Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death
Abstract
Objectives
The aim of the study was to compare incidence rates (IRs) of AIDS/death in patients with and without treatment interruption (TI) of combination antiretroviral therapy (cART) for periods of 3 months or more for different categories of CD4 cell count and viral load, and to determine risk factors for clinical progression to AIDS/death.
Methods
Patients starting cART with a CD4 cell count and a viral load available within 6 months of starting cART were included in the study. The IR and risk factors of TI were determined. We assessed the incidence rate ratios (IRRs) for TI and AIDS/death events using Poisson regression models.
Results
Of 3811 patients included in the study, 26% were ART-naïve prior to cART. The median date of starting cART was July 1997, the median CD4 cell count was 226 cells/μL and the median viral load was 4.36 log10 HIV-1 RNA copies/mL. We observed 1243 interruptions and 403 AIDS-events/deaths. The IR of AIDS/death was higher in patients with lower CD4 cell counts or higher viral loads, regardless of TI. After adjusting for baseline factors, the IR of AIDS/death was significantly higher in the TI group than in the non-TI group [IRR 2.63; 95% confidence interval (CI) 2.01–3.44; P<0.0001]; this could be explained by current CD4 cell counts and viral loads, as the CD4 cell count- and viral load-adjusted IRR was 1.14 (95% CI 0.86–1.51; P=0.37). Within the TI group, patients with a current CD4 cell count of <200 cells/μL had a 3-fold higher risk of AIDS/death than those with a CD4 cell count of 200–350 cells/μL, whereas patients with a current CD4 cell count of >350 cells/μL had a 4-fold lower risk of disease progression.
Conclusions
TI is common in clinical practice. The risk of AIDS/death increased more than 2-fold for patients stopping all cART regimen drugs for 3 months or more. Among patients experiencing a TI, those with low CD4 cell counts, high viral loads or prior AIDS had an increased risk of AIDS/death. Hence, TI should be discouraged and closely monitored if it occurs.
Introduction
The long-term effects, durability and potential limitations of combination antiretroviral therapy (cART) are far from known. In clinical practice, it is difficult for many patients to adhere to continuous, long-term therapy [1], and up to 50% of patients who have started cART discontinue part or all of their initial regimen within 12 months of starting it. This is often a consequence of the patient's own choice [2–6], but can also be related to the drug toxicities and metabolic side effects associated with cART use [7–11]. These toxicities and side effects may increase the risk of interruption or stopping of therapy [12]. As treatment interruptions (TIs) reduce exposure to antiretroviral drugs, TIs can potentially improve toxicity profiles such as lipodystrophy, body shape modifications and metabolic side effects related to prolonged use of therapy [12–14]. ‘Drug holidays’ have also been reported to improve quality of life by reducing the stress of the continuous timing of the drugs [15].
Until recently there have been few published data on TIs and their association with disease progression to AIDS or death in clinical practice, and results have not been entirely consistent [16–21]. The SMART study recently reported that CD4-guided episodic ART use with the aim of maintaining CD4 cell counts >250 cells/μL is associated with a 2-fold increased short-term risk of clinical progression of HIV disease compared with continuous ART [22, published after acceptance of this manuscript]. Whereas the findings in the protocolled randomized trial support recommendations not to interrupt therapy, there may be profound differences between a structured treatment interruption (STI) as part of a controlled experiment or clinical trial, and an unplanned or planned TI within the clinic setting. In this study, we focus on TIs in the clinical setting, which is less precise but is more likely to represent what happens in routine clinical practice.
Hence we aimed to assess the rate of TIs within a heterogenous patient population with considerable total follow-up time, and the clinical consequences thereof, i.e. the risk of AIDS and death associated with TIs. Furthermore, we assessed the independent risk factors for disease progression within the group of patients who interrupted therapy.
Methods
General methods
The EuroSIDA study is a prospective study of 11928 patients with HIV-1 infection in 82 centres across Europe, Argentina and Israel; detailed information about the study has been published [23]. To date, six cohorts of patients have been recruited. For each cohort, centres provided data for consecutive patients seen in out-patient clinics, until a predefined number of patients were enrolled from each centre with a prescheduled clinic appointment. For cohorts I–III, eligible patients were those with a CD4 cell count <500 cells/μL in the 4 months prior to enrolment. This restriction was removed for cohorts IV–VI. Information from patient notes was provided on a standardized data collection form at baseline and every 6 months thereafter. Follow up was until September 2005. At every follow-up visit, CD4 counts and viral load measurements routinely taken since the last follow up were reported. Height, weight, haemoglobin concentrations and other laboratory parameters were also routinely collected. For all patients, the date of starting and stopping every antiretroviral drug was recorded, as was the use of drugs for prophylaxis against opportunistic infections. Dates of diagnosis of all AIDS-defining illnesses were also recorded, including subsequent diagnoses, using the 1993 clinical definition of AIDS from the Centers for Disease Control and Prevention (CDC) [24]. A rigorous quality assurance programme has been established that includes data control at the coordinating centre, site visits to check patient selection, and cross-checking of data against patient notes. All cases of AIDS and death are validated by source verification. Informed consent and ethics committee approval were obtained according to national guidelines.
Statistical methods
Patients eligible for the present analyses were patients who started cART during prospective follow up, had a CD4 cell count and viral load measured in the 6 months prior to starting cART, and had some prospective follow up. cART was defined as a regimen including a single nonnucleoside reverse transcriptase inhibitor (NNRTI), a single protease inhibitor (PI), a ritonavir-boosted PI, or abacavir plus exactly two nucleoside reverse transcriptase inhibitors (NRTIs).
A TI was defined as an interruption of all drugs in the cART regimen for 3 months or more. Interruptions of only some of the drugs in the regimen or TIs of less than 3 months were not counted as interruptions. A 3-month time-lag period for a TI was used to reduce the potential bias caused by terminally ill patients stopping drugs prior to death. Patient follow up began at initiation of cART and ended at the last visit or death, and patients could have more than one TI. Poisson regression, with adjustment for multiple events per patient, was used to determine the incidence of TIs. The following factors were assessed in univariable analyses: gender, risk group, race, age, date of starting cART, time since starting cART, prior AIDS diagnosis, region of Europe, CD4 cell count and viral load at cART initiation, nadir CD4 cell count, prior ART usage, cART regimen, and hepatitis B virus (HBV) and hepatitis C virus (HCV) status. In addition, HBV and HCV status, diagnosis of AIDS, CD4 cell count and viral load were investigated as time-updated values. Factors that had a P-value of <0.1 in univariable analyses were then included in multivariable analyses.
Poisson regression was also used to investigate the relationship between a TI and disease progression (new AIDS/death). In this analysis, patient follow-up began at cART initiation and ended at the last visit or at new AIDS/death. Patients were removed from this analysis when they were not under current follow-up, which was defined as 3 months after either a CD4 cell count or viral load measurement. Patients were re-included in the analysis when they had a new, current CD4 cell count or viral load measured. A similar method for model selection was used as described above. This methodology was also used to determine the factors related to disease progression (new AIDS/death) among those patients with at least one TI. Patient follow-up began at the date of the first TI and ended at the last visit, or at diagnosis of new AIDS/death.
Various sensitivity analyses were performed including analysis of AIDS and death as separate endpoints. In addition, analyses with no time-lag period (0 months) and analysis with increased time-lag period (6 months) for the composite endpoint AIDS/death were carried out.
Results
Patient characteristics
A total of 3811 patients fulfilled the inclusion criteria, and are described in Table 1. The patients experiencing TIs were similar to patients not having a TI, although in the TI group there was a slightly higher proportion of patients belonging to the injecting drug use (IDU) category and, consistent with this, also a higher percentage of TI patients who were HCV positive. The overall CD4 cell count nadir was 150 cells/μL [interquartile range (IQR) 60–243 cells/μL] and was similar in the two groups. The median CD4 cell count at cART initiation was slightly lower in the TI group. In contrast, in the non-TI group, there was a higher proportion of patients who had experienced AIDS prior to cART initiation. The patients in both the TI and non-TI groups started similar cART regimens, with the majority of patients starting a single PI-containing cART regimen.
| Without interruption | With interruption* | Total | P-value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| All | 2932 | 76.9 | 879 | 23.1 | 3811 | 100 | – |
| Gender | |||||||
| Male | 2296 | 78.3 | 622 | 70.8 | 2918 | 76.6 | <0.0001 |
| Female | 636 | 21.7 | 257 | 29.2 | 893 | 23.4 | |
| Exposure group | |||||||
| Homosexual | 1374 | 46.9 | 346 | 39.4 | 1720 | 45.1 | <0.0001 |
| IDU | 588 | 20.1 | 254 | 28.9 | 842 | 22.1 | |
| Heterosexual | 754 | 25.7 | 231 | 26.3 | 985 | 25.9 | |
| Other | 216 | 7.4 | 48 | 5.5 | 264 | 6.9 | |
| Region | |||||||
| Southern Europe/Argentina | 864 | 29.5 | 243 | 27.7 | 1107 | 29.1 | 0.0002 |
| Central Europe | 774 | 26.4 | 267 | 30.4 | 1041 | 27.3 | |
| Northern Europe | 1068 | 36.4 | 335 | 38.1 | 1403 | 36.8 | |
| Eastern Europe | 226 | 7.7 | 34 | 3.9 | 260 | 6.8 | |
| Hepatitis B virus status at starting cART | |||||||
| Negative | 1991 | 67.9 | 582 | 66.2 | 2573 | 67.5 | 0.19 |
| Positive | 195 | 6.7 | 74 | 8.4 | 269 | 7.1 | |
| Unknown | 746 | 25.4 | 223 | 25.4 | 969 | 25.4 | |
| Hepatitis C virus status at starting cART | |||||||
| Negative | 1337 | 45.6 | 328 | 37.3 | 1665 | 43.7 | <0.0001 |
| Positive | 513 | 17.5 | 228 | 25.9 | 741 | 19.4 | |
| Unknown | 1082 | 36.9 | 323 | 36.8 | 1405 | 36.9 | |
| AIDS before cART | 800 | 27.3 | 182 | 20.7 | 982 | 25.8 | <0.0001 |
| Treatment naïve | 786 | 26.8 | 209 | 23.8 | 995 | 26.1 | 0.073 |
| cART regimen | |||||||
| Single PI | 1914 | 65.3 | 597 | 67.9 | 2511 | 65.9 | 0.057 |
| Dual PI | 371 | 12.7 | 79 | 9.0 | 450 | 11.8 | |
| Single NNRTI | 580 | 19.8 | 182 | 20.7 | 762 | 20.0 | |
| Triple NRTI | 67 | 2.3 | 21 | 2.4 | 88 | 2.3 | |
| Without interruption | With interruption* | Total | P-value | ||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | ||
| CD4 cell count at cART initiation | |||||||
| (cells/μL) | 220 | 108–337 | 251 | 136–379 | 226 | 114–347 | <0.0001 |
| Viral load at cART initiation | |||||||
| (log10 copies/mL) | 4.37 | 3.40–5.04 | 4.33 | 3.52–4.98 | 4.36 | 3.42–5.05 | 0.86 |
| Age (years) | 38.0 | 33.3–45.3 | 36.2 | 31.8–42.3 | 37.6 | 32.9–44.6 | <0.0001 |
| Date of cART initiation | |||||||
| (month/year) | 10/97 | 2/97–12/99 | 10/97 | 2/97–2/99 | 10/97 | 2/97–7/99 | 0.025 |
- IDU, injecting drug use; IQR, interquartile range; IRR, incidence rate ratio; cART, combination antiretroviral therapy, defined as a regimen including a single nonnucleoside reverse transcriptase inhibitor (NNRTI), a single protease inhibitor (PI), a ritonavir-boosted PI, or abacavir plus exactly two nucleoside reverse transcriptase inhibitors (NRTIs).
- * Interruption for therapy is defined as interruption of all drugs in the cART regimen for three months or more.
A total of 2816 patients (73.9%) had previously received ART drugs before starting cART.
Treatment interruption
There were 1243 treatment interruptions during 20845 person-years of follow up (PYFU) [an incidence rate (IR) of 6.0 per 100 PYFU; 95% confidence interval (CI) 5.7–6.3]. A total of 954 patients experienced one interruption, and 206 had a second, a median time of 18 months after the first (IQR 11–34 months). A further 63 patients had a third TI a median of 16 months after the second (IQR 11–27 months). Twelve, five and three patients had a fourth, fifth and sixth TI, respectively. At 12 months after starting cART, 5.6% of patients were estimated to have stopped cART for 3 or more months (95% CI 4.9–6.3%) and this increased to 10.6% (9.6–11.6%) at 24 months, 15.7% (14.5–16.9%) at 36 months and 19.8% (18.4–21.2%) at 48 months. There was no change over time in the incidence of TI; for example, in the first 12 months after starting cART, the incidence of TIs was 5.7 per 100 PYFU (95% CI 4.9–6.5), compared with 6.0 per 100 PYFU (95% CI 5.6–6.4) after this time (P=0.12, Poisson regression).
Factors associated with ≥3 months of interruption of cART
Table 2 shows the resulting incidence rate ratios (IRRs) of the univariable and time-updated multivariable analyses of the factors associated with ≥3 months of interruption of cART. Of note, after adjustment, patients with a higher current log viral load had an 86% higher incidence of TI (95% CI 77–95%; P<0.0001), while patients with a 50% higher current CD4 cell count had an 8% increased incidence of TI (95% CI 2–14%; P=0.062). Further, female patients had a 35% higher incidence of TIs than male patients (95% CI 19–54%; P<0.0001), whereas being 10 years older was associated with an 11% decreased incidence of TI (95% CI 4–17%; P=0.0014). Compared with patients from Southern Europe or Argentina, those from Central Europe had a 75% increased incidence of TI (95% CI 49–105%; P<0.0001), while those from Northern Europe had a 58% increased incidence of TI (95% CI 36–85%; P<0.0001). One year more recent initiation of cART was associated with an increased incidence of TI of 9% (95% CI 4–14%; P<0.0001). There were also some differences according to the cART regimen started. Compared with patients starting a single PI-based regimen, those who started an NNRTI-based regimen had a 35% increased incidence of TI (95% CI 14–61%; P=0.0006).
| Univariable | Multivariable;time-updated | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P-value | IRR | 95% CI | P-value | |
| Gender | ||||||
| Male | 1.00 | – | – | 1.00 | – | – |
| Female | 1.59 | 1.36–1.79 | <0.0001 | 1.35 | 0.19–1.54 | <0.0001 |
| Exposure group | ||||||
| Other | 1.00 | – | – | 1.00 | – | – |
| IDU | 1.62 | 1.41–1.86 | <0.0001 | 1.14 | 0.96–1.36 | 0.13 |
| Race | ||||||
| White | 1.00 | – | – | 1.00 | – | – |
| Other | 1.21 | 1.03–1.43 | 0.020 | 1.06 | 0.90–1.24 | 0.49 |
| Region | ||||||
| Southern Europe/Argentina | 1.00 | – | – | 1.00 | – | – |
| Central Europe | 1.32 | 1.12–1.56 | 0.0010 | 1.75 | 1.49–2.05 | <0.0001 |
| Northern Europe | 1.07 | 0.91–1.25 | 0.42 | 1.58 | 1.36–1.85 | <0.0001 |
| Eastern Europe | 1.22 | 0.87–1.72 | 0.25 | 0.84 | 0.60–1.17 | 0.30 |
| cART | ||||||
| Single PI | 1.00 | – | – | 1.00 | – | – |
| Dual PI | 1.11 | 0.88–1.39 | 0.38 | 1.07 | 0.85–1.33 | 0.57 |
| Single NNRTI | 1.52 | 1.29–1.79 | <0.0001 | 1.35 | 1.14–1.61 | 0.0006 |
| Triple NRTI | 1.23 | 0.83–1.84 | 0.31 | 1.06 | 0.72–1.54 | 0.78 |
| Hepatitis C virus status* | ||||||
| Negative | 1.00 | – | – | 1.00 | – | – |
| Positive | 1.64 | 1.42–1.89 | <0.0001 | 1.39 | 1.17–1.66 | 0.0002 |
| Unknown | 0.98 | 0.83–1.15 | 0.78 | 0.90 | 0.78–1.05 | 0.19 |
| AIDS diagnosis* | 1.54 | 1.23–1.92 | <0.0001 | 1.34 | 1.08–1.66 | 0.072 |
| CD4 cell count* | ||||||
| 50% higher | 0.79 | 0.76–0.82 | <0.0001 | 1.08 | 1.02–1.14 | 0.0062 |
| Viral load* | ||||||
| Log higher | 1.79 | 1.72–1.86 | <0.0001 | 1.86 | 1.77–1.95 | <0.0001 |
| Age | ||||||
| 10 years older | 0.81 | 0.75–0.87 | <0.0001 | 0.89 | 0.83–0.96 | 0.0014 |
| Date of starting cart | ||||||
| 1 year later | 1.08 | 1.04–1.11 | <0.0001 | 1.09 | 1.04–1.14 | <0.0001 |
- CI, confidence interval; IDU, injecting drug use; IRR, incidence rate ratio; cART, combination antiretroviral therapy, defined as a regimen including a single nonnucleoside reverse transcriptase inhibitor (NNRTI), a single protease inhibitor (PI), a ritonavir-boosted PI, or abacavir plus exactly two nucleoside reverse transcriptase inhibitors (NRTIs).
- * Variable included as time-updated.
Disease progression to AIDS or death
There were 403 clinical events of AIDS/deaths in 13 192 PYFU; (IR 3.7; 95% CI 3.5–4.0); 251 events were deaths (61.8%). The PYFU in this analysis were restricted to consecutive 3-month intervals following each CD4 or viral load measurement. Seventy-seven (19.0%) of the clinical events occurred during a TI and 329 events occurred whilst on treatment (81.0%), giving IRs of 4.7 (95% CI 3.7–5.8) and 2.8 (95% CI 2.5–3.2) per 100 PYFU, respectively (Fig. 1). The median CD4 cell count and viral load at diagnosis of an event were 160 cells/μL (IQR 60–313 cells/μL) and 3.63 log10 HIV-1 RNA copies/mL (IQR 2.00–4.99 log10 copies/mL) for patients on cART and 120 cells/μL (IQR 51–238 cells/μL) and 4.58 log10 copies/mL (IQR 2.70–5.23 log10 copies/mL) for those off cART, respectively (P=0.020 and P=0.0003, respectively).
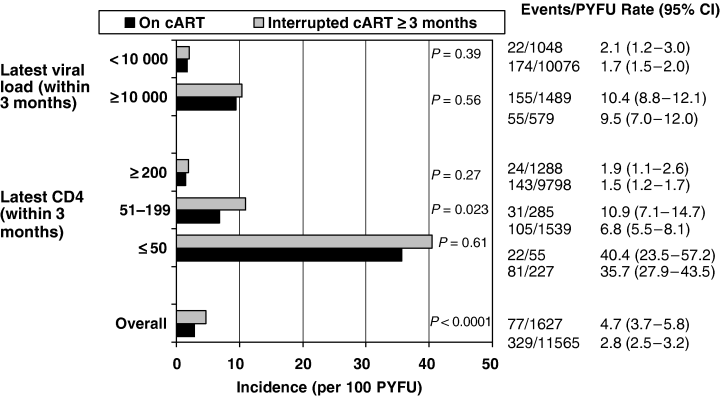
Incidence of clinical disease progression to AIDS or death among patients in the treatment interruption (TI) and non-TI groups. PYFU, person-years of follow up.
When IRs were analysed according to the current CD4 cell count, patients with a current CD4 cell count between 50 and 200 cells/μL in the TI group were found to be at significantly higher risk of new AIDS events or death compared with patients without a TI (Fig. 1). The risk of clinical progression was lower among patients with CD4 cell counts above 200 cells/μL, regardless of whether patients had experienced a TI or not; 1.5 (95% CI 1.2–1.7) compared with 1.9 (95% CI 1.1–2.6) per 100 PYFU (P=0.27).
General factors associated with AIDS or death in patients on cART
Figure 2 shows the IRRs from Poisson regression models comparing the IR of clinical disease progression in patients with TI(s) with that in patients without TIs. In the univariable analysis, patients in the TI group had a 1.66-fold (95% CI 1.30–2.13; P<0.0001) higher incidence of AIDS or death compared with patients in the non-TI group. After adjustment for confounding factors known at baseline (exposure group, cART regimen started, HBV and HCV status, prior AIDS diagnosis, age, time since started cART, date started cART and prior antiretroviral treatment, CD4 cell count and viral load), the IRR increased slightly (IRR 2.63; 95% CI 2.01–3.44; P<0.0001). After adjustment for current CD4 cell count and HBV and HCV status, the IRR dropped to 1.45 (95% CI 1.11–1.91; P=0.0071), while adjusting for current viral load and HBV and HCV status resulted in an IRR of 1.33 (95% CI 1.00–1.77; P=0.049). Adjustment for both current CD4 cell count and viral load and current HBV and HCV status reduced the IRR further to 1.14 (95% CI 0.86–1.51; P=0.37). This suggests that the majority of the increased risk of disease progression after interruption of cART could be explained by changes in current viral load and CD4 cell count following the interruption.
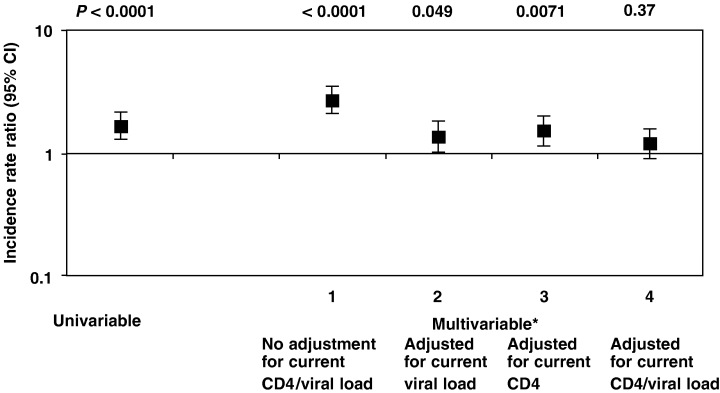
Incidence rate ratios of clinical disease progression among patients with a treatment interruption compared with those without. *The multivariable models were adjusted for exposure group, hepatitis B/C virus status, prior AIDS, combination antiretroviral therapy (cART) regimen, age, date of starting cART, and prior antiretroviral therapy. Current CD4 count or viral load means latest CD4 count or viral load measured within the last 3 months.
Factors associated with disease progression in the TI group
Factors associated with clinical disease progression were assessed amongst people with one or more TIs of ≥3 months (Table 3). Patients with a current CD4 cell count of 200 cells/μL or lower had a 2.95-fold higher incidence of new AIDS/death (95% CI 1.65–5.26; P=0.0003), while patients with a current CD4 cell count above 350 cells/μL had almost an 80% lower incidence (95% CI 61–93%; P<0.0001), when compared with patients with a current CD4 cell count between 201 and 350 cells/μL. Similarly, amongst patients experiencing one or more TIs, a current higher log viral load was associated with a 36% increased incidence of clinical disease progression (95% CI 14–62%; P=0.0007).
| Univariable | Multivariable;time-updated | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P-value | IRR | 95% CI | P-value | |
| Exposure group | ||||||
| Other | 1.00 | – | – | 1.00 | – | – |
| IDU | 1.70 | 1.07–2.69 | 0.024 | 1.61 | 0.77–3.33 | 0.20 |
| Hepatitis C virus status* | ||||||
| Negative | 1.00 | – | – | 1.00 | – | – |
| Positive | 1.36 | 0.83–2.24 | 0.22 | 0.83 | 0.39–1.79 | 0.63 |
| Unknown | 1.21 | 0.65–2.26 | 0.54 | 1.09 | 0.57–2.08 | |
| Prior AIDS | ||||||
| Yes | 1.67 | 1.02–2.74 | 0.042 | 0.86 | 0.51–1.45 | 0.58 |
| Age | ||||||
| 10 years older | 1.24 | 0.98–1.56 | 0.072 | 1.35 | 1.05–1.73 | 0.018 |
| Date started cART | ||||||
| 1 year later | 0.82 | 0.65–0.97 | 0.032 | 0.86 | 0.70–1.05 | 0.14 |
| CD4 cell count* | ||||||
| ≤200 | 3.98 | 2.31–6.88 | <0.0001 | 2.94 | 1.65–5.26 | 0.0003 |
| 201–350 | 1.00 | – | – | 1.00 | – | – |
| >350 | 0.17 | 0.10–0.29 | <0.0001 | 0.23 | 0.13–0.39 | <0.0001 |
| Viral load* | ||||||
| Log higher | 1.76 | 1.48–2.09 | <0.0001 | 1.36 | 1.14–1.62 | 0.0007 |
- CI, confidence interval; IDU, injecting drug use; IRR, incidence rate ratio; cART, combination antiretroviral therapy, defined as a regimen including a single nonnucleoside reverse transcriptase inhibitor, a single protease inhibitor (PI), a ritonavir-boosted PI, or abacavir plus exactly two nucleoside reverse transcriptase inhibitors.
- * Variable included as time-updated.
Results of sensitivity analyses
The results using a 6-month time-lag period instead of a 3-month time-lag period were consistent, suggesting that patients being terminally ill, and consequently interrupting therapy, did not cause a misclassification bias (data not shown). Similar results to those described above were also found when death and AIDS were used as endpoints separately.
Discussion
TI incidence and characteristics
As many as one in five patients interrupted all antiretroviral therapy for at least 3 months during an average of 5.5 years of follow up since starting cART. The proportion of patients experiencing a TI was lower in our predominantly ART-experienced population than among pre-cART naïve patients in Italy [16], and is probably in part explained by the lower proportion of injecting drug users in the EuroSIDA cohort than in the ICoNA cohort. In our trans-European clinical setting, the rate of a first TI was higher than found in clinical trials often characterized by strict inclusion and exclusion criteria [25,26].
Factors associated with a TI
Female gender and IDU were associated with a higher risk of TI. The gender difference may be explained by women having a higher risk of toxicities as a result of lower weight [2,27,28]. Furthermore, younger age [2], recent initiation of cART [21] and use of NNRTI-based regimens were associated with an increased risk of TI.
Surprisingly, patients from Central and Northern Europe had a higher incidence of TIs than patients from Southern and Eastern Europe, where rates were similar, after taking other differences into account. This may reflect a lack of international guidelines on how best to handle a patient wanting or experiencing a TI having led to different therapeutic strategies in different parts of Europe, but may also be explained by other differences in patient characteristics for which we could not control.
Factors associated with disease progression to AIDS or death
Most importantly, our data have shown a detrimental effect of interruption of all drugs in the cART regimen on the risk of disease progression to new clinical AIDS events or death. These results are consistent with previous reports [16,19,21,29,30].
Moreover, although older patients were less likely to interrupt therapy, those who did in fact interrupt therapy were at higher risk of experiencing an AIDS event or death.
Among those who interrupted therapy, there was no difference in the risk of clinical progression between the genders.
TI per se was associated with a 2.6-fold increased risk of clinical disease progression, after adjustment for all factors other than current CD4 cell count and viral load. Adjustment for both current CD4 cell count and viral load reduced the IRR further to 1.14 (95% CI 0.86–1.51; P=0.37), suggesting that the majority of the increased risk of disease progression after interruption of cART is explained by changes in the current viral load and CD4 cell count following the interruption. TIs are of particular concern in patients at an a priori high risk of disease progression, namely patients with low CD4 counts and high viral load as a consequence of the interruption [31–33].
Limitations, strengths and bias (sensitivity analyses)
The TIs recorded in EuroSIDA are generally unplanned. However, it is possible that patients do have TIs and just do not tell the clinician. As we are looking at TIs with a duration of more than 3 months, this potential bias would only tend to make us slightly underestimate the difference between the groups [21].
The strength of our study compared with earlier published data is the fact that our results are based on a large number of patients in a heterogeneous European HIV-infected population. Further, as our IRR calculations are adjusted for the current viral load and CD4 cell count, measured within the previous 3 months, our results are not only consistent but clinically more useful, than earlier published results in prediction of the risk of disease progression in a setting in which patients are seen every few months and clinicians wish to assess the short-term risk of disease progression if the patient undergoes a TI. Currently, there are not sufficient data to explore the risk of clinical progression after a TI in all CD4 cell count strata, and this is in particular the case for patients with a CD4 cell count above 200 cells/μL, because of the very low number of events among such patients regardless of whether they are on cART or not. Until recently, only a few randomized trials, mainly looking at heavily treatment-experienced or salvage patients, had reported on structured TIs of cART in patients with CD4 cell counts over 200 cells/μL, and the conclusion has been that the TI strategies should be evaluated in larger trials [18,34,35].
The SMART study was designed to compare a ‘viral suppression strategy’ (experimental group) and a ‘drug conserving strategy’ (control group, the latter allowing structured TIs among patients until their CD4 cell counts dropped below 250 cells/μL) [22,36]. The first results from the SMART study have recently been presented, and suggest that TI is associated with a 2-fold increased risk of HIV disease progression and/or death, and severe complications (cardiac, renal or hepatic). Bearing these results in mind, even patients with CD4 cell counts above 200 cells/μL, who have a low to moderate risk, should be monitored more closely when interrupting therapy for 3 months or more. However, the risk of clinical progression to AIDS or death following a TI in the clinical setting increases substantially for patients with CD4 cell counts below 200 cells/μL compared with patients with CD4 cell counts above 200 cells/μL.
Conclusions
We found that it was common for patients on cART to interrupt therapy for 3 months or more in clinical practice. The risk of progression to new clinical AIDS events or death was more than 2-fold higher for people experiencing a TI of at least 3 months compared with people who did not. This difference was closely associated with the current viral load and CD4 cell count, with the majority of the risk of a TI being explained by changes in CD4 cell count and viral load.
Among patients experiencing a TI, our results show that, in particular, patients with low CD4 cell counts, high viral loads and prior AIDS have an increased risk of disease or death, and therefore TI should be discouraged in these patients. It the patient does discontinue therapy, he or she should be closely monitored.




