Prevalence of etravirine mutations and impact on response to treatment in routine clinical care: the Swiss HIV Cohort Study (SHCS)
Abstract
Objectives
Etravirine (ETV) is a novel nonnucleoside reverse transcriptase inhibitor (NNRTI) with reduced cross-resistance to first-generation NNRTIs, which has been primarily studied in randomized clinical trials and not in routine clinical settings.
Methods
ETV resistance-associated mutations (RAMs) were investigated by analysing 6072 genotypic tests. The antiviral activity of ETV was predicted using different interpretation systems: International AIDS Society-USA (IAS-USA), Stanford, Rega and Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS).
Results
The prevalence of ETV RAMs was higher in NNRTI-exposed patients [44.9%, 95% confidence interval (CI) 41.0–48.9%] than in treatment-naïve patients (9.6%, 95% CI 8.5–10.7%). ETV RAMs in treatment-naïve patients mainly represent polymorphism, as prevalence estimates in genotypic tests for treatment-naïve patients with documented recent (<1 year) infection, who had acquired HIV before the introduction of NNRTIs, were almost identical (9.8%, 95% CI 3.3–21.4). Discontinuation of NNRTI treatment led to a marked drop in the detection of ETV RAMs, from 51.7% (95% CI 40.8–62.6%) to 34.5% (95% CI 24.6–45.4%, P=0.032). Differences in prevalence among subtypes were found for V90I and V179T (P<0.001). Estimates of restricted virological response to ETV varied among algorithms in patients with exposure to efavirenz (EFV)/nevirapine (NVP), ranging from 3.8% (95% CI 2.5–5.6%) for ANRS to 56.2% (95% CI 52.2–60.1%) for Stanford. The predicted activity of ETV decreased as the sensitivity of potential optimized background regimens decreased. The presence of major IAS-USA mutations (L100I, K101E/H/P and Y181C/I/V) reduced the treatment response at week 24.
Conclusions
Most ETV RAMs in drug-naïve patients are polymorphisms rather than transmitted RAMs. Uncertainty regarding predictions of antiviral activity for ETV in NNRTI-treated patients remains high. The lowest activity was predicted for patients harbouring extensive multidrug-resistant viruses, thus limiting ETV use in those who are most in need.
Introduction
Nonnucleoside reverse transcriptase inhibitors (NNRTIs) are important components in the drug combination schemes that are currently used in the treatment of HIV-1 infections [1,2]. Because of the overlapping resistance profiles of currently approved NNRTI drugs, cross-resistance can emerge rapidly, which excludes future use of this drug class [3–5]. Recently, the situation has changed with the introduction of the diarylpyrimidine derivative etravirine (ETV), a novel, next-generation NNRTI [6]. ETV was designed for activity against wild-type HIV-1 and against strains harbouring NNRTI resistance-inducing mutations selected by nevirapine (NVP) or efavirenz (EFV). Its resistance-associated mutation (RAM) pattern is partially different from that of other NNRTIs and its genetic barrier, defined as the number of mutations required to confer full resistance, seems to be considerably higher [7,8]. Clinical studies showed that the presence of three or more ETV RAMs led to substantially decreased virological response [9–12]. Current International AIDS Society-USA (IAS-USA) recommendations list the following 14 ETV RAMs: V90I, A98G, L100I, K101E/P, V106I, V179D/F/T, Y181C/I/V and G190A/S [13,14]. Recently, Vingerhoets et al. [11] have updated the list of mutations and added K101H, E138A and M230L. Of note, this list does not include K103N, which confers high-level resistance to both EFV and NVP.
The potential eligibility of patients for treatment with ETV in routine clinical practice has not been systematically assessed for large, well-characterized samples of drug-naïve and treatment-experienced individuals. For this reason, we interrogated the resistance database from the Swiss HIV Cohort Study (SHCS) to determine the prevalence of ETV RAMs and to predict the potential eligibility of treatment-naïve and treatment-experienced patients for treatment with ETV. Moreover, we compared different algorithms that predict susceptibility to ETV based on available genotypic resistance tests (GRTs). As it has been shown repeatedly that new antiretroviral drugs should be accompanied by at least two additional active compounds for optimal virological response to salvage treatment [15–19], we further identified patients who had failed NNRTIs previously with at least two remaining drugs for the background regimen in addition to ETV.
Methods
Data and patient selection
Our analysis included clinical and genotypic data collected up to May 2008. The SHCS is a nationwide, clinic-based cohort study with continuous enrolment and at least bi-annual study visits [20]. The SHCS has been approved by ethical committees of all participating institutions and written informed consent has been obtained from participants. The SHCS resistance database contains all genotypic HIV resistance tests performed by the four authorized laboratories in Switzerland, stored in SmartGene's (Zug, Switzerland) Integrated Database Network System (IDNS version 3.4.0) [21]. Resistance tests performed between January 1999 and May 2008 were included in this study. Patients with recent infection were defined as presenting with either documented acute infection or a documented seroconversion within 1 year as described in detail elsewhere [22].
Analysis
Because GRTs were obtained under various circumstances, we stratified our analysis by whether patients were treatment naïve (group A) or antiretroviral therapy (ART) exposed at the time of resistance testing. Samples from treatment-experienced patients were grouped further according to NNRTI exposure. Group B included resistance tests performed in treatment-experienced patients never exposed to any NNRTI, group C included resistance tests performed while patients were receiving NNRTI treatment and group D included tests performed after exposure to NNRTI (58.4% on treatment without NNRTIs, and 41.6% off treatment). We only considered tests that were performed after at least 30 days of continuous exposure to ART since the last treatment modification. Patients could appear in more than one group, but only the latest resistance test of a patient was included if several tests per patient were available for the same group.
Resistance mutations against ETV were defined according to the mutation list of the IAS-USA [13,14]. We further classified ETV RAMs into ETV-specific mutations, which do not show cross-resistance to EFV or NVP based on the IAS-USA recommendations, and nonspecific mutations, which also confer resistance to NVP and EFV. The ETV-specific mutations included V90I, A98G, K101E/H/P, V106I, E138A, V179D/F/T, Y181V and M230L, and ETV nonspecific mutations consisted of L100I, Y181C/I and G190S/A.
GRTs were categorized into fully susceptible, intermediately resistant, and fully resistant to a specific antiretroviral drug with three frequently used interpretation algorithms: the Stanford algorithm, version 5.0.1 [23], the RegaV7 1.1 algorithm [24] and the Agence Nationale de Recherches sur le Sida et les hépatites virales (ANRS) (http://www.hivfrenchresistance.org/index.html) version 16 algorithm as implemented in the Stanford HIV Drug Resistance algorithm comparison tool (http://hivdb.stanford.edu/pages/algs/HIValg.html). As a fourth classification following the IAS-USA spring 2008 guidelines, we considered viruses with at least three IAS-USA ETV RAMs as fully resistant to ETV and viruses with one or two IAS-USA ETV RAMS as intermediately resistant. The newest update of the IAS-USA guidelines, for December 2008, has assigned relative weights to the following mutations based on in vitro and in vivo data: L100I, K101E/H/P and Y181C/I/V [13,14]. In analogy to major protease inhibitor (PI) mutations it has been shown that these weighted ETV mutations do induce a substantial reduction in phenotypic drug susceptibility compared with the other nonweighted ETV mutations [25–27]. Thus, for simplicity, we will call ETV RAMs at these three positions ‘major ETV mutations’. To assess the availability of active antiretroviral compounds for combination with ETV, results obtained using the Stanford interpretation algorithm were mapped to a genotypic sensitivity score (GSS) for all approved drugs except enfuvirtide, maraviroc and raltegravir. Drugs with a score of 0 were considered inactive because of full resistance of the virus to that compound, 0.5 indicated intermediate antiretroviral activity and 1 related to full activity based on GRTs. It was assumed that ETV-based salvage therapy following EFV or NVP failure should consist of the two highest scoring NRTI drugs and one of the highest scoring boosted PIs as background treatment. Thus a maximum score of 3 could be achieved for a fully active background regimen and a score of 0 if all drugs were considered inactive. Virological failure was defined as viral rebound after previous suppression with two consecutive viral loads>500 HIV-1 RNA copies/mL or a single value>500 copies/mL followed by a stop or a modification of the current therapy.
Statistical analysis was performed with stata 10 SE (StataCorp, College Station, TX). Proportions were compared with Fisher's exact test. All confidence intervals (CIs) were two-sided 95% confidence intervals and calculated with the Clopper–Pearson method. The level of significance was set at P<0.05.
Results
Prevalence of RAMs for ETV
We included 6072 resistance tests from the SHCS drug resistance database in our analyses: 3020 (49.7%) GRTs performed in treatment-naïve patients (group A), 1667 (27.5%) performed in NNRTI-naïve patients exposed to ART without NNRTIs (group B), 639 (10.5%) performed in patients on NNRTI treatment (group C) and 746 (12.3%) performed in patients with past NNRTI experience (group D) (Fig. 1).
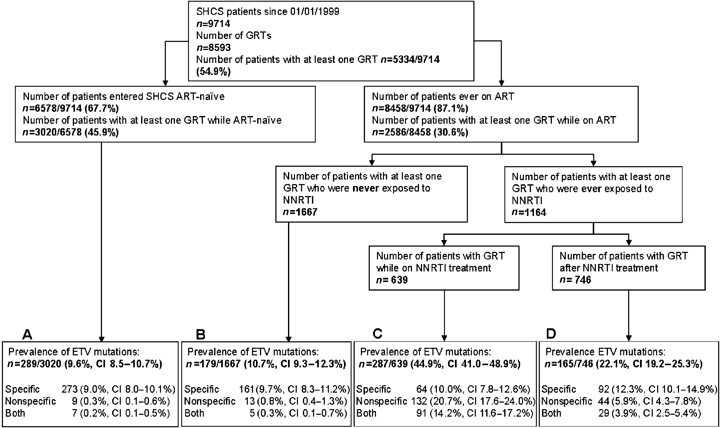
Study design and prevalence of etravirine resistance-associated mutations (ETV RAMs). The study population was subdivided into four groups depending on the circumstances of the genetic resistance test (GRT). Group A: GRT performed in treatment-naïve patients; group B: GRT performed in treatment-experienced patients never exposed to any nonnucleoside reverse transcriptase inhibitor (NNRTI); group C: GRT performed in patients receiving NNRTIs; group D: GRT performed at least 30 days after exposure to NNRTIs. ETV RAMs were defined according to the mutation list of the International AIDS Society-USA (IAS) and subdivided into specific (mutations that affect ETV only) and nonspecific ETV RAMs [mutations that also confer resistance to nevirapine (NVP)/efavirenz (EFV)]. 95% confidence intervals (CIs) are indicated. SHCS, Swiss HIV Cohort Study; ART, antiretroviral therapy.
ETV RAMs occurred three times more frequently in NNRTI-experienced patients (groups C and D: 32.6%, CI 30.2–35.2%) compared with NNRTI-naïve patients (groups A and B: 10.0%, CI 9.1–10.9%, P<0.001). In patients with virological failure on EFV/NVP the prevalence was 51.9% (CI 45.7–58.2%). The prevalence of nonspecific ETV RAMs, which also confer resistance to other approved NNRTIs, was higher in NNRTI-experienced patients (groups C and D: 21.4%, CI 19.2–23.6%) than in NNRTI-naïve patients (groups A and B: 0.7%, CI 0.5–1.0%, P<0.001). The increase in frequency of specific ETV RAMs following exposure to NNRTI was less pronounced, but still highly significant (groups A and B: 9.5%, CI 8.7–10.4%; groups C and D: 19.9%, CI 17.9–22.1%, P<0.001). Among patients with at least one ETV RAM, single mutations were most common (84.8%, CI 83.9–85.7%). The acquisition of three or more ETV RAMs, which would indicate high-level resistance to ETV according to IAS-USA, was most common in groups C and D (8.2%, CI 5.8–11.1%) and occurred significantly less frequently in NNRTI-naïve patients (groups A and B: 0.9%, CI 0.2–2.2%, P<0.001). Also, the occurrence of at least one major ETV RAM according to IAS-USA (L100I, K101E/H/P and Y181C/I/V) was higher in groups C and D (18.8%, CI 16.7–20.9%) compared with NNRTI-naïve patients (groups A and B: 0.9%, P<0.001).
As illustrated in Figure 2, the most prevalent mutations in NNRTI-naïve patients (groups A and B) were the ETV-specific mutations E138A (group A: 2.8%; group B: 2.8%), V106I (group A: 2.3%; group B: 1.7%) and V90I (group A: 2.1%; group B: 2.9%), whereas in patients tested while receiving NNRTIs (group C) or after NNRTI exposure (group D) mutations selected by EFV and NVP were mainly dominating: L100I (8.3%), Y181C (15.0%) and G190A (12.5%) in group C and V90I (3.9%), Y181C (5.0%) and G190A (3.2%) in group D. Interestingly, the two ETV RAMs V179F and Y181V were not found at all in our large study sample.
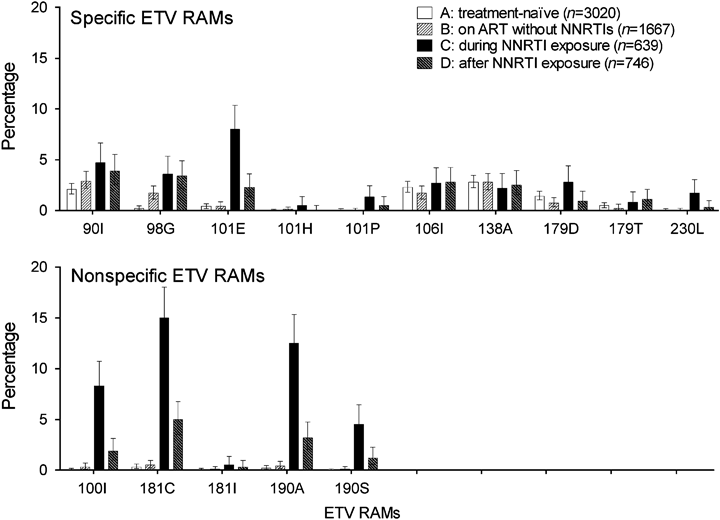
Percentage of etravirine resistance-associated mutations (ETV RAMs) based on genotypic resistance tests in treatment-naïve patients (group A), nonnucleoside reverse transcriptase inhibitor (NNRTI)-naïve patients (group B), patients exposed to NNRTIs (group C) and patients who had stopped treatment with NNRTIs (group D). The following ETV RAMs did not occur: 179F and 181V. ART, antiretroviral therapy.
Association between ETV RAMs and HIV subtypes
We further analysed associations between ETV RAMs and HIV subtypes based on genotypic tests in treatment-naïve patients (group A, n=3020). Most samples were subtype B (78.1%), followed by subtypes CRF02_AG (6.2%), A (5.5%), C (5.4%) and CRF01_AE (4.7%). Based on Fisher's exact test and Bonferroni adjustment of P-values for multiple testing, two mutations were found to be significantly different among subtypes, V90I and V179T (both P<0.001). V90I was most prevalent in subtype CRF02_AG (7.9%, CI 4.3–13.2%) compared with subtypes A (2.1%, CI 0.4–5.9%) and B (1.9%, CI 1.4–2.6%) and did not occur in subtype C. V179T was most common in subtypes A (4.1%, CI 1.5–8.7%) and CRF01_AE (3.2%, CI 0.9–8.1) and was not found in subtypes CRF02_AG, B and C.
ETV RAMs in patients experiencing virological failure with EFV or NVP
We further focused our analysis on patients who had failed NVP- or EFV-containing therapies. In total, 258 GRTs were performed in patients with a virological failure, 182 (70.5%) of whom were exposed to EFV and 76 (29.5%) to NVP.
Frequencies of ETV-specific RAMs did not differ markedly according to exposure to EFV or NVP: V90I (4.9% on EFV vs. 5.3% on NVP, P=1.000), A98G (5.5%vs. 6.6%, P=0.773), K101E (8.2%vs. 11.8%, P=0.357), K101H (0.5%vs. 1.3%, P=0.503), K101P (3.3%vs. 0%, P=0.184), V106I (2.7%vs. 3.9%, P=0.697), E138A (1.6 vs. 5.5%, P=0.200), V179D (4.4%vs. 1.3%, P=0.289), V179T (0.5%vs. 1.3%, P=0.503) and M230L (3.8%vs. 0%, P=0.109). Certain nonspecific ETV mutations, however, preferentially occurred on treatment with specific NNRTIs, such as L100I (14.8% on EFV vs. 1.3% on NVP, P=0.001), Y181C (9.3% on EFV vs. 28.9% on NVP, P<0.001), and G190A (10.4%vs. 22.4%, P=0.017). The mutations Y181I (0% on EFV vs. 1.3% on NVP, P=0.295) and G190S (8.8% on EFV vs. 3.9% on NVP, P=0.203) occurred at similar frequencies in the two treatment groups.
Persistence of ETV RAMs following exposure to EFV or NVP
To analyse the persistence of ETV RAMs, we selected patients with a virological failure on EFV or NVP who had received a GRT during exposure to NNRTI and a later GRT performed while they were on a therapy not containing NNRTI or off treatment (n=87). The median time between stop of NNRTIs and the second GRT was 897 days [interquartile range (IQR) 356–1555 days].
The prevalence of one or more ETV RAMs significantly decreased after cessation of exposure to NNRTIs, from 51.7% (CI 40.8–62.6%) to 34.5% (CI 24.6–45.4%, P=0.032). The appearance of ETV RAMs tended to be less frequent in patients who had stopped ART (25%, CI 7.3–52.4%, n=16) compared with patients who had continued ART without NNRTIs (36.6%, CI 25.5–48.9%, n=71). Overall, 55.6% (CI 40.0–70.4%) of patients had lost at least one ETV RAM after stop of NNRTIs. As shown in Figure 3, the frequency of almost all ETV RAMs decreased after stop of NNRTI drugs.
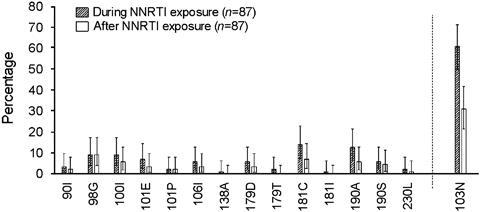
Changes in frequency of etravirine resistance-associated mutations (ETV RAMs) and K103N after discontinuation of nonnucleoside reverse transcriptase inhibitor (NNRTI) therapy. Patients with two genotypic resistance tests (GRTs), one performed during exposure to NNRTIs and a second performed at least 30 days after discontinuation of NNRTI treatment, were included (n=87). For comparison, the NNRTI mutation K103N is listed. K103N does not affect the antiviral activity of ETV, but is known to disappear after discontinuation of NNRTI treatment [33].
Eligibility for ETV in the SHCS population based on different algorithms
We further aimed to determine the potential eligibility for treatment with ETV for 6072 GRTs performed based on the Stanford algorithm. Overall, ETV was considered fully active in 4599 (98.1%, CI 97.7–98.5%) NNRTI-naïve patients and in 518 (69.4%, CI 66.1–72.8%) patients with past NNRTI experience, but only in 262 (41.0%, CI 37.2–44.8%) patients with GRTs while being exposed to NNRTI.
For further analysis, we focused on patients with GRTs obtained while receiving EFV/NVP. Of all four interpretation methods the Stanford algorithm predicted the highest proportion of intermediate/full resistance in GRTs (56.2%, CI 52.2–60.1%). The predictions obtained with the IAS-USA recommendations (44.7%, CI 40.8–48.7%), the Rega 7.1 algorithm (23.3%, CI 20.1–26.8%) and the ANRS algorithm (3.8%, CI 2.5–5.6%) were generally much lower (Fig. 4). The predicted frequency of full resistance was comparable across interpretation methods (Stanford: 3.8%, CI 2.5–5.6%; IAS-USA: 4.4%, CI 3.0–6.3%; Rega: 5.7%, CI 4.0–7.8%), with the exception of the ANRS algorithm, which classified 0.5% (CI 0.1–1.4%) of the samples as fully resistant. As shown in Figure 4, taking either EFV or NVP did not have a differential effect on the estimated antiviral activity of ETV.
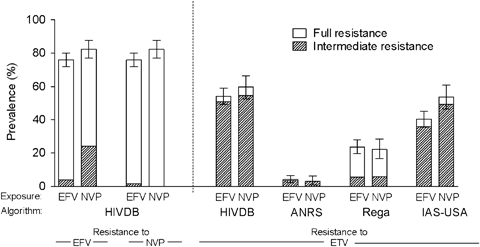
Proportion of genotypic resistance tests (GRTs) indicating intermediate or full resistance against etravirine (ETV), efavirenz (EFV) or nevirapine (NVP) according to different interpretation algorithms. The analysis was stratified by whether the patient was receiving EFV (n=428) or NVP (n=203) at the time of sampling. ANRS, Agence Nationale de Recherches sur le Sida et les hépatites virales; HIVDB, Stanford HIV Drug Resistance Data Base; IAS-USA, International AIDS Society-USA.
Transmission of ETV drug resistance mutations
We analysed the prevalence of ETV RAMs in 763 GRTs in recently infected, treatment-naïve patients [22]. The prevalences of specific and nonspecific ETV mutations were 9.0% (CI 7.1–11.3%) and 0.9% (CI 0.3–1.9%), respectively, without any significant time trends (by the Cochran–Armitage test; not shown). Of note, the ETV RAMs V90I, A98G, E138A and V179D/T were detected in five of 51 (9.8%) plasma samples obtained before 1998, when NNRTIs were not yet registered in Switzerland. Thus, the detection of ETV RAMs in recently infected patients probably reflects polymorphism rather than transmitted RAMs.
Estimated benefit of ETV in patients with different effective background regimens
Stratified by the GSS of the optimized background regimen, we aimed to identify the percentage of patients exposed to NNRTIs (group C) with a virus fully susceptible to ETV. The GSS of an optimized background treatment regimen was defined based on cumulative information from GRTs and consisted of the highest scoring of two NRTIs and one boosted PI. Based on the Stanford algorithm, only 22.9% (CI 15.4–32.0%) of samples with little residual antiviral activity of the background treatment (GSS<2) were fully susceptible to ETV, whereas significantly more viruses were susceptible (45.9%, CI 41.6–50.3%, P<0.001) in samples with more potent backbone regimens (GSS≥2), indicating that the additional benefit of ETV may be limited for those in most need. As shown in Figure 5, other algorithms predicted higher benefits of ETV, however, and the frequency of patients with fully susceptible virus did not correlate with the magnitude of the GSS for the background regimens (ANRS algorithm, range 86.7–98.3%; Rega algorithm, range 60.0–83.8%). Of note, 81.9–84.9% of patients achieved a GSS of ≥2 for the background regimen.
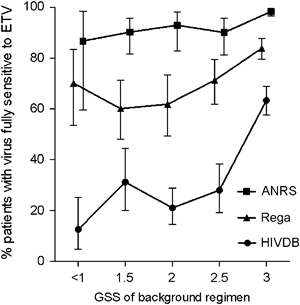
Proportion of patients with a genotypic resistance test performed while receiving nevirapine (NVP) or efavirenz (EFV) (n=631) and with a virus fully susceptible to etravirine (ETV). The analysis was stratified by a cumulative genotypic sensitivity score (GSS) for a hypothetical optimized background regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and one protease inhibitor (PI). A GSS of 0 indicates full resistance and 1 indicates full susceptibility. ANRS, Agence Nationale de Recherches sur le Sida et les hépatites virales; HIVDB, Stanford HIV Drug Resistance Data Base; IAS-USA, International AIDS Society-USA.
Clinical response
An intent-to-treat analysis (n=71) for the week 24 response showed an overall viral suppression rate of 71.8% (CI 59.9–81.9%). The presence of L100I, K101E/H/P or Y181C/I/V was more predictive for the short-term outcome (viral load<50 copies/mL after 24 weeks of follow-up) than the GSS calculated using the Stanford algorithm. Patients with one of the above-mentioned mutations had a suppression rate of 52.9% (CI 27.8–77.1%) compared with 77.8% (64.4–88.0%) when these mutations were absent (P=0.065). In contrast, the GSS for ETV as estimated using the Stanford algorithm was not predictive for a virological response: response rates for fully susceptible viruses were 71.4% (CI 51.3–86.8%) compared with 72.1% (CI 56.3–84.7%, P=1.000) for those with intermediate/full resistance. This tendency was confirmed by a multivariable logistic regression including HIV RNA at the start of ETV treatment, the GSS of the background regimen and the presence of L100I, K101E/H/P or Y181C/I/V. Failure to achieve undetectable HIV RNA was better predicted by the presence of one of the above-mentioned mutations [odds ratio (OR) 2.8, CI 0.8–9.4] than by the Stanford algorithm (OR for intermediately/fully ETV-resistant HIV 0.7, CI 0.2–2.2).
Discussion
In this data set from a highly representative cohort study, the prevalence of ETV RAMs was higher in NNRTI-treated patients compared with NNRTI-naïve patients. ETV RAMs were lost at a relatively high rate once NNRTI drugs were stopped. Uncertainty remains for predictions of ETV activity, because analyses with different algorithms led to widely varying results.
The prevalence of ETV RAMs was 44.9% in patients tested while receiving NNRTIs, and 51.9% in patients failing on NNRTIs. Other studies have reported higher prevalence estimates of ETV RAMs in patients failing on NNRTIs, ranging between 61.7 and 74.1% [28,29]. Also, the occurrence of three or more ETV RAMs was less common in our study [12,29,30]. The most likely explanation for the lower frequency of ETV mutations found in the SHCS might be that treatment failures in the SHCS were managed aggressively early on [15,21] and that NNRTIs were rarely maintained in failing drug regimens, even when no other options were available. Two ETV mutations were associated with the largest impact on response, namely Y181I and Y181V [31]. The former mutation was rare and the latter did not appear in our study sample. In addition, four mutations were also associated with a higher negative impact on response and their prevalence was increased with exposure to NNRTI treatment, namely L100I (8.3%), K101P (1.3%), Y181C (15.0%) and M230L (1.7%). It should be mentioned that the prevalence of K101E increased under NNRTI treatment, even though K101E is not associated with NNRTI resistance [31].
The fact that certain ETV-specific IAS-USA mutations such as 90I and 106I were already present at relative high frequencies in samples from treatment-naïve patients and their persistence in longitudinal samples suggest that these represent polymorphisms. This hypothesis is further strongly supported by the high frequency of transmitted ETV RAMs in our large study group with documented recent infection, especially as the prevalence of ETV RAMs did not differ before and after registration of NNRTIs in Switzerland. Whether these polymorphisms really have an impact on ETV activity in clinical practice has to be further investigated in data sets with longer follow-up.
It should be noted that ETV RAMs that also affect other approved NNRTIs were lost at a relatively high rate once NNRTI drugs were stopped, as our analysis of GRTs performed on and off treatment containing NNRTIs has demonstrated. Overall, 55.6% of patients with sequential GRTs lost at least one ETV RAM, and hence GRTs performed distantly from NNRTI-containing therapy should be interpreted with caution because of the possible presence of minor viral variants with drug resistance mutations.
Overall, 631 GRTs were performed while patients were receiving EFV (n=428) or NVP (n=203). When analysed with the Stanford algorithm, 67.5% of GRTs indicated full resistance to EFV and 76.9% full resistance to NVP, whereas full resistance to ETV was observed in 3.8% of GRTs. Full susceptibility to ETV was only predicted for 43.8% of GRTs in patients on EFV/NVP by the Stanford algorithm, suggesting considerable cross-resistance of ETV with approved NNRTIs. However, the other interpretation methods, namely ANRS, Rega and IAS-USA, gave less weight to the potential overlap of resistance mutations between ETV and EFV/NVP, thus yielding higher predictions for the proportion of viruses fully susceptible to ETV. Therefore, depending on the interpretation method used, we estimated that 43.8–96.2% of patients with a GRT performed while receiving potent NNRTI drug therapy would be unaffected by the presence of resistance mutations and 3.3–54.9% could still obtain some benefit from treatment with ETV.
This low concordance among interpretation methods for predicting the antiviral activity of ETV reflects the current uncertainty about how much the resistance profiles of ETV and EFV/NVP are overlapping. Moreover, the set of mutations that may affect the viral response to ETV have not yet been clearly defined outside of the Duett studies [9,10]. All these issues are highly relevant for decision-making in clinical practice, and further studies are warranted.
ETV is currently approved for use in salvage treatments. The application of a novel antiretroviral drug is especially interesting in patients with previous virological failure on NNRTIs and few alternative treatment possibilities. Depending on the interpretation algorithm, our calculations showed that the effectiveness of ETV decreased almost linearly in highly treatment-experienced patients with decreasing potency of an optimized background regimen.
The presence of major ETV IAS-USA mutations (L100I, K101E/H/P and Y181C/I/V) reduced the treatment response at week 24. The occurrence of these mutations was more predictive for clinical outcome than the Stanford algorithm. Although our sample size was relatively small for this analysis (n=71), a recent study investigating short-term responses at week 8 in a larger number of patients (n=243) supports our findings [32].
Because of its good tolerability, a further potential application for ETV could be in patients with intolerance to EFV and NVP. In our study sample, 11% of patients had to cease treatment within 90 days of NNRTI initiation because of intolerance. ETV may be a safer alternative for these patients, because adverse events such as rashes are less severe and their incidence is lower compared with EFV treatment [8].
This study has some limitations. Not all GRTs in ART-experienced patients were performed because of virological failures. We repeated the main analyses for those patients who failed NNRTI treatment virologically, with virological failure defined as two consecutive on-treatment viral load measurements >500 copies/mL after at least 180 days of treatment. Of note, our results did not change. Moreover, as sequential GRTs were available for relatively few ART-exposed patients, eligibility may have been somewhat overestimated. However, the SHCS drug resistance database generally has a very high degree of completeness, with GRTs for more than 65% of all patients with a history of virological failures (V. von Wyl and H. F. Günthard, unpublished data).
In conclusion, here we clearly demonstrate that ETV RAMs in drug-naïve patients mainly reflect polymorphisms and probably do not confer high levels of resistance to ETV by themselves. Moreover, we have shown that only small differences exist in the prevalence of ETV RAMs among subtypes. Most importantly, caution is needed in interpreting GRTs performed off NNRTI treatment, because the frequency of ETV RAMs diminishes if the selection pressure is removed. Furthermore, considerable uncertainty remains in predicting the antiviral activity of ETV, underlined by widely discrepant results obtained using different interpretation systems. The newly defined weighted IAS-USA ETV mutations, however, were associated with enhanced clinical relevance compared with nonweighted mutations. Thus, the presence of these weighted mutations should particularly caution clinicians to use ETV, in particular, in patients with extensive multidrug resistance. In this patient group, the predicted activity of ETV was lowest, thus limiting the use of ETV in those patients most in need.
Acknowledgements
We thank the patients participating in the SHCS for their commitment, all the study nurses and study physicians for their invaluable work, the staff at the data centre and in particular Martin Rickenbach for data management, all the staff at the resistance laboratories for their high-quality work and SmartGene for providing an impeccable database service. Furthermore, we thank Joseph Wong for critical reading of the manuscript.
Conflicts of interest: The authors declare that they have no conflict of interest in connection with this study.
Funding: This study has been financed in the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (SNF grant 3345-062041). Further support was provided by the SNF grant 3247B0-112594/1 (to HFG, SY and BL), SHCS projects 470, 528 and 569, the SHCS Research Foundation, and by a further research grant from the Union Bank of Switzerland in the name of a donor to HFG. This word was further supported by the European Community's Seventh Framework Programme (FP7/2007-2013) under the project: Collaborative HIV and Anti-HIV Drug Resistance Network (CHAIN) grant agreement no. 223131. The funding agencies had no part in conducting the study or in preparing the manuscript.




