) | 230 (±62.5) | −2.35 (±41.2) | −0.06 | 0.96 |
| 9–12 | 43 | 42 (98%) | 225 (±74.9) | 43 (100%) | 142 (±39.4) | 83.5 (±43.5) | 1.92 | 0.06 |
| 13–24 | 34 | 34 (100%) | 219 (±74.2) | 34 (100%) | 105 (±29.2) | 114 (±46.6) | 2.44 | 0.02 |
| Part 2 (>24 months) | ||||||||
| Pregnancy | 30 | 30 (100%) | 33.9 (±6.0) | 28 (93%) | 17.4 (±2.6) | 16.5 (±3.7) | 4.42 | <0.001 |
| ID, no reactivation | 30 | 29 (97%) | 26.9 (±4.7) | 28 (93%) | 12.3 (±1.8) | 14.6 (±3.2) | 4.62 | <0.001 |
| ID, reactivation | 20 | 20 (100%) | 912 (±340) | 20 (100%) | 325 (±101) | 587 (±246) | 2.40 | 0.03 |
- ID, immunodepressed.
- aChi-square test, p <0.001.
Evolution of the Ax/Ac ratio as a function of the interval between infection and sampling
It was possible to calculate the Ax/Ac ratio even when the results for both of these automated tests were higher than the positivity threshold recommended by the manufacturers. In the present study, it was possible to determine this ratio for 263 of 304 sera taken from pregnant women who had been followed-up for seroconversion, and for 76 of 80 sera taken from patients with chronic infection. In comparison, it was possible to measure the AI for 256 of 304 and 76 of 80 sera, respectively.
For the sera with accurately dated infections (part 1), the mean Ax/Ac ratios (±SE) were 2.50 (±0.26), 2.14 (±0.13), 2.33 (±0.22), 1.34 (±0.09), 1.32 (±0.10), 0.92 (±0.08), 0.74 (±0.07), respectively, for sera taken at 1, 2, 3, 4–5, 6–8, 9–12 and 13–24 months after infection. For sera taken from patients with chronic infection (>24 months; part 2), the mean Ax/Ac ratios were much lower, being 0.56 (±0.03), 0.44 (±0.04) and 0.53 (±0.04) for pregnant women and patients with immunodepression, with and without reactivation, respectively.
Taking a ratio of 1 as the threshold (receiver operator characteristic analysis; data not shown), 1/50 (2%), 2/55 (3.6%) and 0/36 (0%), respectively, of the sera taken 1, 2 and 3 months after infection had a ratio <1 (Table 3), with the three sera yielding an Ax/Ac ratio of <1 having been taken from the same patient. Moreover, only 12 of 141 sera from these three groups had ratios of 1–1.2 (threshold + 2 SE). In contrast, of the sera taken from patients with chronic infection (>24 months), 80/80 (100%) yielded an Ax/Ac ratio <1 (Table 3). The correlation between the Ax/Ac ratio and the time between infection and sampling was significant and inversely proportional (r = −0.59; p <0.001) (Fig. 1).
| Time after infection (months ± 15 days) | No. of sera | Mean ratio (Axsym Toxo-IgGM/ Access Toxo-IgG) (±SE) | % with ratio <1 | Mean avidity index (±SE) | % with avidity index >0.3 |
|---|---|---|---|---|---|
| Part 1 | |||||
| 1 | 50 | 2.50 (±0.26) | 2 | 0.032 (±0.003) | 0 |
| 2 | 55 | 2.14 (±0.13) | 3.6 | 0.050 (±0.005) | 0 |
| 3 | 36 | 2.33 (±0.22) | 0 | 0.071 (±0.007) | 0 |
| 4–5 | 45 | 1.34 (±0.09) | 26.6 | 0.098 (±0.009) | 2.2 |
| 6–8 | 41 | 1.32 (±0.10) | 34.1 | 0.143 (±0.016) | 5 |
| 9–12 | 43 | 0.92 (±0.08) | 59.5 | 0.27 (±0.017) | 46.5 |
| 13–24 | 34 | 0.74 (±0.07) | 82.3 | 0.32 (±0.017) | 61.7 |
| Part 2 (>24 months) | |||||
| Pregnancy | 30 | 0.56 (±0.03) | 100 | 0.52 (±0.019) | 96.7 |
| ID, no reactivation | 30 | 0.53 (±0.04) | 100 | 0.52 (±0.022) | 96.7 |
| ID, reactivation | 20 | 0.44 (±0.04) | 100 | 0.60 (±0.013) | 100 |
- ID, immunodepressed.
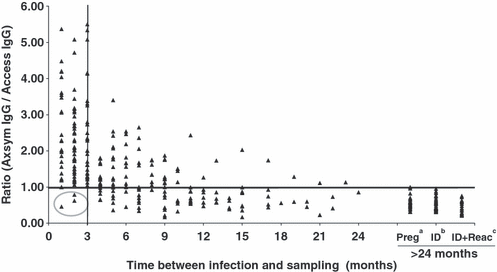
Evolution of the Axsym IgG/Access IgG ratio, based on the time between infection and sampling, for 384 selected sera. Correlation between the ratio and time between infection and sampling was r = −0.59 (p <0.001). The three circled sera came from the same pregnant woman. aPreg, pregnancy; bID, immunodepressed; cID+React, ID with reactivation.
Evolution of the Ax/Ac ratio as a function of treatment
The influence of treatment on the kinetics of the serological titres and the assay ratios was investigated. Prophylactic treatment (spiramycin alone for >15 days) significantly reduced the serological titres according to both assays to a similar degree (Table 4). In contrast, there was no significant difference in the kinetics of the Ax/Ac ratios between the treated and untreated patients (p >0.2). For the non-treated pregnant women who were diagnosed either at delivery or after a spontaneous abortion (13 cases, 50 sera), the mean Ax/Ac ratios (±SE) were 2.49 (±0.55), 2.11 (±0.20), 2.32 (±0.45), 1.61 (±0.48), 1.64 (±0.36), 1.02 (±0.13) and 0.88 (±0.29) for the sera taken 1, 2, 3, 4–5, 6–8, 9–12 and 13–24 months after infection, respectively. These results were comparable to those for the treated women (66 cases, 254 sera) with mean Ax/Ac ratios (±SE) of 2.60 (±0.29), 2.16 (±0.16), 2.33 (±0.25), 1.30 (±0.08), 1.25 (±0.10), 0.90 (±0.09) and 0.74 (±0.08), respectively.
| Time after infection (months ± 15 days) | Treateda | Non-treatedb | Access Toxo-IgG | Axsym Toxo-IgG | ||||
|---|---|---|---|---|---|---|---|---|
| No. of sera | No. of sera | TreatedaMean IgG, IU/mL (±SE) | Non-treatedbMean IgG, IU/mL (±SE) | Student’s t-test,p | TreatedaMean IgG, IU/mL (±SE) | Non-treatedbMean IgG, IU/mL (±SE) | Student’s t-test,p | |
| 1 | 43 | 7 | 10.5 (±1.8) | 10.8 (±5.1) | NS | 28.5 (±5.8) | 31.8 (±17.5) | NS |
| 2 | 41 | 14 | 75 (±13) | 155 (±53) | 0.02 | 150 (±27) | 281 (±79) | NS |
| 3 | 28 | 8 | 168 (±31) | 353 (±110) | 0.04 | 334 (±56) | 708 (±198) | 0.02 |
| 4–5 | 40 | 5 | 232 (±60) | 795 (±318) | <0.01 | 218 (±37) | 935 (±307) | <0.01 |
| 6–8 | 35 | 6 | 106 (±28) | 1234 (±785) | <0.01 | 111 (±26) | 889 (±268) | <0.01 |
| 9–12 | 37 | 6 | 194 (±70) | 416 (±321) | NS | 110 (±28) | 337 (±220) | 0.04 |
| 13–24 | 30 | 4 | 209 (±82) | 294 (±125) | NS | 94 (±32) | 190 (±59) | NS |
- NS, not significant.
- aSpiramycin 9 MIU/day for ≥15 days.
- bNon-treated cases, diagnosis at delivery or at spontaneous abortion.
Comparison with the Toxo-IgG Avidity Vidas assay
As with the Ax/Ac ratio, there was a strong correlation between the AI and the time between infection and sampling (r = 0.90; p <0.001) (Fig. 2). However, the kinetics of the AI were slow, with an increase of c. 0.02/month in the first few months after infection (Table 3). The threshold of 0.3 was rarely attained before 6 months (1/45 sera for the 4–5-month group), whereas the Ax/Ac ratio decreased rapidly to below the proposed threshold of 1 in a larger number of cases (12/45 for the 4–5-month group). This slower evolution of the AI, compared with the Ax/Ac ratio, was confirmed for samples with longer intervals between infection and sampling. Whereas only 2/41 (5%), 20/43 (46.5%) and 21/34 (61.7%), respectively, of the sera from the 6–8-, 9–12- and 13–24-month groups attained an AI threshold of 0.3, the Ax/Ac ratio of <1 was attained in 14/41 (34.1%), 26/43 (59.5%) and 28/34 (82.3%) of sera, respectively (Table 3).
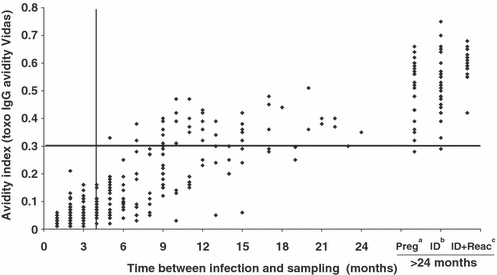
Evolution of the Toxo-IgG avidity Vidas results, based on the time between infection and sampling, for 384 selected sera. Correlation between Toxo-IgG avidity index and time between infection and sampling: r = 0.90 (p <0.001). aPreg, pregnancy; bID, immunodepressed; cID+React, ID with reactivation.
Discussion
Serological results that are positive for both IgM and IgG are difficult to interpret during the first trimester of pregnancy in the absence of a known preceding Toxoplasma infection. This serological profile is relatively common, representing >1% of the cases seen in our laboratory (data not shown). This is related to the availability of increasingly sensitive IgM detection techniques, which are able to detect residual IgM >1 year after seroconversion [5,20,21], and the relatively high prevalence of toxoplasmosis in France of c. 50% [22]. However, even if this IgM+ and IgG+ profile is a warning sign, it is only rarely associated with an acute infection at the onset of pregnancy. In such cases, the AI is useful [1,3,16], in that it allows recent infection to be excluded for >50% of the cases (data not shown). The AI test is reliable and, to our knowledge, with the exception of a recent report concerning a particular Vidas Toxo-IgG IV avidity assay [11], there have been no documented cases of seroconversion in <4 months that were associated with an AI of >0.3. Globally, the AI allows the exclusion of any risk of congenital toxoplasmosis. However, the present study confirms previous reports that the AI evolves slowly [10,11,16]. Among pregnant women presenting with positive IgM and IgG and an AI of <0.3 (0.5% in our laboratory; data not shown), a large number were probably infected before pregnancy, and thus are not at risk of maternofetal transmission. For these cases, a possible solution would be to decrease the AI threshold to 0.2 [10]. This permits more women to be reassured without introducing a significant risk, since only one of 141 sera taken after 1, 2, and 3 months in the present study had an AI >0.2 (i.e., 0.21).
An alternative approach could involve the coupling and comparison of two serological techniques that use different antigenic targets [17,18]. In the present study, two automated tests, Axsym Toxo-IgG and Access Toxo-IgG, were compared. Axsym Toxo-IgG becomes positive early, whereas Access Toxo-IgG allows an old infection to be confirmed with more assurance (titres elevated two-fold). The early positivity of Axsym Toxo-IgG assay has been described previously [23–26], and may be linked to the nature of the antigen used by the manufacturer, which is mainly membrane-associated [23]. The difference in the kinetics of the two tests may be related to the difference in the antigens used. Little information is available concerning the exact composition and preparation method of the antigens used in these assays, but there are only minor technical differences between the two tests. Axsym Toxo-IgG uses latex microbeads, whereas Access Toxo-IgG uses polyethylene microbeads covered with metallic oxide (paramagnetic beads). The diameters of the microparticles used in the two tests are also very similar (0.5–1 μm).
Based on the results obtained, it is proposed that the ratio of Axsym Toxo-IgG and Access Toxo-IgG can be used as an additional test for dating toxoplasmosis. It is important to note that treatment reduces the titres obtained with both techniques to a similar degree, but does not modify the ratio between the two tests (Table 4). In all cases, the ratio was high during the first 3 months of infection, and then decreased with time. This ratio has the advantage of developing much more rapidly than the AI, but does not guarantee 100% exclusion. Thus, three and 15 of the 141 sera taken after 1, 2 and 3 months had abnormally low ratios of <1 (the proposed threshold) and <1.20 (threshold + 2 SE), respectively (Fig. 1). However, all three sera with an Ax/Ac ratio of <1 were from the same patient, and ten of the 15 sera with Ax/Ac ratios <1.2 had very low serological titres of <30 IU/mL. The Ax/Ac ratio must therefore be interpreted with caution for sera with titres <30 IU/mL. Nevertheless, it is suggested that the Ax/Ac ratio can be used as a second-line exclusion test for cases with an AI of <0.3 in the first trimester of pregnancy (Fig. 3). This test is applicable when Axsym Toxo-IgG and Access Toxo-IgG both indicate >30 IU/mL. In such cases an Ax/Ac ratio of <1 allows the possibility of a recent infection within the previous 3 months to be excluded. In the absence of seroconversion studies with a much larger cohort, a further serological control is necessary after 10 days for cases presenting with IgM+, IgG+, AI <0.3 and an Ax/Ac ratio <1 in order to confirm the absence of a rise in serological titres (Fig. 3).

Proposed algorithm for the follow-up of pregnant women presenting with anti-Toxoplasma IgM and IgG in the first serum. (*denotes the percentage of pregnant women (n = 4000) with this profile at the onset of pregnancy in our laboratory).
This new approach to dating Toxoplasma infection presents the following advantages: (i) optimisation of the technique is simple and it could be performed in many laboratories; (ii) similar results might be obtained by comparing other serological assays, provided that one assay detects IgG maturation earlier than the other; (iii) the approach could be developed commercially so that two tests could be performed using the same automated machine; and (iv) as with the avidity test, it is possible to date infection at the onset of pregnancy with the first sample, thereby enabling the start of any necessary prophylactic treatment without waiting for a second sample, while at the same time allowing the exclusion of recent infection occurring within 3 months of the onset of pregnancy. This will help to reassure patients and prevent unnecessary and costly prophylactic treatment and follow-up.
Acknowledgements
We are grateful to the individuals, especially M. Wallon, G. Belot and their laboratory team, who provided us with some missing sera in the course of the maternal follow-up. We acknowledge the staff of the Laboratory of Parasitology for their skilful technical assistance. No information has been provided by the authors concerning the existence or absence of conflicting or dual interests.




