Impact of reverse transcriptase resistance on the efficacy of TMC125 (etravirine) with two nucleoside reverse transcriptase inhibitors in protease inhibitor-naïve, nonnucleoside reverse transcriptase inhibitor-experienced patients: study TMC125-C227*
*TMC125-C227 is registered with ClinicalTrials.gov (NCT00225303).
Abstract
Objectives
TMC125-C227, an exploratory phase II, randomized, controlled, open-label trial, compared the efficacy and safety of TMC125 (etravirine) with an investigator-selected protease inhibitor (PI) in nonnucleoside reverse transcriptase inhibitor (NNRTI)-resistant, protease inhibitor-naïve, HIV-1-infected patients.
Methods
Patients were randomized to TMC125 800 mg twice a day (bid) (phase II formulation; n=59) or the control PI (n=57), plus two nucleoside reverse transcriptase inhibitors (NRTIs).
Results
In an unplanned interim analysis, patients receiving TMC125 demonstrated suboptimal virological responses relative to the control PI. Therefore, trial enrolment was stopped prematurely and TMC125 treatment discontinued after a median of 14.3 weeks. In this first-line NNRTI-failure population, baseline NRTI and NNRTI resistance was high and reduced virological responses were observed relative to the control PI. No statistically significant relationship was observed between TMC125 exposure and virological response at week 12. TMC125 was better tolerated than a boosted PI for gastrointestinal-, lipid- and liver-related events.
Conclusions
In a PI-naïve population, with baseline NRTI and NNRTI resistance and NRTI recycling, TMC125 was not as effective as first use of a PI. Therefore the use of TMC125 plus NRTIs alone may not be optimal in PI-naïve patients with first-line virological failure on an NNRTI-based regimen. Baseline two-class resistance, rather than pharmacokinetics or other factors, was the most likely reason for suboptimal responses.
Introduction
The use of first-generation nonnucleoside reverse transcriptase inhibitors (NNRTIs) in treatment-experienced patients is limited by a low genetic barrier to developing resistance and extensive cross-resistance within the class [1,2]. High levels of NNRTI resistance have been found in treatment-naïve [3–7] and treatment-experienced [8,9] patients from diverse clinical cohorts. Therefore, a significant need exists for an NNRTI that retains potency after the development of resistance or treatment failure with first-generation NNRTIs. To date, only a limited number of sequencing studies in patients experiencing failure of their first NNRTI-containing regimen have been reported [10–12]. This may in part be attributable to differences in standard practice and variations in patient access to NNRTI regimens among countries [13–15], which makes it difficult to recruit patients with first-line NNRTI treatment failure. Difficulty in recruiting this patient population has been encountered in another trial [16]. Regardless of the difficulty, studies in patients with first-line NNRTI treatment failure are needed to guide treatment strategies.
TMC125 (etravirine) is an NNRTI recently approved for clinical use, with antiviral activity against virus resistant to other available NNRTIs [17]. Its efficacy and safety in treatment-experienced, NNRTI-resistant patients have been demonstrated in phase IIb and III trials [18–20]. In the phase IIb TMC125–C223 study, TMC125 treatment led to a significantly greater reduction in plasma viral load than control treatment at 24 weeks [18]. In the large phase III DUET-1 and -2 trials, TMC125 combined with other antiretroviral drugs significantly improved virological suppression at 24 weeks relative to placebo [19,20]. Based on the findings of the phase IIb study, the exploratory phase II TMC125–C227 trial was conducted to compare the efficacy and safety of TMC125 against those of a protease inhibitor (PI) in HIV-1-infected, PI-naïve patients with first-line NNRTI treatment failure, including those in resource-limited settings. Of note, in response to efficacy concerns raised by an investigator, the trial sponsor (Tibotec, Mechelen, Belgium) conducted an unplanned interim analysis in which more patients in the control PI group had achieved an undetectable plasma viral load (<50 copies/mL) than in the TMC125 group. Based on these findings, the trial sponsor decided to discontinue TMC125 treatment, switch patients from TMC125 to an approved PI-based therapy and cease enrolment of new patients. This decision was endorsed by the independent Data and Safety Monitoring Board (DSMB). We report the final results of the study, including pre- and post-treatment switch data.
Methods
Patients
Patients were at least 18 years old, with documented HIV-1 infection and plasma viral load >1000 copies/mL at screening. They were PI naïve, with documented genotypic evidence of NNRTI resistance (either at screening or historically), treatment failure on a first-line NNRTI regimen or treatment interruption after a first-line NNRTI regimen failure. Additionally, patients who had previously been treated with an NNRTI to prevent mother-to-child transmission (MTCT) were allowed to participate if they showed NNRTI resistance; this population was limited to no more than 25% of the total study population. Patients with chronic hepatitis B and/or C virus infection were permitted, provided that their levels of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT) were less than three times the upper limit of normal (ULN).
Patients were excluded if they had a life expectancy of <6 months, had an active AIDS-defining illness at screening or baseline or a clinically significant disease that would have compromised the trial outcome, had previously permanently discontinued any NNRTI therapy because of skin events, had serum creatinine levels greater than twice the ULN, had acute hepatitis A, B or C, or had evidence of significantly decreased hepatic function or decompensation. Female patients who were pregnant, breast feeding or of childbearing age but not using effective contraception were also excluded from the trial.
Written informed consent was obtained from all patients. The protocol was reviewed and approved by an independent ethics committee based on applicable regulations and the trial was conducted in accordance with the Declaration of Helsinki.
Study design
This was a phase II, multinational, randomized, active-controlled, open-label, exploratory trial investigating the efficacy and safety of TMC125 in PI-naïve, NNRTI-resistant, HIV-1-infected patients. The TMC125 dose used in this study [800 mg twice a day (bid)] was based on an early formulation, which had a similar exposure to the 200 mg bid formulation used subsequently in the phase III DUET trials [19,20].
Patients were randomized at a 1:1 ratio to TMC125 800 mg bid (administered postprandially) or to an investigator-selected PI (ritonavir-boosted or unboosted), both in combination with an optimized background regimen (OBR) composed of two investigator-selected NRTIs. Dual-boosted PI regimens were not permitted. Investigator selection of NRTIs was guided by baseline resistance testing; patients had to show viral sensitivity to the selected NRTIs based on the screening resistance test (Virco BVBA, Mechelen, Belgium; see ‘Resistance determination’ section). Previously used NRTIs were allowed but the drugs had to show viral sensitivity on the resistance report. Other NNRTIs and investigational antiretrovirals were not allowed. Randomization was stratified for treatment status at screening and for region (region 1: Thailand and South Africa; region 2: Russia, Spain and the UK; region 3: Argentina, Brazil and Mexico).
The discontinuation of TMC125 intake and switch to control PI treatment divided the timeline of the study into two treatment phases: pre-switch and post-switch. In the TMC125 group, the pre-switch treatment phase covered the period from the first to the last day of TMC125 intake. The TMC125 group subsequently received a PI-based regimen in the post-switch treatment phase (24 weeks), which lasted from the day of the treatment switch visit until the day of last contact. In the control group, the duration of the pre-switch treatment phase was reduced at the time of TMC125 treatment switch to 24 weeks from the planned 48 weeks. As the control group did not switch treatment, they did not participate in a post-switch treatment phase.
In both treatment groups, dose adjustments for NRTIs and within-class NRTI substitutions were permitted for tolerability reasons only. No dose adjustments were permitted for TMC125. In the control group, dose adjustments to the investigator-selected PI were allowed for tolerability reasons only; within-class substitutions were not permitted for PIs.
Efficacy, safety and pharmacokinetic assessments
Plasma viral load was measured at baseline, on weeks 2 and 4, and every 4 weeks if applicable until week 24 using the Roche Cobas Amplicor HIV-1 Monitor™ (Roche Molecular Systems, Pleasanton, CA, USA). Samples were collected in standard, dry ethylenediaminetetraacetic acid tubes. Immunological change was assessed by measuring CD4 cell count every 4 weeks. Safety assessments included the reporting of adverse events and HIV-related events, laboratory abnormalities and physical examinations. Patients were withdrawn from the study for pregnancy, a disallowed change of antiretroviral therapy, a recurring grade 3 or 4 ALT or AST elevation, a grade 4 adverse event or laboratory abnormality, a persistent grade 3 elevation of amylase and/or lipase, any grade 3 or 4 skin event or allergic reaction, or signs of clinical hepatitis where TMC125 could not be excluded as contributing to the cause. The DSMB monitored the overall safety and efficacy of the trial every 12 weeks, and also received weekly reports of all grade 3 and 4 adverse events, serious adverse events and any cutaneous events.
Pharmacokinetic assessments were conducted in all patients randomized to TMC125. Blood samples for TMC125 plasma concentrations were collected on weeks 4, 12 and 24 as applicable. At week 4, a sample was obtained at 0 (trough) and 1 h post-dose; all other samples were collected at random time-points. TMC125 plasma concentrations were determined using a validated liquid chromatography with tandem mass spectrometry method. The area under the plasma concentration–time curve over 12 h (AUC12 h) and trough concentration (C0 h) were estimated using NONMEM® version V level 1.1 (ICON Development Solutions, Ellicott City, MD, USA).
Resistance determination
Phenotype and genotype were determined using Antivirogram® and virco®TYPE HIV-1 (both from Virco BVBA, Mechelen, Belgium), respectively, at baseline, at week 24 and at the time of withdrawal from the study. A screening virco®TYPE HIV-1 was provided to investigators to guide the selection of the administered NRTIs. During the recruitment period, Virco introduced a new version of the virco®TYPE HIV-1 report with clinical cut-offs (CCOs). However, the initial version of the resistance report was used throughout the screening period for consistency. Although data were also analysed according to the version provided at screening, the analyses presented here were performed using the more recent virco®TYPE with linear modelling (virco®TYPE HIV-1 LM) with CCOs [21]. The results with the virco®TYPE HIV-1 LM show (a) sensitive antiretroviral defined as reduced or maximal response (i.e. below the upper CCO), and (b) sensitive antiretroviral defined as maximal response only (i.e. below the lower CCO).
The presence of NRTI resistance-associated mutations (RAMs) was assessed according to the International AIDS Society USA (IAS-USA) Fall 2005 list [22]: M41L*, E44D, A62V, K65R, D67N*, 69ins, K70R*, L74V, V75I, F77L, Y115F, F116Y, V118I, Q151M, M184I/V, L210W*, T215F*/Y* and K219E*/Q* [the asterisk denotes thymidine analogue mutations (TAMs)]. The sensitivity of the NRTIs for the background regimen was determined based on the screening resistance test. The results of resistance testing at screening were used to determine patient inclusion in the trial while the results of resistance testing at baseline were used in the analysis of the data in the trial.
The widely used IAS-USA 2006 list of mutations [23] features 14 NNRTI RAMs (L100I, K103N, V106A/M, V108I, Y181C/I, Y188C/H/L, G190A/S, P225H and P236L). This trial used an extended list of 27 NNRTI RAMs at screening (A98G, L100I, K101E/P/Q, K103H/N/S/T, V106A/M, V108I, Y181C/I/V, Y188C/H/L, G190A/E/S, P225H, M230L, P236L, K238N/T and Y318F). The extended list was based on a broad review of data from clinical and in vitro analyses, which was undertaken to reflect the emerging resistance to NNRTIs [24].
A post hoc analysis was conducted to determine the effect of the number of baseline TMC125 RAMs on the virological response of patients treated with TMC125. A list of 13 TMC125 RAMs (V90I, A98G, L100I, K101E, K101P, V106I, V179D, V179F, Y181C, Y181I, Y181V, G190A and G190S) has been identified during the analysis of the larger DUET trials [25]. These mutations were not known during the initiation or conduct of the TMC125-C227 study.
Statistical analysis
In the original analysis plan, a sample size of 300 patients was expected to provide approximately 80% power to show noninferiority between TMC125 and control PI treatment (2.5% one-sided significance level and delta of 15%). Because enrolment was prematurely terminated, the target sample size and endpoints planned for the evaluation of the primary objectives of the trial could not be achieved. Only observed data were used in all analyses and no imputation methods were used for missing data. Where baseline data were missing, they were imputed with screening values.
The original primary efficacy parameter of this study was the proportion of patients with undetectable plasma viral load (<50 copies/mL) at week 24. Because of the early discontinuation of TMC125 treatment, the number of patients treated for at least 24 weeks in the TMC125 group was small (n=8), making the primary efficacy parameter invalid. The analysis was modified to focus on change in log10 viral load from baseline at week 12. For the overall trial population, virological response rates were compared between treatment groups using Fisher's exact test. An analysis of covariance model for the change in viral load from baseline was performed with factors consisting of treatment group, imputed baseline log10 plasma viral load, region and treatment status at screening. Mean changes in CD4 count compared with baseline are described. To evaluate the suboptimal response in the TMC125 group during the pre-switch treatment phase, pre-specified subgroup analyses of the primary endpoint were performed based on baseline resistance parameters and patient characteristics.
Results
Patient disposition and baseline characteristics
The study was conducted between 1 March 2005 and 11 July 2006. Of 250 patients screened, 116 were randomized and treated in the pre-switch treatment phase (Fig. 1). The patients who were screened but not randomized did not fulfil all of the inclusion criteria. The majority (82%) of patients were recruited from resource-limited settings, including South Africa (41%), Thailand (16%), Brazil (23%) and Mexico (2%). Ten patients discontinued the study before the treatment switch [six (10%) from the TMC125 group and four (7%) from the control PI group]. A total of 106 patients (53 in each group) completed the pre-switch treatment phase. All 53 patients from the TMC125 group subsequently participated in the post-switch treatment phase; 50 completed the post-switch treatment phase and three patients were withdrawn.
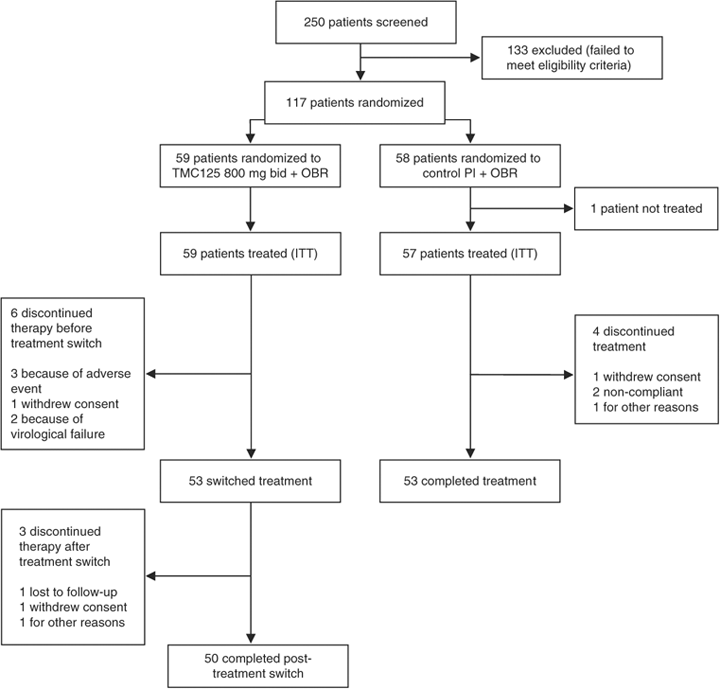
Patient disposition. ITT, intention to treat; OBR, optimized background regimen; PI, protease inhibitor.
Because of the premature discontinuation of TMC125 treatment and changes to the planned duration of control treatment, there was substantial inter- and intra-group variability in the duration of therapy. At the time that TMC125 treatment was discontinued, the median (range) duration of treatment was 14.3 (3.9–32.1) weeks for the TMC125 group. For the control group, the median duration of treatment was 27.1 (11.1–47.3) weeks. For the TMC125 patients who participated in the post-switch treatment phase, the treatment in the post-switch period lasted for a median of 24.1 (5.0–30.0) weeks.
Demographic and baseline characteristics were generally well balanced between the groups (Table 1), except for median CD4 cell count (lower in the TMC125 group) and number of patients under Centers for Disease Control and Prevention (CDC) category C (higher in the TMC125 group but the difference was small). The median duration of previous antiretroviral therapy at screening was 27 months for NNRTIs and 28 months for NRTIs.
| TMC125 (n=59) | Control PI (n=57) | All patients (n=116) | |
|---|---|---|---|
| Patient demographics | |||
| Male [n (%)] | 28 (47.5) | 29 (50.9) | 57 (49.1) |
| Age (years) [median (range)] | 34.0 (23–50) | 36.0 (20–61) | 34.0 (20–61) |
| Black [n (%)] | 24 (40.7) | 23 (40.4) | 47 (40.5) |
| Caucasian [n (%)] | 20 (33.9) | 21 (36.8) | 41 (35.3) |
| Asian [n (%)] | 10 (16.9) | 11 (19.3) | 21 (18.1) |
| Disease characteristics | |||
| Viral load (log10 copies/mL) [median (range)] | 4.31 (2.3–5.8) | 4.33 (2.5–5.8) | 4.32 (2.3–5.8) |
| CD4 count (cells/μL) [median (range)] | 180.0 (7–698) | 245.0 (9–527) | 227.5 (7–698) |
| Duration of HIV infection (years) [median (range)]* | 3.3 (0.37–8.64) | 3.0 (1.15–11.16) | 3.1 (0.37–11.16) |
| Hepatitis B and/or C virus coinfected [n (%)] | 3 (5.1) | 3 (5.3) | 6 (5.2) |
| CDC category C [n (%)] | 15 (25.4) | 11 (19.3) | 26 (22.4) |
| Treatment status at screening [n (%)] | |||
| Treatment interruption after a first-line NNRTI regimen | 7 (11.9) | 8 (14.0) | 15 (12.9) |
| Treatment interruption after receiving an NNRTI for prevention of MTCT | 2 (3.4) | 2 (3.5) | 4 (3.4) |
| First-line NNRTI regimen | 50 (84.7) | 47 (82.5) | 97 (83.6) |
| Regional distribution of patients [n (%)] | |||
| Asia and South Africa | |||
| Thailand | 8 (13.6) | 10 (17.5) | 18 (15.5) |
| South Africa | 26 (44.1) | 22 (38.6) | 48 (41.4) |
| Europe | |||
| Russia | 2 (3.4) | 3 (5.3) | 5 (4.3) |
| Spain | 4 (6.8) | 2 (3.5) | 6 (5.2) |
| UK | 0 (0.0) | 2 (3.5) | 2 (1.7) |
| Latin America | |||
| Argentina | 4 (6.8) | 4 (7.0) | 8 (6.9) |
| Brazil | 14 (23.7) | 13 (22.8) | 27 (23.3) |
| Mexico | 1 (1.7) | 1 (1.8) | 2 (1.7) |
| Clade [n (%)] | |||
| A1 | 0 (0.0) | 3 (5.3) | 3 (2.6) |
| AE | 7 (11.9) | 9 (15.8) | 16 (13.8) |
| B | 21 (35.6) | 21 (36.8) | 42 (36.2) |
| BF | 2 (3.4) | 1 (1.8) | 3 (2.6) |
| C | 27 (45.8) | 22 (38.6) | 49 (42.2) |
| F1 | 2 (3.4) | 1 (1.8) | 3 (2.6) |
| Mutations at baseline [median (range)] | |||
| All RT mutations | 30 (11–47) | 31 (14–51) | 30 (11–51) |
| NNRTI RAMs [24] | 2 (0–4) | 2 (0–4) | 2 (0–4) |
| IAS-USA NNRTI RAMs [23] | 1 (0–3) | 1 (0–4) | 1 (0–4) |
| IAS-USA NRTI RAMs [22] | 2 (0–6) | 1 (0–7) | 1 (0–7) |
| NRTI TAM-1 mutations | 0 (0–4) | 0 (0–5) | 0 (0–5) |
| NRTI TAM-2 mutations | 1 (0–5) | 1 (0–6) | 1 (0–6) |
| TMC125 RAMs [25] | 1 (0–3) | 1 (0–3) | 1 (0–3) |
| IAS-USA PI RAMs | 2 (0–5) | 3 (0–6) | 3 (0–6) |
| IAS-USA primary PI mutations | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Number of previously used antiretroviral drugs [n (%)] | |||
| Any antiretroviral drug | |||
| 1 | 2 (3.4) | 2 (3.5) | 4 (3.4) |
| 3 | 41 (69.5) | 36 (63.2) | 77 (66.4) |
| 4 | 10 (16.9) | 11 (19.3) | 21 (18.1) |
| 5 | 6 (10.2) | 8 (14.0) | 14 (12.1) |
| NNRTI | |||
| 1 | 51 (86.4) | 51 (89.5) | 102 (87.9) |
| 2 | 8 (13.6) | 6 (10.5) | 14 (12.1) |
| NRTI | |||
| 0 | 2 (3.4) | 2 (3.5) | 4 (3.4) |
| 2 | 46 (78.0) | 39 (68.4) | 85 (73.3) |
| 3 | 8 (13.6) | 12 (21.1) | 20 (17.2) |
| 4 | 3 (5.1) | 4 (7.0) | 7 (6.0) |
| PI | |||
| 1 | 0 (0.0) | 1 (1.8)† | 1 (0.9) |
| Previous antiretroviral therapy (months) [median (range)]‡ | |||
| Any antiretroviral drug | 31.8 (0–103) | 31.7 (0–97) | 31.7 (0–103) |
| NNRTI | 31.8 (0–103) | 27.3 (0–81) | 30.7 (0–103) |
| NRTI | 31.4 (2–103) | 31.9 (9–97) | 31.7 (2–103) |
| PI | – | 0.03† | 0.03† |
- * n=19 in TMC125 group; n=24 in control group.
- † One patient had previously taken three capsules of lopinavir/ritonavir (protocol violation).
- ‡ n=58 in TMC125 group.
- CDC, Centers for Disease Control and Prevention; IAS, International AIDS Society; MTCT, mother-to-child transmission; NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RAM, resistance-associated mutation; RT, reverse transcriptase; TAM, thymidine analogue mutation.
In this first-line NNRTI failure population, the prevalence of baseline NRTI and NNRTI RAMs was higher than in countries where monitoring of viral load is standard of care and virological failure is determined early (Table 1). The proportion of patients with at least two NNRTI RAMs was 69.5 and 71.9% in the TMC125 and control groups, respectively, and the proportion with at least two NRTI RAMs was 50.8 and 36.8%, respectively. The most frequently observed NNRTI RAMs (proportion of patients with the mutation in the TMC125 and control groups, respectively) were K103N (42.4 and 57.9%), Y181C (20.3 and 17.5%), K101E (16.9 and 8.8%) and G190A (22.0 and 22.8%). The number of NNRTI and NRTI RAMs was higher in patients from South Africa and Thailand than in patients from other countries (Fig. 2), although the number of patients in some countries was too small to make an adequate comparison.
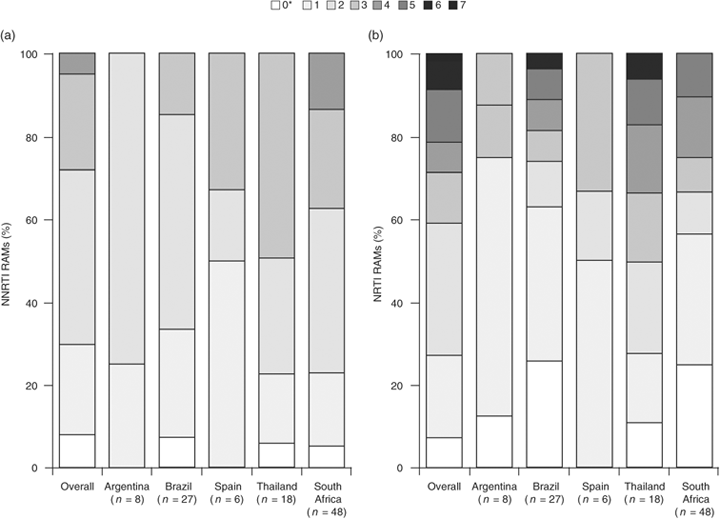
Number of detectable resistance-associated mutations (RAMs) at baseline by country. (a) Nonnucleoside reverse transcriptase inhibitor (NNRTI) RAMs; (b) nucleoside reverse transcriptase inhibitor (NRTI) RAMs. *All patients had NNRTI RAMs at screening or from prior genotyping. Data for Russia, UK and Mexico are not shown because of small sample sizes (n<4 each).
The individual NRTIs selected for use in the OBR were similar between the treatment groups. The NRTIs used by patients in the study were zidovudine (55%), tenofovir (45%), didanosine (31%), abacavir (24%), lamivudine (23%), stavudine (22%) and emtricitabine (1%). Overall, the three most common NRTI combinations were zidovudine/didanosine (17%), zidovudine/tenofovir (16%) and zidovudine/lamivudine (12%). A substantial number of patients recycled NRTIs (i.e. NRTIs had been used in a treatment regimen prior to baseline) in both treatment groups. In the TMC125 group, 37% of patients recycled one NRTI and 9% recycled two NRTIs, compared with 35 and 12%, respectively, in the control group. There were notable differences between the groups in the number of active NRTIs (maximal response only; by vircoTYPE® HIV-1 LM): more patients in the TMC125 group (31%) than in the control group (23%) had no active NRTI in the OBR whereas fewer patients used one or two active NRTIs in the TMC125 group (32 and 37%, respectively) than in the control group (37 and 40%). In the control group, 96.5% of patients used a ritonavir-boosted PI and 3.5% used an unboosted PI. Lopinavir (63%), atazanavir (32%), nelfinavir (4%) and amprenavir (2%) were the PIs used in this group.
Pre-switch treatment phase
Virological response
Virological responses are illustrated in Fig. 3. In the pre-switch treatment phase, the mean change in viral load from baseline in the control group was −2.16 and −2.13 log10 copies/mL at weeks 12 (n=53) and 24 (n=52), respectively. In the TMC125 group, the mean viral load reduction from baseline at week 12 was −1.39 log10 copies/mL (n=40), an intergroup least square (LS) means difference of 0.79 [primary endpoint; 95% confidence interval (CI) 0.38, 1.21]. After week 12, plasma viral load increased towards the baseline value; the mean change in viral load was −0.99 log10 copies/mL at week 20 (n=17) and −1.51 log10 copies/mL at week 24 (n=8).
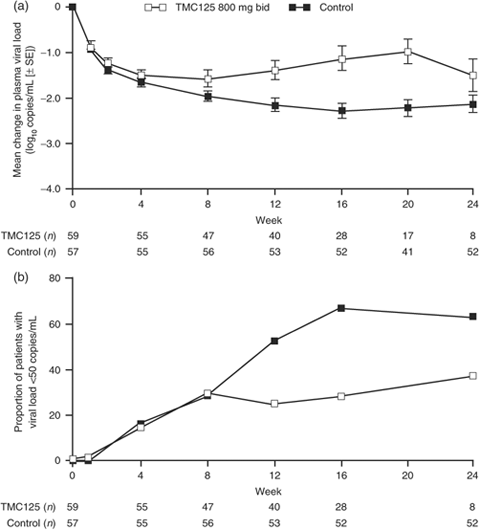
Virological responses from baseline up to week 24 (observed data). (a) Mean change from baseline in plasma viral load. (b) Proportion of patients with viral load <50 copies/mL. bid, twice a day; SE, standard error.
The proportion of patients achieving viral load <50 copies/mL is shown in Fig. 3b and is comparable between treatment groups up to week 8 (approximately 30% in both groups). However, as seen with the primary endpoint, the proportion of responders continued to increase from week 12 onwards in the control group, while in the TMC125 group it reached a plateau. For example, the week 12 inter-group difference in the proportion of patients with viral load <50 copies/mL was −27.8% (95% CI−46.8%, −8.8%). To further investigate the efficacy results from the TMC125 group, virological response (mean change in log10 viral load over time) was analysed by baseline resistance, sensitivity of the background regimen and country.
In a post hoc multivariate analysis of baseline resistance data for the TMC125 group, a reduced virological response was observed at week 12 in patients who had any of the following baseline characteristics: at least one detectable NNRTI RAM; the presence of Y181C (in combination with other NNRTI RAMs) but not K103N; baseline TMC125 fold change in 50% effective concentration (FC) equal to or greater than the arbitrary threshold of 10; and a higher number of baseline IAS-USA NRTI RAMs, TAMs or TMC125 RAMs.
Although the presence of at least one NNRTI RAM was associated with a reduced virological response, there was no clear correlation of response with the total number of NNRTI RAMs; median change from baseline in log10 viral load was similar at week 12 despite the presence of one, two, three or four NNRTI RAMs at baseline. In contrast, responses declined as the number of TMC125 RAMs present at baseline increased, with three or more TMC125 RAMs being required to substantially affect viral load reduction from baseline (Fig. 4). An increased virological response with TMC125 was observed in those patients with a baseline TMC125 FC<4 (arbitrary threshold); the mean change in viral load from baseline at week 12 was −1.82 log10 copies/mL for patients with TMC125 FC<4 (n=24) vs. −0.96 log10 copies/mL with TMC125 FC≥4 (n=11).
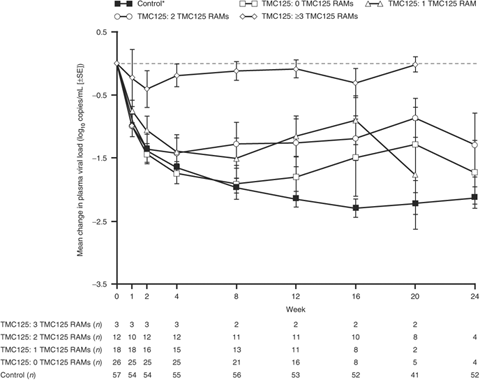
Mean change in plasma viral load [±standard error (SE)] by number of baseline TMC125 resistance-associated mutations (RAMs) (observed data; post hoc analysis). TMC125 RAMs include V90I, A98G, L100I, K101E, K101P, V106I, V179D, V179F, Y181C, Y181I, Y181V, G190A and G190S. These RAMs were identified using data from the DUET trials, which were conducted after the C227 study [25]. *Patients in the control group received an investigator-selected protease inhibitor (PI) plus two nucleoside transcriptase inhibitors (NRTIs).
NRTI resistance also had a substantial effect on virological response. As shown in Fig. 5a, an increasing number of TAMs and/or M184V, M184I or K65R had a negative impact on virological response. Similarly, an increased virological response with TMC125 was observed in patients using two active NRTIs in the background regimen. There was also a correlation between decreased baseline phenotypic sensitivity to TMC125 and an increased number of NRTI resistance mutations (TAMs, M184I/V and/or K65R; Fig. 5b). These combined factors were associated with reduced viral load change from baseline at week 12.
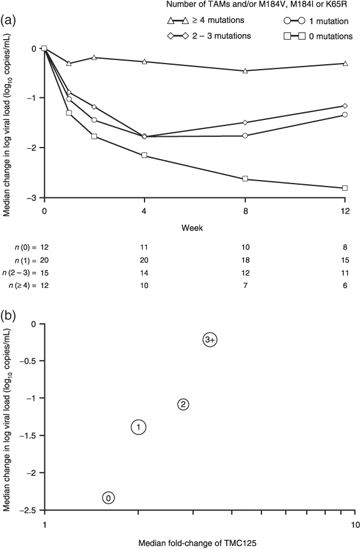
Effect of baseline resistance on plasma viral load change from baseline in the TMC125 arm. (a) Response according to number of nucleoside reverse transcriptase inhibitor (NRTI) mutations [number of thymidine analogue mutations (TAMs) and/or M184V, M184I or K65R]. (b) Combined effect of baseline TMC125 and NRTI resistance (observed data; post hoc analysis). The label inside the bubble denotes the number of mutations (TAM mutation plus M184I, M184V and K65R). The size of the bubble is driven by the number of included patients.
Virological response varied by country and this subanalysis included TMC125-treated patients from Argentina (n=4), Brazil (n=14), South Africa (n=26), Spain (n=4) and Thailand (n=8). The availability of data at each time-point varied according to the length of the enrolment period in each country prior to the discontinuation of TMC125; patients from Brazil randomized to TMC125 were treated for not more than 8 weeks at the time of discontinuation. Russia, Mexico and the UK were not included in the subanalysis because there were fewer than four patients from each country. At week 4, patients receiving TMC125 from all five countries had a mean viral load reduction greater than −1 log10 copies/mL from baseline. After achieving a mean reduction in log10 viral load of −1.91 log10 copies/mL from baseline at week 8 (n=21), patients from South Africa experienced a viral load rebound that was earlier than in Argentina, Brazil or Spain and continued until week 20 (−0.98 log10 copies/mL; n=8). For patients from Thailand, the mean reduction in log10 viral load from baseline was much less pronounced compared with that for other countries (−1.18 log10 copies/mL at week 4); these patients experienced a viral load rebound at weeks 12 and 16, with a mean reduction of approximately −0.5 log10 copies/mL. Similar to the inter-country variability, virological response varied according to HIV-1 subtype with a mean reduction in viral load from baseline at week 12 of +1.16, −0.64, −1.48, −1.61 and −2.77 log10 copies/mL among patients with clades F1 (n=1), AE (n=7), B (n=12), C (n=18) and BF (n=2), respectively.
Immunological response
Before the treatment switch, patients in the TMC125 group had a lower mean change from baseline in CD4 cell count at week 12 than patients in the control group: 42 cells/μL (n=38 of 59 patients) vs. 79 cells/μL (n=52 of 57 patients). At 24 weeks, there was a smaller difference in mean CD4 count between the two treatment groups: 112 cells/μL (n=6 of 59 patients) in the TMC125 group vs. 123 cells/μL (n=51 of 57 patients) in the control group.
Pharmacokinetics
Population pharmacokinetic analysis was performed on 51 patients who received TMC125 before the treatment switch. A complete data set could not be obtained because of early trial discontinuation. For TMC125, the estimated mean AUC12 h and C0 h were 4609 ng × h/mL and 275 ng/mL, respectively. Age, weight, sex, race, region and use of tenofovir in the background regimen did not affect AUC12 h or C0 h, but there was a trend for lower AUC12 h and C0 h with increasing body weight.
The relationship between pharmacokinetic parameters and plasma viral load was assessed. The mean reduction in viral load from baseline appeared to be higher in patients with exposure to TMC125 above the median AUC12 h (>4521 ng × h/mL). However, no statistically significant relationship between AUC12 h and change from baseline log10 viral load at week 12 was observed.
Safety and tolerability
Overall, 81.4% of patients taking TMC125 and 89.5% of control patients reported at least one adverse event during the pre-switch period (Table 2). The most frequently reported adverse events (occurring in at least 10% of patients regardless of severity and cause) in the TMC125 group were nausea, diarrhoea and headache; in the control PI group, these were diarrhoea, loose stools, upper respiratory tract infection, headache and nausea.
| TMC125 (n=59) | Control PI (n=57) | |
|---|---|---|
| Duration of treatment (weeks) [median (range)] | 14.3 (3.9–32.1) | 27.1 (11.1–47.3) |
| AEs, any cause [n (%)] | ||
| At least one AE | 48 (81.4) | 51 (89.5) |
| AEs leading to permanent discontinuation | 3 (5.1) | 0 (0.0) |
| Any grade 3 or 4 AE | 8 (13.6) | 10 (17.5) |
| Any serious AE | 2 (3.4) | 2 (3.5) |
| Deaths (any cause) | 0 (0.0) | 0 (0.0) |
| AEs, any grade and cause (incidence ≥10%) [n (%)] | ||
| Nausea | 10 (16.9) | 6 (10.5) |
| Diarrhoea | 8 (13.6) | 16 (28.1) |
| Headache | 8 (13.6) | 10 (17.5) |
| Upper respiratory tract infection | 5 (8.5) | 10 (17.5) |
| Loose stools | 2 (3.4) | 10 (17.5) |
| Grade 3 or 4 AEs, any cause (incidence >1 patient in either treatment group) [n (%)] | ||
| Hyperbilirubinaemia | 0 (0.0) | 2 (3.5) |
| Hypercholesterolaemia | 0 (0.0) | 2 (3.5) |
| Angioneurotic oedema | 2 (3.4) | 0 (0.0) |
- AE, adverse event; PI, protease inhibitor.
The majority of adverse events were grade 1 or 2 in severity. Grade 3 or 4 adverse events were mostly single events and did not occur in more than two patients. The most frequent adverse events thought possibly related to TMC125 treatment were nausea, upper and lower respiratory tract infections and headache. Four patients (two from each group) reported at least one serious adverse event. Three patients permanently discontinued TMC125 treatment because of adverse events. The first patient developed pulmonary tuberculosis, which was considered unrelated to TMC125 treatment. The second patient developed maculopapular rash (considered possibly related to TMC125 and doubtfully related to OBR) and grade 3 angioneurotic oedema (probably related to TMC125 and possibly related to OBR). The third patient developed grade 3 angioneurotic oedema, which was considered probably related to both TMC125 and the OBR (abacavir and zidovudine). The cases of rash and angioneurotic oedema resolved soon after treatment discontinuation, and no patients died during the trial.
Adverse events of specific interest for TMC125 and PIs include rash, and gastrointestinal and hepatobiliary disorders. Rash (any type and regardless of cause) was reported in 11.9% of patients in the TMC125 group and in 3.5% of the control group (8.4% difference; 95% CI−1.2%, 17.9%). Gastrointestinal disorders occurred less frequently in the TMC125 group than in the control group (39.0%vs. 59.6%; difference −20.6%; 95% CI −38.5%, −2.9%; Table 2). No hepatobiliary disorders of any grade or cause were reported in the TMC125 group. In the control group, 8.8% of patients developed jaundice (5.3% grade 1, 1.8% grade 3 and 1.8% grade 4) while 3.5% had grade 3 hyperbilirubinaemia.
There were no clinically relevant changes over time in mean values for laboratory parameters for patients in the TMC125 group. There was a trend for an increase in the mean creatinine values in both groups. However, grade 3 or 4 lipid and bilirubin abnormalities (any cause) were less frequent with TMC125 than with control PI therapy.
Post-switch treatment phase
In patients in the TMC125 group who were switched to an approved PI-based regimen, the mean change in viral load following the switch was −0.56 log10 copies/mL after 12 weeks (n=39) and −0.53 log10 copies/mL after 24 weeks (n=49). The mean increase in CD4 count from baseline was 65 cells/μL at week 12 (n=39) and 35 cells/μL at week 24 (n=47).
Discussion
In this exploratory study of TMC125 in PI-naïve individuals with NNRTI and NRTI resistance, the patients in the TMC125 group, whilst demonstrating an initial, substantial mean decrease in viral load, had lower virological responses at week 12 compared with patients in the control PI group. This is probably attributable to the combination of baseline NRTI and NNRTI resistance, in patients using a regimen comprised only of these agents. In addition, many patients recycled previously used NRTIs. The combination of NRTI resistance and NRTI recycling compromised the activity of the background regimen, which negatively affected the virological response over time to the NNRTI-based (TMC125) treatment relative to the control PI treatment. As the study population was PI-naïve, the PI component of therapy was likely to be highly active in patients in the control group, and the effect of baseline NRTI resistance was not as apparent as in the patients in the TMC125 group. Therefore the use of TMC125 plus NRTIs alone may not be optimal in PI-naïve patients with first-line virological failure on an NNRTI-based regimen.
Our efficacy findings are in contrast to those of the phase IIb TMC125-C223 trial and the two large, placebo-controlled, phase III clinical trials DUET-1 and -2, both of which investigated the efficacy and tolerability of TMC125 in treatment-experienced patients with documented NNRTI and PI resistance [18–20]. In the TMC125-C223 study, the proportion of patients who achieved undetectable viral load was significantly greater in the TMC125 than in the control group [18]. The success of TMC125 treatment in the TMC125-C223 study formed the basis for conducting the TMC125-C227 trial. The efficacy of TMC125 in a treatment-experienced population was confirmed in the primary 24-week analysis of DUET-1 and -2 [19,20]. In DUET-1, 56% of patients receiving TMC125 compared with 39% of patients receiving placebo, both given in addition to a background antiretroviral regimen, achieved the primary efficacy parameter of confirmed viral load <50 copies/mL (P=0.005) [19]. Similarly, in DUET-2, significantly more patients in the TMC125 group than in the placebo group achieved confirmed viral load <50 copies/mL at week 24 (62 vs. 44%, respectively; P=0.0003) [20].
When interpreting the data from the TMC125-C227 trial it is important to note that the number of NRTI mutations present tended to increase with increasing resistance to TMC125 (Fig. 5b). It is therefore likely that the combination of NRTI and TMC125 resistance compromised the activity of a regimen consisting only of these agents, particularly when compared with a PI-based regimen in this PI-naïve population. With the availability of resistance data from the DUET trials, patient suitability for TMC125 treatment can now be more accurately assessed. Indeed, a post hoc analysis reported here shows that virological responses in the presence of zero, one or two TMC125 RAMs were greater than the responses achieved in the presence of three TMC125 RAMs (Fig. 4).
Overall, TMC125 was well tolerated in the TMC125-C227 trial, consistent with published results of other TMC125 studies [19,20,26,27]. Tolerability of TMC125 was better than that of the control PI regimen in terms of gastrointestinal or lipid events. Although TMC125 treatment was associated with a higher incidence of rash than the control PI in this study, it has also been shown that the rash is manageable, generally mild to moderate, and most patients can continue treatment. These findings are consistent with the tolerability findings in the DUET trials.
This study had several limitations. The open-label design may have contributed to patient assessment biases. The enrolment of PI-naïve patients with combined baseline NRTI and NNRTI resistance skewed the virological response in favour of the control PI treatment. In addition, there were inter-group differences in NRTI activity. Finally, the early discontinuation of TMC125 treatment made the duration of exposure to the study drugs unequal between treatment arms and curtailed the gathering of data, which could have been used to fully assess treatment response and predictors of response.
The results of our study demonstrated the limitation of TMC125 treatment in combination with only two NRTIs in the setting of high levels of NNRTI and NRTI resistance. This underscores the need to adequately monitor patients for virological failure to limit the development of viral resistance, thus limiting future treatment options. If such monitoring is not possible, then the most active regimen likely to succeed should be considered. It must be emphasized that, in contrast to the findings in this study, the results of the DUET trials show that TMC125 remains highly active against a large number of NNRTI-resistant viruses, and with a different background regimen than administered in the current trial, resulted in superior virological results compared with the background regimen without TMC125. The good tolerability profile of TMC125 was confirmed when compared with placebo in the phase III trials.
Therefore, our findings encourage the use of TMC125 with an active antiretroviral regimen to ensure optimal efficacy. They also suggest that the use of TMC125 plus NRTIs alone may not be optimal in PI-naïve patients with first-line virological failure on an NNRTI-based regimen. Finally, they support frequent monitoring for virological failure and early treatment switch to lessen the likelihood of developing high-level drug resistance and limiting future treatment options. All of these findings are in line with current treatment guidelines in developed countries [28,29].
Acknowledgements
The authors would like to thank the patients and their families, the investigators, the study centre staff, the DSMB, Diego Miralles, Marie-Pierre de Béthune, Richard M. W. Hoetelmans and the Tibotec study team. We also thank Kerry Padilla and Joanne Williams (medical writers, Gardiner-Caldwell Communications, Macclesfield, UK) for their assistance in drafting the manuscript and collating author contributions. Financial assistance to support this service was provided by the study sponsor, Tibotec BVBA, Mechelen, Belgium.
Potential conflicts of interest
KR has recently received research grants, funding and/or honoraria, or has been a consultant or advisor to, or received lecture sponsorships from, Abbott, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, GlaxoSmithKline, Hoffmann-La Roche, Janssen-Cilag, Merck Sharpe & Dohme, Tibotec and Virco. RJP has recently been an advisor to GlaxoSmithKline and Hoffmann-La Roche and is an investigator in clinical trials sponsored by Abbott, Gilead, Tibotec, Pfizer, Schering-Plough and Bristol-Myers Squibb. GHL has no involvement with the pharmaceutical industry. FC is an investigator in clinical trials sponsored by Tibotec and several other pharmaceutical companies. PD has received research grants, funding and/or honoraria, or has been a consultant or advisor to, or received lecture sponsorships from, GlaxoSmithKline, Bristol-Myers Squibb, Gilead, Boehringer Ingelheim, Janssen Cilag, Merck Sharpe & Dohme and Pfizer. SL has received research grants/funding, or has been a consultant or advisor to, or received lecture sponsorships from, Bristol-Myers Squibb, Roche and Janssen-Cilag. WP has received research grants/funding from Tibotec. JV, MP, IP, GDeS and BW are all employees of Tibotec BVBA, Mechelen, Belgium. TNK is an employee of Tibotec, Inc., Yardley, PA, USA.
Appendix
Appendix: the TMC125-C227 study group
In addition to the authors, the TMC125-C227 study group included the following investigators and coordinators –Argentina: Pedro Cahn, Patricia Patterson, Carlos Zala, Alejandro Krolewiecki, Claudia Elena Ochoa, Pablo Parenti, Claudia Suarez, Marcelo Losso, Silvia Ivalo, Adriana Duran, Leonardo Lourteau, Javier Toibaro, Isabel Cassetti, Claudia Vanzulli, Marcelo Laurido, Gabriela Bugarin, Gabriela Trinidad, Analia Urueña, Rosa Bologna and Jorge Benetucci; Brazil: Artur Timerman, Ricardo Vipich, Sandra Tardochi, Juvencio Furtado, José Valdez Madruga, Roberta Noguiera, Suzane Leme, Denise Estevam, Marta Ramalho, Fabio Araujo, Mylva Fonsi, Eduardo Franco, Rita Sartio, Maura Silveira, Dinalda Monteiro, Mariana Carvalho, Mariangela Resende, Monica de Moraes, William Abreu, Ana Lucia Krzesinski, Clóvis Arns da Cunha, Rodrigo Reis, Jaime Rocha, Maria Kuchiki, Paula Toledo, Silvia De Rossi, Mirian Carvalho, Monica da Silva, Beatriz Grinsztejn, Jorge Ribeiro, Jose Pilotto, Anita Silva, Junia Rodrigues, Ruth Friedman, Valeria Rolla, Brenda Hoagland, Carla Vorsatz, Monica Barros and Sandra Cardoso; Mexico: Jaime Andrade Villanueva, Gemma Chávez, Luz Alicia Gonzalez and Fernando Amador; Russia: Elena Vinogradova, Natalia Zakharova, Anna Vinogradova, Olga Leonova, Ludmila Krutitskaya, Natalia Badosova, Yelena Semushina, Alexey Yakovlev, Nadezhda Burova, Ludmila Abrosimova, Zoya Kingo, Grigory Moshkovich, Stella Minaeva, Mariya Goryaeva, Oleg Kozyrev, Igor Malyuzhenko, Maria Malyuzhenko, Oleg Romanenko, Margarita Makarova, Elina Zamyatina, Faniya Siraziyeva, Rostislav Mikusev, Vladimir Koksin and Valery Shoshokin; South Africa: Lerato Mohapi, Mellicent Khosa, Ditabe Mohohlo, Prudensce Ive, Melanie-Ann John, Dennis Rubel, Ian Sanne, Cynthia Firnhaberr, David Spencer, Joyce Marshall, Mukesh Raniga, Ezio Baraldi and Mauricio Itturalde, Spain: Daniel Podzamczer, Elena Corbera, Montserrat Plaza, Pilar Guillen, Montse Fuster, Maria-Antonia Sambeat, Gracia García, Josep Cadafalch, Maria del Mar Gutiérrez Macià, Koldo Aguirrebengoa, Josune Goicoetxea, Miguel Montejo, Ignacio de los Santos Gil, Raquel Carrillo Gómez and Jesús Sanz; Thailand: Chaiwat Ungsedhapand, Chris Duncomb, Juntanat Ananworanich, Saskia Autar, Anchalee Avihingsanon and Torsak Bunupuradah; UK: Phillip Hay, Prasad Velisetty, Derek Macallan, Tariq Sadiq, Richard Lau, Paul Brennan-Benson, Mark Pakianathan, Janet Mantell, Brian Gazzard, Desmond Maitland, Akil Jackson and Alastair Teague.




