Detection of cytomegalovirus reactivation in cancer patients receiving chemotherapy
Abstract
While increasing numbers of cytomegalovirus (CMV)-associated diseases are occurring in patients undergoing conventional chemotherapy, information regarding CMV reactivation is limited. This pilot study was conducted to investigate CMV reactivation induced by chemotherapy. Seven blood samples were collected from each of 15 patients with newly diagnosed malignant disease, at baseline before chemotherapy, and once every month after chemotherapy was commenced. CMV viral loads in leukocytes were determined by real-time PCR. Host responses to changes in viral loads were assessed by assaying CMV-specific IgG titres and tumour necrosis factor (TNF)-α and interferon (IFN)-γ levels in each of the blood samples, and by scoring the number of CMV-associated clinical symptoms that developed. All except one patient experienced CMV reactivation during the course of chemotherapy, with the average viral load peaking after the third course of treatment. Titres of CMV-specific IgG increased in line with the increase in viral load. Plasma levels of TNF-α and IFN-γ initially decreased from baseline, and then rose to peak levels at the same time as, or shortly after, the highest viral loads were recorded. Clinical symptoms potentially attributable to CMV infection appeared as the viral load increased. It was concluded that the incidence of CMV reactivation in patients receiving conventional chemotherapy is high. Reactivation is not asymptomatic, but was self-limiting in most of these cases. Increases in plasma TNF-α and IFN-γ occur after reactivation, but not before.
Introduction
Cytomegalovirus (CMV) establishes a life-long relationship with its host via a latent infection. Patients with compromised immunity, including those with human immunodeficiency virus infection, malignancies and organ transplants, or individuals receiving immunosuppressive therapy, are at high risk for reactivation of latent CMV. At present, most knowledge regarding CMV reactivation has come from studies of transplant recipients and AIDS patients [1–3]. However, increasing numbers of patients receiving chemotherapy who experience symptomatic CMV reactivation have been observed, although there are few published data concerning this phenomenon [4].
CMV reactivation is usually defined on the basis of direct or indirect evidence of virus activity (e.g., seroconversion, isolation of the virus from any body site, or direct detection of CMV antigen). Individuals experiencing CMV reactivation may develop generalised signs and symptoms (e.g., fever, malaise, leukopenia), various site-specific CMV-associated syndromes (e.g., interstitial pneumonitis, hepatitis, gastroenteritis, or retinitis) [5], or a potentially fatal disseminated infection, particularly if the patient is immunosuppressed [6]. Real-time quantitative PCR provides some advantages for measuring CMV DNA as compared to traditional methods, e.g., pp65-antigenaemia [7], viral culture and mRNA sequence-based amplification [8]. In the present study, patients with newly diagnosed cancer were monitored to determine whether the CMV viral load increased when they received chemotherapy and to evaluate host responses to changes in viral load.
Materials and methods
Patients
The study was approved by the Mackay Memorial Hospital Institutional Review Board (Taipei, Taiwan), and informed consent was obtained from all participants (Clinical Trial Registration: NCT00366717; http://www.clinicaltrials.gov). Patients with newly diagnosed malignant disease who were expected to receive chemotherapy for ≥6 months were eligible for inclusion in the study. Between May 2005 and January 2006, 20 patients with various malignancies were recruited, of whom 15 completed the study. All patients received chemotherapy approximately once a month. Blood (c. 10 mL) was collected from each participant before the initiation of chemotherapy, and once-monthly after each course of chemotherapy. In total, seven blood samples were collected from each of the participants. Patients 1, 2, 3, 5, 7, 9, 10, 13, 14 and 15 received cisplatin 100 mg/m2 on day 1, and 5-fluorouracil 1000 mg/m2/day on days 1–4. Patients 4 and 6 received 5-fluorouracil 400 mg/m2/day on days 1–5, and leukovorin 200 mg/m2/day on days 1–5. Patient 8 received epirubicin 50 mg/m2 on day 1, and vincristine 1.4 mg/m2 on days 1 and 8. Patient 11 received cisplatin 120 mg/m2 on day 1, and etoposide 100 mg/m2/day on days 1–3. Patient 12 received gefitinib 500 mg/day for 14 days, followed by 14 days of no treatment.
Quantitative PCR
Total genomic DNA was extracted from buffy coat samples using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions, and was stored at −40°C. In brief, buffy coat samples were prepared by low-speed centrifugation of whole blood for 10 min at room temperature, followed by lysis of red blood cells with lysis buffer (0.16 M ammonium chloride, 0.01 M sodium bicarbonate, 0.1 mM EDTA) following removal of plasma. The cells were washed twice with phosphate-buffered saline. Total cells were counted and resuspended in phosphate-buffered saline to a final concentration of 2.5 × 107 cells/mL. Quantification of CMV DNA copy numbers was performed by amplifying nucleotides 106047–106119 of the UL73 gene using real-time TaqMan PCR (ABI Prism 7000; Applied Biosystems, Foster City, CA, USA) with forward primer 5′-CCTGGTGGACTATGCTTAATG, reverse primer 5′-GGAAGCAGCAATGTCGTAGTACAAT and TaqMan MGB probe 5′-FAM-ATTCTCATGGGAGCTTTT. Each reaction mixture contained 300 nM CMV primers, 200 nM probe and TaqMan PCR Universal Master Mix containing AmpliTaq Gold DNA polymerase, dNTPs with deoxyuridine triphosphate, AmpErase uracil-N-glycosylase and optimised buffers (Applied Biosystems). TaqMan Exogenous Internal Positive Control Reagent (Applied Biosystems) was added to distinguish negative reactions from PCR inhibition. PCR conditions comprised 50°C for 2 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. To establish a standard curve, a plasmid containing the target sequence of the CMV UL73 gene was constructed using pGEM-T Easy (Promega, Madison, WI, USA). Purified plasmid DNA was quantified using a spectrophotometer, and the number of plasmid copies was calculated. Quantification of CMV DNA in the test samples was achieved by comparison with serial ten-fold dilutions of the previously quantified plasmid standards. The plasmid standards and test samples were assayed in duplicate, with the final data presented as copy numbers of DNA/μL of buffy coat (copies/μL).
Measurement of CMV-specific IgG
CMV IgG titres in plasma were measured using an AxSYM CMV IgG kit (Abbott Laboratories, Chicago, IL, USA), based on microparticle enzyme immunoassay technology. Anti-CMV IgG levels were expressed as numbers of antibody units/mL. These units were derived from the standard calibration prepared from human samples by Abbott Laboratories. Samples with zero to highest concentrations are defined as 0–250 absorbance units (AU)/mL. Values of >250 AU/mL were obtained after serial dilution.
Evidence of host response
Measurement of tumour necrosis factor (TNF)-α and interferon (IFN)-γ. Blood samples were anticoagulated with EDTA. Plasma was separated from blood cells by low-speed centrifugation for 10 min. All samples were stored at −70°C. Plasma TNF-α and IFN-γ levels were measured using commercially available quantitative sandwich immunoassay kits (R&D Systems, Minneapolis, MN, USA).
CMV-associated clinical findings. Patients were questioned concerning symptoms such as anorexia, diarrhoea, pyrexia, malaise and respiratory symptoms, and laboratory data, including abnormal liver function tests, thrombocytopenia, monocytosis and abnormal chest radiograph findings, were recorded. For the present study, it was assumed that the higher the number of potentially CMV-associated clinical findings, the higher the possibility of reactivated CMV infection. Thus, each of the possible findings listed above was assessed at each visit, with a score of one being given to each. The maximum score was nine. The score was averaged for all 15 participants at each visit, and the averages for all seven visits were compared.
Statistics
All results are presented as means ± standard error. Changes over time (months) in the outcome measures were analysed by repeated-measures analysis of variance (ANOVA), followed by Newman–Keals post-test using PRISM v.3.0 statistical software (GraphPad, San Diego, CA, USA), with p <0.05 being regarded as statistically significant.
Results
Patient characteristics
Of the 15 participants (11 men; mean age 54.5 ± 9.04 years, range 38–71 years), 11 had head and neck cancers, two had lung cancer, one had lymphoma and one had rectal cancer. The chemotherapy regimen received by each patient was standard treatment for each specific cancer, with the exception of patient 7, who received gefitinib (Table 1). All participants received 3.3 mg of dexamethasone intravenously once before each course of chemotherapy. One participant (patient 12) received intermittent high-dose corticosteroids during the study period for underlying rheumatoid arthritis.
| Patient | Age (years) | Gender | Disease | Chemotherapy regimen | Outcome | Maximum recorded cytomegalovirus load (copies/mL) |
|---|---|---|---|---|---|---|
| 1 | 52 | M | Lung cancer | Cisplatin, 5-FU | Death | 275 060 (3)a |
| 2 | 49 | M | Oral cancer | Cisplatin, 5-FU | Death | 215 190 (3) |
| 3 | 54 | M | NPC | Cisplatin, 5-FU | Death | 34 140 (3) |
| 4 | 70 | M | NPC | 5-FU, leucovorin | Survival | 19 440 (3) |
| 5 | 52 | M | NPC | Cisplatin, 5-FU | Death | 19 440 (3) |
| 6 | 71 | F | Rectal cancer | 5-FU, leucovorin | Survival | 6 320 (2) |
| 7 | 58 | M | NPC | Cisplatin, 5-FU | Survival | 2 800 (3) |
| 8 | 48 | F | Lymphoma | Epirubicin, vincristine | Death | 2 510 (3) |
| 9 | 65 | F | NPC | Cisplatin, 5-FU | Survival | 2 510 (4) |
| 10 | 49 | M | HPC | Cisplatin, 5-FU | Survival | 1 540 (2) |
| 11 | 53 | M | Lung cancer | Cisplatin, etoposide | Death | 1 130 (3) |
| 12 | 38 | M | Oral cancer | Gefitinib | Survival | 280 (4) |
| 13 | 50 | M | Tongue cancer | Cisplatin, 5-FU | Survival | 270 (3) |
| 14 | 61 | M | NPC | Cisplatin, 5-FU | Survival | 200 (4) |
| 15 | 47 | F | NPC | Cisplatin, 5-FU | Survival | 0 |
- NPC, nasopharyngeal cancer; HPC, hypopharyngeal cancer; 5-FU, fluorouracil.
- aNumber in parentheses indicates the chemotherapy course with the maximal viral load.
Dynamics of CMV DNA in leukocytes during chemotherapy
Ten of the 15 participants had no detectable viral DNA in their baseline leukocyte samples before starting chemotherapy. The viral load increased in 14 of 15 patients after chemotherapy commenced, reaching a peak after the third course (Fig. 1) in ten participants. The highest CMV viral loads were >100 000 copies/mL in two patients. As the viral load increased, the patients experienced more symptoms and showed more abnormal laboratory tests. Patient 3 experienced anorexia, malaise and diarrhoea, and had an abnormal liver function test. Patient 5 experienced anorexia, pyrexia, dyspnoea and malaise. The leukocyte viral loads subsequently decreased gradually without any antiviral therapy, indicating that CMV reactivation in these patients was self-limiting. As indicated in Table 1, the highest CMV DNA loads were found in patients who died (median 26 790 copies/mL; range 1130–275 060), while lower CMV loads were associated with patients who survived (median 1540 copies/mL; range 0–19 440).
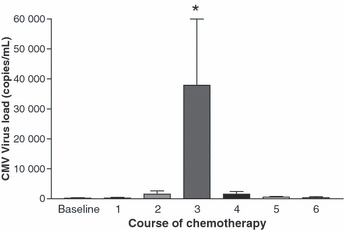
Mean cytomegalovirus (CMV) counts in leukocytes as determined by real-time quantitative PCR at baseline and after each course of chemotherapy. Ten of 15 participants had undetectable virus at baseline before starting chemotherapy. The peak recorded viral load was found after the third course of chemotherapy, with ten patients reaching a maximum viral load after the third to fourth course of chemotherapy. *p <0.05, course 3 compared to courses 0, 1, 2, 4, 5 and 6.
Host response
CMV-specific IgG. All participants were seropositive for CMV-specific IgG before chemotherapy (Fig. 2). The titres rose in 11 of the subjects after chemotherapy was commenced. In most patients, the peak anti-CMV IgG titre was detected after the appearance of peak viral loads. These increased titres, especially those peaking after peak viral loads, indicated an active host immune response to CMV replication.

Mean cytomegalovirus (CMV)-specific IgG in baseline blood samples and after each course of chemotherapy. The recorded anti-CMV IgG titre of nine patients peaked after the recorded viral load peak.
TNF-α and IFN-γ. TNF-α and IFN-γ levels decreased from baseline after initiation of chemotherapy, but then rose above baseline to maximum levels after the fourth course of chemotherapy (3, 4). TNF-α levels peaked simultaneously with the peak viral load in three patients, and after the peak viral load in ten patients. Peak IFN-γ levels occurred at the same time as the peak viral load in one patient, but after the peak viral load in seven patients. The timing of the rise in the cytokine levels in relation to the rise in virus titres suggests that TNF-α and IFN-γ levels increased in plasma in response to CMV replication. This is consistent with a normal host immune response to a replicating virus.
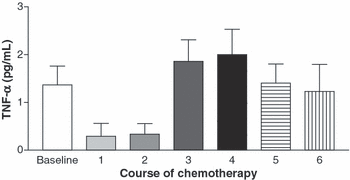
Mean tumour necrosis factor (TNF)-α plasma levels at baseline and after each course of chemotherapy. The level peaked in three patients at the same time as the recorded peak viral load, and in ten patients after the recorded viral load peak.
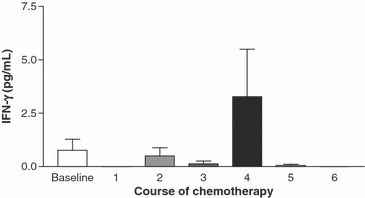
Mean interferon (IFN)-γ plasma levels at baseline and after each course of chemotherapy. Seven (46.7%) participants had the highest levels of IFN-γ after the recorded viral load peak.
Clinical findings. Scores for symptoms and abnormal laboratory data that may have been associated with CMV reactivation peaked after the second course of chemotherapy before the peak viral load. All CMV-associated symptoms resolved, except in one participant who had persistent fever and respiratory symptoms after the sixth course of chemotherapy (Fig. 5). The appearance of symptoms and abnormal test results thus paralleled the increasing viral load, but then resolved as antibody titres and serum cytokines increased (Table 2).

Mean combined score for cytomegalovirus-associated symptoms and laboratory findings at baseline and after each course of chemotherapy. The peak score preceded the recorded peak number of viral particles. *p <0.05, course 2 compared to courses 0, 1, 4, 5 and 6. # p <0.05, course 3 compared to courses 0, 1, 4, 5 and 6.
| Baseline | 1 | 2 | 3 | 4 | 5 | 6 | p value | |
|---|---|---|---|---|---|---|---|---|
| CMV viral load (copies/mL) | 273.2 ± 186.0 | 335.2 ± 242.0 | 1816 ± 1234 | 43 681 ± 25 203 | 1799 ± 1001 | 644.2 ± 245.0 | 449.0 ± 401.0 | 0.013 |
| CMV-specific IgG (AU/mL) | 223.8 ± 42.6 | 240.8 ± 49.1 | 233.3 ± 55.3 | 316.7 ± 49.6 | 572.8 ± 158.0 | 449.8 ± 130.7 | 258.4 ± 17.3 | 0.011 |
| TNF-α (pg/mL) | 1.366 ± 0.394 | 0.292 ± 0.270 | 0.334 ± 0.223 | 1.858 ± 0.454 | 2.000 ± 0.530 | 1.689 ± 0.428 | 1.228 ± 0.568 | 0.023 |
| IFN-γ (pg/mL) | 0.053 ± 0.053 | 0 | 0.513 ± 0.443 | 0.150 ± 0.150 | 3.779 ± 2.546 | 0 | 0.968 ± 0.968 | 0.109 |
| Symptom score | 0.067 ± 0.067 | 0.400 ± 0.235 | 1.933 ± 0.452 | 1.267 ± 0.331 | 0.467 ± 0.133 | 0.400 ± 0.214 | 0.133 ± 0.133 | <0.001 |
- AU, absorbance units; TNF, tumour necrosis factor; IFN, interferon.
Discussion
This pilot study demonstrated that all except one of the CMV-seropositive patients experienced CMV reactivation during the course of chemotherapy. Similar observations have been reported in adults with haematological malignancies, among whom CMV pneumonia is an important cause of mortality [9,10]. The incidence of CMV pneumonia in such patients (<3%) is thought to be far less than in patients receiving solid-organ transplantation (>15%). There are also a number of reports concerning CMV reactivation in patients receiving chemotherapy; however, these are mostly related to stem-cell transplantation [2], solid-organ transplantation [7] or specific immunosuppressants [11].
Increasing use of cytoreductive therapy that significantly suppresses cellular immunity is believed to be one of the key factors that expose patients to a greater risk of severe CMV disease. However, none of the participants in the present study received major immunosuppressive agents, e.g., purine analogues, cyclophosphamide or large doses of systemic steroids. It is believed that the present findings are most consistent with a scenario in which the patients’ latent CMV was reactivated when chemotherapy was commenced, as clearly demonstrated by the increasing viral load. At the same time that the viral load was increasing, the patients began experiencing non-specific symptoms and laboratory abnormalities were found.
It has been shown previously that the existence of high CMV DNA levels in the blood is a major risk-factor for developing CMV disease. The viral loads in two of the patients in the present study rose to >100 000 copies/mL. This represents a critical threshold at which even relatively small increases in viral load correspond to rapid increases in the probability of disease, which is a phenomenon termed the ‘threshold concept of CMV disease’ [11]. As the viral load peaked, the patients experienced more symptoms, but without obvious CMV disease. It is possible that the immunity against viral disease was not fully suppressed by the non-cytoreductive chemotherapy. In these patients, these non-specific symptoms and laboratory abnormalities cannot, in themselves, be proven to be CMV-induced. However, an increase in anti-CMV IgG antibody titres and cytokines followed the increased viral load. As these peaked, the symptoms largely resolved (except in one patient) and they did not reappear during the rest of the course of chemotherapy. The highest symptom scores, although relatively low with a mean score of 2/9, were found to precede the highest viral load. This implies that the patients’ immunity was responding actively to the replicating virus, and the symptoms began to subside while the continuing elevated viral load was halted by multiple defence systems. Clearly, it is not possible to reach specific conclusions concerning the value of the symptom scores on the basis of this small study, but these data provide a basis for further studies.
Most patients had measurable baseline plasma levels of TNF-α and IFN-γ; these became virtually undetectable after the first or second course of chemotherapy, but then rose and peaked with, or just after, the peak in CMV viral load. As with the symptoms and laboratory abnormalities, this time course suggests that the cytokines were expressed in response to CMV replication, as might be expected as part of the normal host immune response against a replicating virus [12–14]. However, these findings are inconsistent with the theory that inflammatory cytokines, such as TNF-α, are major factors leading to the reactivation of latent CMV [15,16]. In addition, an increased risk of CMV reactivation is often seen in transplantation patients with elevated levels of TNF-α [17]. TNF-α has been implicated in mediating acute graft vs. host disease, which remains a major cause of death in patients receiving allogeneic blood stem-cell transplantation.
Treatment with the human chimeric monoclonal antibody infliximab blocks the function of TNF-α and has been used to treat steroid-refractory acute graft vs. host disease [18]. However, infliximab does not appear to decrease the incidence of CMV-related infection in patients with graft vs. host disease. Indeed, reports are accumulating of patients treated with anti-TNF-α antibody who have developed severe CMV disease [19–21], such that caution has been expressed concerning this treatment [22]. It was thought originally that measurement of plasma TNF-α might predict CMV reactivation during conventional chemotherapy, whereas the results in the present series suggest that increases in TNF-α levels in this setting mainly reflect the host immune response to a replicating virus rather than virus reactivation. Similarly, the rise in CMV-specific IgG, with peak levels occurring mostly after viral loads peaked, may not limit viral replication, but can be interpreted as indicating an active host immune reaction to CMV replication.
The present study was not designed to explore the question of treatment for patients receiving conventional chemotherapy who have clinically apparent reactivation of CMV. Reactivation of CMV and Epstein–Barr virus is considered to be rare in patients with solid tumours who are receiving conventional chemotherapy, and prophylactic therapy is therefore not recommended [23]. Certainly, treatment should be given to patients with established infection, particularly when it is severe. However, none of the patients in the present study had documented CMV disease according to current definitions, although evidence has been presented that they may have been mildly symptomatic because of viral reactivation. Treatment would not normally be indicated for such mild and apparently self-limited disease.
The main limitations of the present study were the small number of patients and the limited categories of malignancies. However, four conclusions can be reached from the findings. First, it appears that CMV reactivation during conventional chemotherapy may occur in a high proportion of patients. Second, this reactivation is not necessarily asymptomatic, but is probably self-limiting in many patients. Third, given the course of events, humoral immunity, as assessed by CMV-specific IgG, seems to respond actively to CMV reactivation. Finally, CMV reactivation is apparently not preceded by an increase in plasma TNF-α and IFN-γ levels, suggesting that these cytokines are expressed as part of the host response to viral replication rather than being a cause of it. Investigations of larger groups of patients with a variety of cancers and different types of chemotherapy are needed to confirm the scope and impact of CMV reactivation during conventional chemotherapy.
Acknowledgements
This work was supported by the Mackay Memorial Hospital, Taipei, Taiwan (MMH-E-94008 and MMH-E-95008). No information has been provided by the authors concerning the existence or absence of conflicting or dual interests.




