Haemoglobin and albumin as markers of HIV disease progression in the highly active antiretrovial therapy era: relationships with gender*
*This work has previously been presented as Abstracts at the 10th Anniversary Conference of the British HIV Association (BHIVA), Cardiff, UK, 15–17 April 2004 (The effect of gender on haemoglobin as a marker of HIV disease progression in the HAART era) and at the 3rd International AIDS Society (IAS) conference on HIV pathogenesis and treatment, Rio de Janeiro, Brazil, 24–27 July 2005 (Albumin as a marker of HIV disease progression and prognosis in women and men following initiation of HAART).
Abstract
Objectives
The aims of the study were to describe gender differences in haemoglobin and albumin and to investigate the prognostic value of these measurements in relation to highly active antiretroviral therapy (HAART).
Methods
Anaemia was defined as haemoglobin <13.5 g/dL for men and <11.5 g/dL for women. Albumin <35 g/L was defined as hypoalbuminaemia. Proportional hazards models were used to describe relationships between these markers and HIV progression and death.
Results
A total of 291 patients had pre-HAART and 1-year measurements. Mean haemoglobin and albumin levels pre-HAART were lower in women than in men (haemoglobin: 11.2 vs 13.2 g/dL, respectively, P<0.0001; albumin: 37.4 vs 40.2 g/L, respectively, P<0.0001), and a higher proportion of women were anaemic and hypoalbuminaemic compared with men. Despite a rise in both markers in the first year on HAART, mean haemoglobin levels remained lower by 2.08 g/dL (P<0.0001) and albumin by 2.88 g/L (P<0.0001) in women. In the 495 patients included in this analysis, haemoglobin and albumin levels were both significantly related to short-term risk of AIDS and death independently of CD4 count [hazards ratio (HR)=0.73/g/dL higher haemoglobin, 95% confidence interval (CI) 0.55–0.82, P<0.0001 and HR=0.87/g/L higher albumin, 95% CI 0.83–0.91, P<0.0001]. The prognostic value did not differ by gender.
Conclusions
Women were more likely to be anaemic and/or hypoalbuminaemic pre-HAART, but post-HAART increases were similar to those in men. Both haemoglobin and albumin were strong independent prognostic factors for risk of AIDS and death, regardless of gender.
Introduction
The management of patients with HIV-1 infection is dependent on the use of markers of disease progression such as the CD4 count and HIV-1 RNA concentration [1,2]. While these remain the main markers in clinical use, they do not fully explain an individual's prognosis [3]. In addition, their measurement requires sophisticated and often expensive laboratory equipment [4,5]. Additional markers of disease progression are therefore required, particularly in resource-poor settings.
Many alternative markers have been proposed, including total lymphocyte count, white blood cell count, packed cell volume (haematocrit) [5] and the level of expression of CD38 on CD8 cells [6]. However, few have been accepted into routine use largely because results linking these markers to disease progression are inconsistent or because the exact mechanism by which they are involved in disease progression is not fully understood [3]. Many investigators have studied the use of haemoglobin as an alternative marker of disease progression [4,5,7–17]. Anaemia is frequently experienced by HIV-positive individuals and has been shown to be a strong independent predictor of disease progression [8,9,14,15] and death [12,18]. Highly active antiretroviral therapy (HAART) is associated with an improvement in anaemia [9–11], but little work has been carried out on the short- and longer-term prognostic value of haemoglobin for progression to AIDS and death amongst those receiving HAART [18].
Serum albumin has also been proposed as an alternative prognostic marker, as hypoalbuminaemia is associated with mortality in many other acute and chronic conditions [19,20]. Feldman and colleagues [21] reported that low serum albumin concentrations were associated with increased mortality over 3 years. These findings were confirmed by Sabin and colleagues [3], who showed that low serum albumin was independently associated with an increased risk of AIDS and death over a longer time period (15–20 years) in individuals with haemophilia coinfected with hepatitis C virus.
Amongst HIV-1-negative individuals, women are known to have lower haemoglobin levels and a higher prevalence of anaemia than men [22]. As the shift in the HIV-1 epidemic has resulted in a large increase in the number of women infected, this may have implications for the incidence of anaemia in those infected with HIV as well as for the use of haemoglobin and albumin as short- and long-term prognostic markers of disease progression and death.
The aim of our study was therefore to examine gender differences in haemoglobin and albumin levels before and after commencing HAART and to investigate the use of anaemia and hypoalbuminaemia as short-term (over a 3-month period) and longer-term (up to 7 years) markers of HIV-1 disease progression and death.
Methods
Patient population
All individuals included in the study were patients at the Royal Free Hospital, London. Demographic, clinical and laboratory information on these patients is collected prospectively and stored on a computer database as patients attend for care. The information is audited every 6–9 months by a trained research assistant who updates and cross-checks the information in the notes with that on the database. The Royal Free Cohort has been described previously [23].
For the purpose of this study, HAART is defined as any regime containing a nonnucleoside reverse transcriptase inhibitor (NNRTI) or a protease inhibitor (PI). Normal haemoglobin was defined as haemoglobin >13.5 g/dL for men and >11.5 g/dL for women, while anaemia was defined as values below these cut-offs. Albumin levels between 35 and 50 g/L were defined as normal, and values below 35 g/L as hypoalbuminaemic in both men and women. Although the reference range for albumin is the same for men and women, it is worth noting that albumin levels are slightly lower in women before the menopause compared with men of the same age [24].
All patients included in the analyses were naïve to antiretroviral therapy at the time of starting HAART. Trends in haemoglobin and albumin levels were plotted over the first 2 years after initiating HAART. However, we chose to focus on changes in haemoglobin and albumin at 1 year after commencing HAART, so formal statistical comparisons were made between levels pre-HAART (the last value in the period between 6 weeks before and 7 days after starting HAART) and 1 year post-HAART (the first value in the period between 1 year post-HAART and 1 year and 6 weeks post-HAART). For these analyses, we therefore only included patients with haemoglobin and albumin values within both of these time intervals. However, for the Cox proportional hazards model, patients were only required to have a pre-HAART haemoglobin and albumin value, thereby increasing the sample size.
Laboratory methods
CD4 cell counts are measured using standard flow cytometry techniques and plasma HIV-1 RNA is measured using a variety of commercially available kits. When first introduced in 1996, viral load monitoring was performed using the Amplicor polymerase chain reaction HIV-1 Monitor Test 1.0 (Roche Products Ltd, Welwyn Garden City, UK) which was later upgraded to the 1.5 version with add-in non-B primers. Currently the Roche ultrasensitive assay is used to measure HIV-1 RNA.
Statistical methods
Basic demographic characteristics of the study population were obtained and comparisons made between men and women using unpaired t-tests, χ2 tests and Wilcoxon rank sum tests, as appropriate.
In order to provide an illustration of haemoglobin and albumin changes in those starting HAART, the average haemoglobin and albumin values were calculated for each 3-month period for the first 2 years after starting HAART in both men and women. All haemoglobin and albumin values measured in each 3-month period were used to calculate these averages. Differences in the mean changes of each marker at 6 months and 2 years between men and women were tested for significance using the unpaired t-test.
We then concentrated on the pre-HAART and 1-year post-HAART time-points. The mean haemoglobin and albumin levels as well as the proportion of patients with anaemia and hypoalbuminaemia at both time-points were calculated for men and women and unpaired t-tests were used to make comparisons of the values between the two genders, and paired t-tests to test whether values had changed significantly over time in men and women.
A number of regression analyses were performed. First, separate univariable and multivariable regression analyses were carried out on the haemoglobin and albumin values at the start of HAART and 1-year post-HAART to investigate whether gender, ethnicity, risk group, CD4 count, HIV-1 RNA load, time since diagnosis, calendar year of HAART, age at start of HAART and previous AIDS diagnosis were associated with haemoglobin and albumin levels at each time-point. Spearman's correlation was also used to study the association between haemoglobin and albumin with the above factors at both time-points. Survival analysis was then used to determine the long- and short-term prognostic value of haemoglobin and albumin levels by considering them as fixed variables (i.e. the baseline measurement, providing information on long-term prognosis) and time-updated variables (i.e. the most recent measurement, providing information on prognosis over the average time between measurements, i.e. every 3 months or so), respectively, adjusting for all the above factors. Follow-up for each patient began on the date of starting HAART and stopped when a new AIDS event was diagnosed, when the patient died or at the date of the patient's last clinic visit, whichever happened first; median follow up after HAART was 2.6 years (1.6–3.8 years, interquartile range [IQR]) for women and 2.7 years (1.5–4.1 years, interquartile range [IQR]) for men. Cox proportional hazards models were used to investigate the prognostic values of haemoglobin and albumin separately for predicting the time to AIDS/death. Finally, we determined the prognostic values of haemoglobin and albumin separately in women and men and then added an interaction term to the model to allow comparisons of the prognostic value of each marker between men and women. All statistical tests were performed using SAS version 8.0 (SAS Institute Inc., Cary, NC).
Results
Of the 3156 patients in the database, 950 were antiretroviral naïve and commenced a HAART regime containing a PI or NNRTI between 1996 and 2003. Whilst haemoglobin and albumin are now measured routinely with other liver function tests every 3 months, they have not always been measured as frequently in the past. Thus, of these 950 patients, 495 (52%) had pre-HAART haemoglobin and albumin values and only 291 (31%) had both pre-HAART and 1-year post-HAART haemoglobin and albumin values. All comparisons of haemoglobin and albumin over the year were performed on the 291 patients.
The baseline characteristics of the 291 patients with both pre-HAART and 1-year post-HAART haemoglobin and albumin values did not differ from those of patients without these values (n=950–291=659). Most of the patients in both groups were men (75 and 78%, respectively) of whom the majority were homosexual and of white ethnicity.
Two hundred and seventeen (75%) of the 291 patients were male; of these, 167 (77%) were of white ethnicity and 161 (74%) were homosexual. Fifty-two (70%) of the 74 women were of black African ethnicity and 68 (92%) were heterosexual. The mean age of the women pre-HAART was 34.1 years [standard deviation (SD) 8.8 years], while the average age in men was 4 years older (mean 38.3 years, SD 7.9 years, P<0.0001). Women had significantly lower CD4 counts both pre-HAART and 1 year after commencing HAART compared with men [pre-HAART: median (interquartile range (IQR)) 127 (38–225) vs 197 (70–298) cells/μL, P=0.003; 1 year post-HAART: 293 (222–439) vs 379 (242–581) cells/μL, P=0.007]. The median viral loads at both time-points were, however, similar in women and men [pre-HAART: 5.4 (IQR 4.8–5.7) vs 5.3 (4.9–5.8) copies/mL, respectively, P=0.42; 1 year post-HAART: 1.7 (1.7–2.6) vs 1.7 (1.7–1.7) copies/mL, respectively; P=0.11]. The women also appeared to commence HAART significantly earlier than men [0.13 (0.06–0.62) vs 0.61 (0.01–4.23) years from diagnosis, P=0.005].
The prognostic values of haemoglobin and albumin as markers of HIV disease progression and death were assessed using Cox's regression analysis. For this analysis, the above restrictive inclusion criteria were relaxed so that patients were not required to have 1-year post-HAART values. This resulted in the addition of a further 204 patients, bringing the total of patients included in this analysis to 495. There were no differences in the baseline characteristics of these additional 204 patients compared with the 291 patients included in the earlier analysis. One hundred and forty-nine (73%) of the 204 patients were men, most of whom were homosexual (116, 78%) and of white ethnicity (117, 79%). Once again, the median CD4 counts pre-HAART were lower in the women than in the men [118 (IQR 38–271) vs 192 (91–364) cells/μL], but the median viral loads were similar in women and men. These values were comparable to those for the patients with haemoglobin and albumin levels at both time-points.
Both haemoglobin and albumin levels rose after the commencement of HAART in men and women (Fig. 1). For both variables, the rise was greatest in the first 6 months post-HAART, when levels then tended to flatten out. The mean changes in haemoglobin and albumin levels at 6 months and 2 years did not differ significantly by gender (6 months: haemoglobin, P=0.61; albumin, P=0.89; 2 years: haemoglobin, P=0.17; albumin, P=0.95).
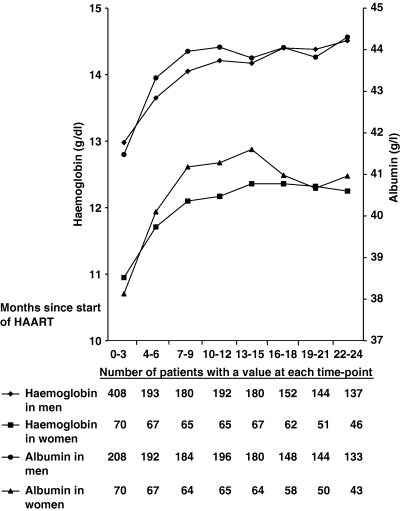
Mean haemoglobin and albumin values at 3-monthly intervals from the time of highly active antiretroviral therapy (HAART) initiation stratified by gender.
Haemoglobin and albumin levels were significantly higher in men compared with women both pre-HAART and 1 year post-HAART (Table 1). There was a significant increase in the haemoglobin level by approximately 1 g/dL in both men and women at 12 months post-HAART (P<0.0001 in each case). Albumin levels also rose significantly over the first year on HAART in both men [mean (SD) increase: 3.4 (5.0) g/L, P<0.0001] and women [3.8 (4.8) g/L, P<0.0001]. Once again, the size of the haemoglobin and albumin rises over the year did not differ significantly by gender (P=0.98 and 0.51, respectively).
| Men | Women | P-value(comparisonbetween menand women) | |
|---|---|---|---|
| Haemoglobin pre-HAART | 13.2 (1.8) | 11.2 (1.6) | <0.0001 |
| (g/dL) [mean (SD)] | |||
| Haemoglobin 1 year post-HAART | 14.2 (1.6) | 12.2 (1.3) | <0.0001 |
| (g/dL) [mean (SD)] | |||
| Haemoglobin difference over 1 year | 0.9 (1.7) | 0.9 (1.3) | |
| (g/dL) [mean (SD)] | |||
| P-value (comparison over time) | <0.0001 | <0.0001 | 0.98 |
| Anaemia pre-HAART [% (n)] | 41.5 (90) | 55.4 (41) | 0.04 |
| Anaemia 1 year post-HAART [% (n)] | 18.0 (39) | 24.3 (18) | 0.24 |
| P-value (comparison over time) | <0.0001 | <0.0001 | |
| Albumin pre-HAART | 40.18 (5.1) | 37.35 (5.0) | <0.0001 |
| (g/L) [mean (SD)] | |||
| Albumin 1 year post-HAART | 43.86 (3.2) | 41.14 (3.9) | <0.0001 |
| (g/L) [mean (SD)] | |||
| Albumin difference over 1 year | 3.4 (5.0) | 3.8 (4.8) | |
| (g/L) [mean (SD)] | |||
| P-value (comparison over time) | <0.0001 | <0.0001 | 0.51 |
| Hypoalbuminaemia pre-HAART [% (n)] | 11.4 (24) | 30.4 (21) | <0.0001 |
| Hypoalbuminaemia 1 year post-HAART [% (n)] | 0.9 (2) | 6.9 (5) | 0.003 |
| P-value (comparison over time) | 0.0001 | 0.0001 | |
- P-values were calculated using paired and unpaired t-tests, as appropriate, and χ2 tests for percentages.
- SD, standard deviation.
A higher proportion of women were anaemic compared with men at each time-point. After 1 year on HAART, however, there was a dramatic decrease in the percentage of men and women with anaemia (Table 1). A higher proportion of women were hypoalbuminaemic both pre-HAART and 12 months later compared with men. One year post-HAART, however, only 6.9% of women and 0.9% of men remained hypoalbuminaemic.
The haemoglobin and albumin levels at both time-points were positively correlated with the CD4 count [Spearman's correlation coefficient (r)=0.45, P<0.0001 and r=0.20, P=0.0007 for pre- and 1-year post-HAART haemoglobin, respectively, and r=0.35, P<0.0001 and r=0.20, P=0.008 for pre- and 1-year post-HAART albumin, respectively]. Time since the patient's first positive HIV diagnosis was also positively correlated with haemoglobin at both time-points but not with albumin; this correlation was weaker than that with the CD4 count. Viral load was negatively and weakly correlated with albumin at both time-points (r=−0.19, P=0.001 and r=−0.12, P=0.05 for pre- and 1-year post-HAART albumin), but no such relationship was seen between viral load and haemoglobin. Further, haemoglobin and albumin were positively correlated with each other at both time-points (r=0.59 pre-HAART, P<0.0001 and r=0.34 1 year post-HAART, P<0.0001). Interestingly, there was no correlation between the change in CD4 count and the change in either haemoglobin or albumin over the year (r=0.10, P=0.11 and r=0.09, P=0.17, respectively). Change in viral load was negatively correlated with change in albumin over the year, but not with change in haemoglobin (r=−0.27, P<0.0001 and r=−0.06, P=0.36, respectively).
In a multivariable linear regression analysis, gender remained independently associated with both pre-HAART haemoglobin and pre-HAART albumin after adjusting for age, CD4 count and viral load at the start of HAART (Table 2). Gender also remained an independent predictor of haemoglobin and albumin levels at 12 months after starting HAART; at this point, women had haemoglobin levels that were 2.08 g/dL lower [95% confidence interval (CI)−2.65, −1.51, P<0.0001] and albumin levels that were 2.88 g/L lower (95% CI−4.07, −1.70, P<0.0001) than men (Table 2). CD4 count and viral load were no longer significantly associated with haemoglobin and albumin levels 1 year post-HAART in the multivariable analysis.
| Haemoglobin | Albumin | |||||
|---|---|---|---|---|---|---|
| Mean change | 95% CI | P-value | Mean change | 95% CI | P-value | |
| Pre-HAART* | ||||||
| Female sex | −1.65 | −2.25, −1.05 | <0.0001 | −2.86 | −4.43, −1.28 | <0.0004 |
| CD4 count | 0.21 | 0.10, 0.32 | <0.0002 | 0.07 | −0.22, 0.37 | 0.62 |
| (per 100 cells/μL higher) | ||||||
| HIV RNA | 0.10 | −0.17, 0.37 | 0.46 | −0.99 | −1.70, −0.27 | 0.007 |
| (per log10 copies/mL higher) | ||||||
| Time since diagnosis | 0.08 | 0.02, 0.15 | 0.01 | 0.06 | −0.12, 0.23 | 0.52 |
| (per additional year) | ||||||
| Age at start of HAART | −0.02 | −0.04, 0.004 | 0.11 | −0.14 | −0.20, −0.08 | <0.0001 |
| (per 10 years older) | ||||||
| Previous AIDS diagnosis | −0.98 | −1.44, 0.53 | <0.0001 | −3.47 | −4.67, −2.28 | <0.0001 |
| Calendar year of starting HAART | −0.003 | −0.25, 0.24 | 0.98 | −1.50 | −2.15, −0.85 | <0.0001 |
| (per additional year) | ||||||
| One-year post-HAART† | ||||||
| Female sex | −2.08 | −2.65, −1.51 | <0.0001 | −2.88 | −4.07, −1.70 | <0.0001 |
| Age at 1-year post-HAART | −0.22 | −0.04, 0.00 | 0.05 | −0.14 | −0.18, −0.09 | <0.0001 |
| (per 10 years older) | ||||||
- * Also adjusted for ethnicity and risk group, which were not significantly associated with either haemoglobin or albumin.
- † † Also adjusted for ethnicity, risk group, time since diagnosis, calendar year of HAART and previous AIDS diagnosis, which were not significantly associated with either haemoglobin or albumin.
- CI, confidence interval.
Unadjusted for other factors, higher pre-HAART haemoglobin and albumin levels were associated with a significantly lower risk of AIDS and death over the subsequent few years (up to 7 years for some patients) [haemoglobin: hazard ratio (HR)=0.76 per g/dL increase, P<0.0001; albumin: HR=0.91 per g/L increase, P=< 0.0001] (Table 3). After adjusting for all other factors, the relationship with haemoglobin became nonsignificant, but that with albumin remained significant. As time-updated covariates, the most recent haemoglobin and albumin levels were both significantly related to the risk of AIDS and death independently of each other and of time-updated CD4 count or previous AIDS diagnosis (haemoglobin: HR=0.73 per g/dL increase, P<0.0001; albumin: HR=0.87 per g/L increase, P<0.0001). After adjusting for haemoglobin, albumin and CD4 count as either fixed or time-updated covariates, gender was not significantly associated with the risk of AIDS and/or death. Haemoglobin levels were significantly associated with the risk of AIDS and death in men but not in women in the time-updated model, adjusted for all other factors (men: HR=0.55 per g/dL increase, 95% CI 0.46, 0.65; women: HR=0.74 per g/dL increase, 95% CI 0.53, 1.03). Albumin levels were also significantly associated with prognosis in women and men separately (men: HR=0.81 per g/L increase, 95% CI 0.77, 0.86; women: HR=0.89 per g/L increase, 95% CI 0.83, 0.95). When the interaction term was applied to compare differences between women and men, there was no gender difference in the prognostic value of either haemoglobin or albumin (P=0.30 and 0.47, respectively).
| Unadjusted analysis | Adjusted analysis* | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Values at the start of HAART | ||||||
| (fixed covariates) | ||||||
| Albumin | 0.91 | 0.88–0.94 | <0.0001 | 0.91 | 0.86–0.97 | 0.003 |
| (per g/L higher) | ||||||
| Haemoglobin | 0.76 | 0.68–0.86 | <0.0001 | 0.95 | 0.78–1.15 | 0.59 |
| (per g/dL higher) | ||||||
| CD4 count | 0.65 | 0.52–0.81 | <0.0001 | 0.66 | 0.50–0.85 | 0.002 |
| (per 100 cells/μL higher) | ||||||
| Updated values over follow up | ||||||
| (time-updated covariates) | ||||||
| Albumin | 0.83 | 0.80–0.86 | <0.0001 | 0.87 | 0.83–0.91 | <0.0001 |
| (per g/L higher) | ||||||
| Haemoglobin | 0.59 | 0.52–0.67 | <0.0001 | 0.73 | 0.55–0.82 | <0.0001 |
| (per g/dL higher) | ||||||
| CD4 count | 0.58 | 0.49–0.68 | <0.0001 | 0.66 | 0.55–0.82 | <0.0001 |
| (per 100 cells/μL higher) | ||||||
- * Adjusted for variables shown plus gender, ethnicity, risk group, viral load, time since diagnosis, calendar year of HAART, age at HAART and previous AIDS diagnosis.
- CI, confidence interval; HAART, highly active antiretroviral therapy; HR, hazard ratio.
Discussion
Our results suggest that haemoglobin and albumin levels are lower in women both before and 1 year after starting HAART. HAART is associated with similar increases in haemoglobin and albumin in both men and women, and with a decrease in the proportion of patients with anaemia and hypoalbuminaemia. Although the absolute differences in haemoglobin seen between the genders may be expected as a result of levels being lower in women even in HIV-negative populations, a higher proportion of women were anaemic at both time-points. Haemoglobin and albumin levels were also strongly associated with HIV-1 disease progression and death in the short term. Over the longer term, however, haemoglobin levels were a weaker predictor of outcome than either the albumin level or the CD4 count. These findings were independent of gender. Thus, once the most recent levels are known, little additional information is gained by knowing the pre-HAART levels of these markers.
A number of studies have previously considered the relationship between anaemia and HAART, and have shown an improvement in anaemia after initiation of HAART [9–11]. Others have investigated haemoglobin and albumin as markers of survival in HIV-infected women. In particular, Berhane and colleagues [18] have recently reported an independent association between anaemia and decreased survival in HIV-1-infected women enrolled in the Women's Interagency HIV study (HR=2.58; P<0.001). The endpoint used in this study was all-cause mortality and the relation between anaemia and survival was assessed for the first 2 years of HAART use, as only 18.7% of women were on HAART for 18 months or longer. In our study, we considered progression to AIDS as well as death as an endpoint and showed that, whilst haemoglobin was not significantly associated with prognosis over the longer term (up to 7 years for some patients) after controlling for the CD4 count, both haemoglobin and albumin provided independent prognostic information over the short term. Thus, knowledge of both markers may be helpful when assessing patient prognosis. Other studies [4,12,13] have shown an association between anaemia and HIV-1 disease progression and death, but the effect of gender on those receiving HAART was not determined in these studies. In patients already on HAART, haemoglobin has been shown to be a short-term marker of disease progression independently of the CD4 count and HIV-1 RNA concentration [14].
Low levels of albumin have also been reported to be associated with shorter survival in HIV-1-infected women in the Women's Interagency HIV Study [21], but once again in this study the only endpoint investigated was all-cause mortality and the follow up was restricted to 3 years. The same group went on to study serial albumin measurements prior to HAART initiation and concluded that albumin was a strong independent predictor of mortality, particularly in the short term [25]. The association between albumin and progression to AIDS was not investigated in this study as the number of AIDS events was small. In a study investigating albumin as a marker of HIV disease progression in individuals with haemophilia, Sabin and colleagues [3] found that albumin was not associated with progression to AIDS in the short term, but was associated with death. However, in their study, albumin was associated with progression to AIDS and death in the long term. In the current study, we combined progression to AIDS and death as a single outcome as these endpoints are related [26], and this may account for the discrepancy between our results and those of the above study.
The pathogenesis of HIV-associated anaemia and hypoalbuminaemia is unclear and is likely to be multifactorial. Poor nutritional status can account for reduced levels of both haemoglobin and albumin. However, this is unlikely to account for the long-term association between albumin and HIV-1 disease progression and death seen in our study. The concentrations of both albumin and haemoglobin may also be regulated by cytokines, which may be affected by HIV-1 infection [3]. As albumin concentrations are a marker of the synthetic ability of the liver, coinfection with hepatitis, tumours of the liver or antiretroviral drugs that alter the synthetic ability of the liver may account for the low levels of albumin associated with HIV-1 disease progression. Although all our patients were on HAART, the antiretrovirals were not considered individually, so patients on some antiretrovirals, in particular nevirapine, may be more likely to have hepatic dysfunction [27]. Women of child-bearing age were more likely to be started on nevirapine because of the teratogenic effects associated with efavirenz [28]. This may partly explain the higher proportion of women with hypoalbuminaemia 1 year after starting HAART. However, these gender differences were not seen when albumin was considered as a prognostic marker. Other causes of HIV-related anaemia may include haemolytic anaemia, changes in erythropoietin synthesis, bone marrow suppression and bleeding [12]. Heavy menstrual bleeding is commonly seen in HIV-infected women as many of these women are of black African ethnicity in whom uterine fibroids are more frequently seen. This may explain the higher proportion of anaemia found amongst women compared with men, but does not appear to have any effect on the prognostic value of haemoglobin in women.
Our study has several limitations. Firstly, the study population used for the comparison of haemoglobin and albumin over the year in men and women was quite small as a result of our rigid entry criteria. This was necessary as patients routinely have blood tests, which include haemoglobin and albumin, at 3-monthly intervals and, if a wider window of entry criteria was selected, other haemoglobin and albumin results (i.e. not the last pre-HAART or first 1-year post-HAART value) may also have been included. In addition, our strict entry criteria would also have eliminated patients who were lost to follow-up or died before reaching the 1-year time-point or who did not have the potential for 1-year follow-up (this included 22.8% of all those with a pre-HAART haemoglobin and albumin result). We tried to overcome this in the Cox model by relaxing the restrictive criteria, which allowed the inclusion of patients without values both pre-HAART and 1 year post-HAART. Secondly, it is the patients with most advanced HIV infection and the lowest CD4 counts who would be commencing antiretroviral treatment. As haemoglobin and albumin levels are correlated with CD4 counts, these patients would be expected to also have the lowest concentrations of haemoglobin and albumin. Thus, the prevalence of anaemia and hypoalbuminaemia in our study may be higher than that among HIV-infected populations as a whole. It may therefore only be possible to generalize our results to HIV-infected patients about to commence HAART. Thirdly, we assessed the prognostic value of haemoglobin and albumin only in antiretroviral-naïve patients commencing HAART for the first time, and therefore it may not be possible to generalize our results to patients who were treatment experienced at the time of starting HAART. Finally, our study was insufficiently powered to investigate relationships between specific drugs or drug classes and haemoglobin and albumin rises. However, as they are independent prognostic markers and are raised by HAART, these are markers that should be measured as secondary endpoints in trials comparing specific drugs.
In summary, despite the lower haemoglobin and albumin levels in women at the start of HAART, haemoglobin and albumin levels were found to rise at similar rates in men and women after they commenced HAART. Haemoglobin and albumin levels are strong prognostic markers of HIV disease progression and death in the short term, but a weaker predictor of outcome in the longer term. The prognostic value of these markers was independent of gender. Therefore, haemoglobin and albumin levels may be useful alternative markers of disease progression, particularly in resource-poor settings.
Acknowledgements
Support for this study was obtained from an unrestricted grant from GlaxoSmithKline.




