Mitochondrial DNA assessment in adipocytes and peripheral blood mononuclear cells of HIV-infected patients with lipodystrophy according to a validated case definition
Abstract
Background
Several studies have compared mitochondrial DNA (mtDNA) content in tissue from HIV-1-infected patients on highly active antiretroviral therapy with and without evidence of lipodystrophy, the diagnosis of which was based on subjective clinical assessment.
Objectives
The aim of this study was to assess the utility of mtDNA quantification as a marker of HIV-associated lipodystrophy as diagnosed using a published validated case definition.
Methods
We assessed mtDNA content in adipocytes from both thigh and lumbar subcutaneous adipose tissue (n=19), and in peripheral blood mononuclear cells (PBMC) (n=26), obtained from 26 HIV-1-infected patients classified as having lipodystrophy (n=17) or not having lipodystrophy (n=9) according to the validated definition derived from the Lipodystrophy Case Definition Study.
Results
The adipocyte and PBMC mtDNA contents did not significantly differ between patients with and without lipodystrophy. Lipodystrophy patients had been treated for significantly longer times, especially with dideoxynucleoside analogues. In both groups, the thigh adipocyte mtDNA content was significantly greater than that of the lumbar region. When all patients were considered together, a statistically significant negative correlation was found between thigh adipocyte mtDNA content and stavudine treatment duration.
Conclusions
Longer exposure to dideoxynucleoside analogues was associated with lipodystrophy, and longer exposure to stavudine was correlated with lower mtDNA content in thigh adipocytes. However, a single measurement of adipocyte mtDNA content in this limited sample of patients could not distinguish between patients with and without clinical lipodystrophy. The observed variation in mtDNA content between different subcutaneous adipose tissue depots argues for harmonization of future studies regarding which depot to biopsy.
Introduction
The introduction of highly active antiretroviral therapy (HAART) has resulted in a decrease of mortality and disease progression rates in HIV-1-infected patients. HAART, however, is also associated with the development of several longer term adverse effects, including changes in body fat distribution, i.e. lipodystrophy [1]. Lipodystrophy in HIV-1-infected patients is characterized by loss of subcutaneous fat from the face, buttocks and extremities and accumulation of visceral fat. These physical changes can represent a major stigma for patients and thereby may adversely affect their quality of life and sustained adherence to therapy.
Both HIV protease inhibitors (PIs) and nucleoside reverse transcriptase inhibitors (NRTIs) have been implicated in the pathogenesis of lipodystrophy. Several studies indicate that longer term exposure to NRTIs plays a key role in the development of one particular aspect of lipodystrophy, namely the wasting of subcutaneous adipose tissue known as peripheral lipoatrophy [2–4].
Mitochondrial toxicity has been suggested as one of the possible mechanisms underlying lipoatrophy [5,6], as a result of inhibition by NRTIs of DNA polymerase gamma, the enzyme responsible for the replication of mitochondrial DNA (mtDNA). Previous studies have determined the capacity of specific NRTIs to inhibit the function of this polymerase, either by acting as a competitive inhibitor or by incorporation into, and termination of, the nascent mtDNA chain. This could eventually lead to defects in the respiratory chain located in the inner membrane of the mitochondria [7]. The individual effect of each NRTI and the differential susceptibility of the various tissues to NRTI-induced mitochondrial toxicity are thought to determine the resulting clinically recognizable pathology.
To date, several studies have reported on mtDNA content in tissue samples of patients with lipodystrophy or, more specifically, lipoatrophy. The results, however, have been inconsistent [8–18]. Whereas some studies reported a lower mtDNA content in adipose tissue from patients with lipodystrophy [9,17], others did not [13,18]. One possible explanation for these differences may be that the classification of patients as being lipodystrophic or not was based on subjective reports by physicians and/or patients.
We therefore performed a study in which we assessed mtDNA content in subcutaneous fat biopsies taken from two body regions, as well as in peripheral blood mononuclear cells (PBMC) obtained from HIV-1-infected patients, classified as having lipodystrophy or not according to the only available validated case definition [19].
Materials and methods
Patient characteristics
The patients enrolled in the current study were originally recruited for the Lipodystrophy Case Definition study (LDCD) [19], the aim of which was to develop a sensitive, specific, objective and broadly applicable case definition of HIV lipodystrophy.
All 26 LDCD participants enrolled at the Academic Medical Centre in Amsterdam, the Netherlands, were older than 17 years of age and without any clinical signs of AIDS and, at the time of enrolment into the LDCD study, individually consented to participate in the current add-on study. Patient characteristics such as age, gender, ethnic origin and history of HIV infection were recorded.
As part of the parent LDCD study, lipodystrophic features were recorded, both from self-reports by patients and clinical examinations by physicians according to a standardized procedure. Initially, patients were classified as being either a case or a control according to a Web-based lipodystrophy scoring system. The resulting groups each consisted of 13 patients. Case patients were classified as such by the presence of at least one lipodystrophic feature, such as moderate or severe fat loss or fat accumulation reported independently by both patient and physician. Importantly, to avoid classifying age-related isolated abdominal obesity as lipodystrophy, central adiposity was not considered as a defining parameter. Patients were assigned to the control group if they did not have lipodystrophy-related features according to both self-assessment and physician's examination. Both cases and controls had to undergo a series of assessments, including whole-body dual-energy X-ray absorptiometry and single-slice abdominal computerized tomography. Details of these procedures have been published [19]. The time at which adipose tissue biopsies and PBMC were obtained coincided with these assessments.
Using the same original assessment data obtained at the time of conducting the LDCD study and for the purpose of the work reported herein, all 26 patients were reclassified once the validated LDCD model had been published and had been made available for public use on the Internet (http://web.med.unsw.edu.au/nchecr/).
According to the final validated diagnostic LDCD model, and in contrast to the original classification, 17 of the 26 patients were reclassified as having lipodystrophy and nine as not having lipodystrophy. Two of the patients reclassified as nonlipodystrophic were naïve to antiretroviral therapy, whilst the remainder were treatment-experienced and had been treated with various HAART regimens at the time of assessment.
Whilst PBMC were obtained from all 26 patients, only 22 patients provided informed consent to have subcutaneous fat biopsies taken from both the lumbar region and the thigh (providing a total of 44 fat biopsies). Demographic, clinical and laboratory data were retrieved from the original LDCD study case report forms.
PBMC and adipose tissue samples
Subcutaneous fat biopsies were taken using a 4-mm punch biopsy needle, from the inner side of the right thigh and the lumbar region. Fat biopsies were immediately snap-frozen and subsequently stored at −80°C until mtDNA assays were performed.
Prior to performing the mtDNA assay, a single operator dissected the skin layer from the biopsy to avoid contamination by epidermal mtDNA. To avoid contamination by mtDNA from connective tissue, i.e. stromal-vascular cells containing the preadipocytes, a collagenase digestion procedure, followed by a differential centrifugation step adapted from Crisp et al. [20], was employed to isolate the adipocytes. The digested biopsy tissue was spun at 350 g for 5 min, leaving the adipocytes suspended in the supernatant, and the stromal-vascular cells deposited in the pellet. Total nucleic acid was then extracted from the adipocyte fraction using the Boom silica-based method [21]. The Retina™ Mitox™ assay (Primagen, Amsterdam, the Netherlands) was then used to amplify mtDNA and nuclear DNA simultaneously.
Viable cryopreserved PBMC samples were examined under a microscope and washed to avoid any significant platelet contamination prior to nucleic acid extraction [22,23]. Subsequent microscopic analysis confirmed less than 5 platelets being present per cell [24]. Total nucleic acid was isolated from an equivalent amount of 3 × 105 cells, yielding a corresponding amount of 3000 cells as input for the real-time duplex NASBA amplification (Retina™ Mitox™).
Both the adipose tissue samples and the PBMC were tested in duplicate. The mean mtDNA copy number of each duplicate measurement was determined by relating assay results to a calibration line included in each run, consisting of a dilution series of plasmid mixes with a dynamic range between 40 and 1600 copies of mtDNA per cell. Results are reported as number of mtDNA copies per cell.
Statistical analysis
Statistical analysis was performed using SPSS for Windows 11.0 SPSS Inc., Chicago, IL, USA. Results are expressed as median and interquartile range. mtDNA values were logarithmically (log10) transformed prior to analysis. Comparison of the lipodystrophy group and the nonlipodystrophy group was performed by means of the nonparametric Mann–Whitney U-test.
Comparison of mtDNA content between subcutaneous thigh and lumbar region adipocytes within the lipodystrophic group and the nonlipodystrophic group was performed by means of the nonparametric Wilcoxon signed-rank test.
An analysis was performed on the group as a whole to determine whether there was any correlation between the content of mtDNA in thigh and lumbar region adipocytes as well as in PBMC on the one hand, and total therapy, total NRTI and total stavudine duration, respectively, on the other hand by means of Spearman's rank correlation test.
Results were considered statistically significant at P<0.05. All reported P-values are two-sided.
Results
Baseline characteristics are reported in Table 1. Patient characteristics such as gender and CD4 cell count were comparable for the lipodystrophic and nonlipodystrophic groups, but patients with lipodystrophy were older (P=0.043).
| Variable | No LD(n=9) | LD(n=17) | P |
|---|---|---|---|
| Female gender | 3 | 3 | 0.366§ |
| Age (years) | 40.28 ± 12.21* | 47.05 ± 7.97 | 0.043‡ |
| Height (cm) | 172 (167–183)† | 173 (171–183) | 0.711‡ |
| Weight (kg) | 80 (63–86)† | 70 (67–74) | 0.517‡ |
| CD4 cell count (cells/μL) | 510 (320–680)† | 690 (415–805) | 0.388‡ |
| Nadir CD4 cell count (cells/μL) | 340 (35–515)† | 240 (150–335) | 0.535‡ |
| Plasma HIV-RNA load (log10 copies/mL) | 2.63 (2.31–4.71)† | 3.58 (2.26–4.47) | 0.713‡ |
| Detectable viral load (n) | 5/9 | 10/17 | 0.378§ |
| HIV disease stage (category C) (n) | 3/9 | 6/17 | 0.639 |
| Total duration of therapy (months) | 35.59 (13.49–46.18)† | 97.70 (58.96–102.88) | 0.000‡ |
| NRTI duration (months) | 32.66 (13.49–40.72)† | 94.61 (58.96–102.60) | 0.000‡ |
| NNRTI duration (months) | 0 (0–25.29)† | 0 (0–17.19) | 0.610‡ |
| PI duration (months) | 20.85 (0–32.22)† | 40.53 (9.95–50.88) | 0.121‡ |
| ddI plus d4T duration (months)# | 0† | 0 | 0.637‡ |
| D-drug duration (months) | 6.94 (0–35.85)† | 46.02 (34.98–77.29) | 0.004‡ |
| d4T duration (months) | 4.93 (0–27.88)† | 24.33 (0–44.50) | 0.416‡ |
- * Age is presented as mean ± standard deviation.
- † † Other data are presented as median and interquartile range (IQR).
- P-values:
- ‡ Mann–Whitney;
- § χ2.
- # Only one nonlipodystrophic and three lipodystrophic patients were on didanosine (ddI) plus stavudine (d4T), the median duration of which was 27.86 and 33.95 (IQR 14–41.1) months, respectively.
- D-drug, dideoxynucleoside analogues; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Patients with lipodystrophy had been treated for a significantly longer time overall (P<0.01), and were exposed to NRTIs for longer times (P<0.01), as well as to dideoxynucleoside analogues (zalcitabine, stavudine and didanosine) (P=0.004). No statistically significant difference was found for the use and duration of treatment with PI and nonnucleoside reverse transcriptase inhibitors (NNRTIs) (Table 1).
mtDNA values obtained from five of the 44 biopsies were not included in the analysis (three from patients with and two from patients without lipodystrophy) because of discordant results of the duplicate measurements.
The mtDNA content of adipocytes from either the lumbar or thigh region and that of PBMC were not statistically significantly different between the two groups (Table 2).
| Tissue | Log10 mtDNA copies/cell | P | |
|---|---|---|---|
| No LD | LD | ||
| Thigh | 2.16 (1.46–2.93) | 1.79 (1.53–2.04) | 0.335 |
| (n=6) | (n=13) | ||
| Lumbar region | 1.84 (0.91–2.14) | 1.30 (0.78–1.90) | 0.258 |
| (n=6) | (n=12) | ||
| PBMC | 2.66 (2.50–2.75) | 2.43 (2.15–2.71) | 0.100 |
| (n=9) | (n=17) | ||
- Data are presented as median and interquartile range (IQR). P-values are for the Mann–Whitney U-test.
- PBMC, peripheral blood mononuclear cells.
The mtDNA content of subcutaneous adipocytes from the thigh was significantly higher than the mtDNA content of the lumbar region adipocytes, in both patients with (P=0.041) and without (P=0.043) lipodystrophy (Fig. 1).
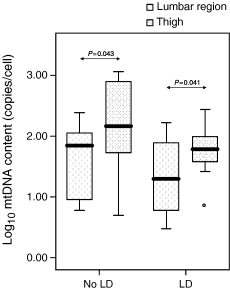
A box-whisker plot showing a comparison of log10 mitochondrial DNA (mtDNA) content (copies/cell) in thigh and lumbar region adipocytes in both lipodystrophic (LD) and nonlipodystrophic (No LD) patients. The plot illustrates the median (black line), the interquartile range (top and bottom of each box) and the reference interval containing 95% of the distribution.
A statistically significant negative correlation (r=−0.482; P=0.037) was found between cumulative duration of stavudine exposure and mtDNA content in thigh adipocytes, as shown in Fig. 2. A trend towards a negative correlation was also observed between cumulative exposures to any antiretroviral therapy (NRTIs in general as well as stavudine in particular) and mtDNA content in both lumbar adipocytes and PBMC, but this did not reach statistical significance (data not reported).
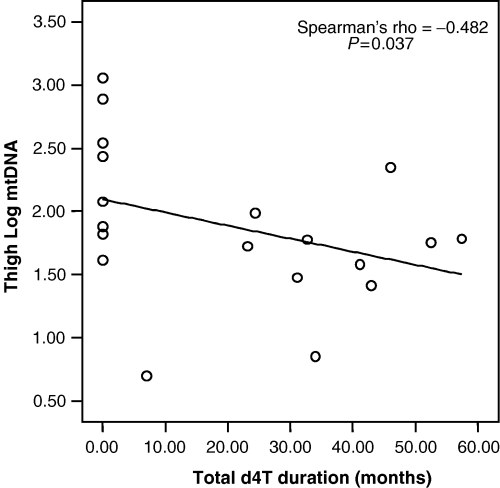
Correlation between log10 mitochondrial DNA (mtDNA) (copies/cell) in thigh adipocytes and stavudine (d4 T) therapy duration for the group as a whole.
Discussion
In this study, in which the presence or absence of lipodystrophy was defined by applying a published validated objective case definition, no statistically significant difference in the mtDNA content of subcutaneous adipocytes obtained from two separate body regions was found between patients with and without lipodystrophy. This result is consistent with those of previous cross-sectional studies [13,18], but differs from those of other studies which did find lower mtDNA content in adipose tissue from patients with lipodystrophy [9,17]. This is possibly a consequence of the limited sample set examined. However, it is likely that these discrepant results can, at least in part, be explained by the lack of a uniform method to define lipodystrophy across studies. Furthermore, the differences in methods of obtaining and handling adipose tissue among the studies might also play a role.
Earlier studies reported that mtDNA depletion occurred in PBMC of HIV-1-infected patients with long-term exposure to HAART, and to a possibly greater extent in PBMC of those with lipodystrophy [18,24]. We did not find a statistically significant difference in PBMC mtDNA content between patients with and without lipodystrophy. Of importance, the various studies throughout the literature of mtDNA in PBMCs report a high variability of mtDNA content [12,26,27]. This discrepancy is likely to be dependent on several factors, one of which may be the different degree to which the various assays account for platelet contamination. Further standardisation of methodology is required in order to allow a better comparison of future studies performed by different groups.
Consistent with other studies [3,13,18], patients with lipodystrophy had received antiretroviral therapy with NRTIs for longer times, in particular antiretroviral therapy with dideoxynucleoside analogues. Given that adipocyte mtDNA content was not significantly different between patients with and without lipodystrophy, data from all patients were pooled to identify any correlation between mtDNA content and duration of exposure to antiretroviral treatment in general, as well as to specific treatment components. In agreement with several reports [3,13,18], a statistically significant negative correlation was found between mtDNA content of thigh adipocytes and cumulative exposure to stavudine.
Interestingly, when we compared the mtDNA content of subcutaneous adipocytes from two body regions, the mtDNA content of adipocytes from the thigh was significantly higher than that of adipocytes from the lumbar region, both in lipodystrophic and in nonlipodystrophic patients (Fig. 1). This highlights the need for standardization of the adipose tissue depot to be sampled in order to allow improved interstudy comparisons.
In conclusion, our results are in agreement with previous studies, insofar as longer exposure to particularly toxic components of antiretroviral regimens is associated with lower mtDNA content in different tissues, including thigh subcutaneous adipocytes and PBMC. The lack of a difference observed in this cross-sectional analysis in adipocyte mtDNA content between those with and without objectively diagnosed lipodystrophy should be interpreted with caution, as it might be attributable to the limited number of patients we were able to include in the study. However, while keeping this limitation in mind, our results indicate that this marker cannot be used as a reliable single time-point measurement to assist in identifying patients with this particular adverse effect of HAART. This said, the finding does not argue against the role that mitochondrial toxicity is thought to play in the pathogenesis of lipodystrophy.
Acknowledgements
We thank the patients for their participation in this study. This project was conducted in collaboration between the Academic Medical Centre and Primagen BV. Financial support was obtained from a private foundation that wishes not to be named and the Dutch Ministry of Economic Affairs under the SENTER BTS programme (project BTS 00113).




