Peri-operative body temperatures in isoflurane-anaesthetized rabbits following ketamine–midazolam or ketamine–medetomidine
Abstract
Objective To investigate alterations in peri-operative body temperatures and oesophageal-skin temperatures in isoflurane-anaesthetized rabbits following either ketamine–midazolam or ketamine–medetomidine induction of anaesthesia.
Animal population Fifty client-owned rabbits, (25 male, 25 female) of different breeds anaesthetized for elective neutering (age range: 3–42 months; mass range: 1.15–4.3 kg).
Study design Randomized, blinded clinical study.
Methods Pre-anaesthetic rectal temperature was measured. A 24 SWG catheter was placed in a marginal ear vein after local anaesthesia. Ketamine (15 mg kg−1) with medetomidine (0.25 mg kg−1) (group KMT) or with midazolam (3 mg kg−1) (group KMZ) was injected intramuscularly (IM). Following endotracheal intubation anaesthesia was maintained with isoflurane in oxygen. Carprofen (3 mg kg−1) and glucose saline (5 mL kg−1 hour−1) were administered through the intravenous catheter. Room temperature and humidity, skin temperature (from tip of pinna) and oesophageal temperature were measured during anaesthesia. Ovariohysterectomy or castration was performed. Rectal temperature was taken when isoflurane was discontinued (time zero) and 30, 60 and 120 minutes thereafter. Atipamezole (0.5 mg kg−1) was administered IM to rabbits in group KMT at zero plus 30 minutes. Mass, averaged room temperature and duration of anaesthesia data were compared using a two-tailed t-test. Age, averaged room humidity, rectal temperature decrease, oesophageal temperature decrease and oesophageal–skin difference data were compared using a Kruskal–Wallis test. p < 0.05 was considered significant.
Results The averaged oesophageal–skin temperature difference was significantly greater in group KMT [median 9.85 °C (range 6.42–13.85 °C)] than in group KMZ [4.38 °C (2.83–10.43 °C)]. Rectal temperature decreased over the anaesthetic period was not significantly different between the two groups; however, oesophageal temperature decrease was significantly less in group KMT [1.1 °C (−0.1–+2.7 °C)] than in group KMZ [1.4 °C (0.6–3.1 °C)].
Conclusions Oesophageal–skin temperature difference is larger in rabbits anaesthetized with ketamine–medetomidine combination than ketamine–midazolam.
Clinical relevance The oesophageal temperature in rabbits anaesthetized with ketamine–medetomidine and isoflurane decreases significantly less than in animals anaesthetized with ketamine–midazolam and isoflurane, during anaesthesia.
Introduction
Rabbits are now the third most commonly anaesthetized pet (Brodbelt et al. 2005). However, their peri-anaesthetic mortality rate (1 in 72) is still higher than that of dogs (1 in 601) (Brodbelt et al. 2005).
Rabbits have a high surface area to volume ratio and a high metabolic rate, and therefore may be predisposed to developing hypothermia peri-operatively if appropriate means to actively warm them or prevent loss of body heat are not undertaken. Hypothermia may contribute to a prolonged recovery from anaesthesia and may have cardiovascular and respiratory effects (Murison 2001). Hypothermia after fentanyl/fluanisone anaesthesia has been investigated, comparing rabbits’ body temperatures after postoperative buprenorphine/saline (Capner et al. 1999). Rectal temperature decrease during anaesthesia with four different protocols (pentobarbitone, ketamine/xylazine, fentanyl/fluanisone/diazepam and halothane anaesthesia) was found to be similar (Peeters et al. 1988). Very little has been published, however, on core-peripheral temperature differences in rabbits anaesthetized with varying anaesthetic protocols.
Ketamine-based combinations appear to be increasingly popular in rabbit anaesthesia as indicated by the number of recent publications (Dupras et al. 2001; Hedenqvist & Roughan 2001; Hedenqvist et al. 2002; Avsaroglu et al. 2003; Henke et al. 2005; Orr et al. 2005). This may be due to the relatively long recovery from anaesthesia, seen in rabbits anaesthetized with midazolam/fentanyl/fluanisone (Hedenqvist et al. 2002).
Medetomidine is an α2-adrenergic agonist. Both α1- and α2-adrenergic receptors are present in the rabbit ear microvasculature and participate in the control of its blood flow (Li et al. 1998). One aim of this study was to observe the effects of the two anaesthetic protocols on the oesophageal–skin temperature differences in these rabbits, as medetomidine may cause peripheral vasoconstriction.
We also aimed at investigating whether there was any difference in peri-operative body temperatures in rabbits undergoing neutering anaesthetized by ketamine–midazolam compared with ketamine–medetomidine with anaesthesia maintained with isoflurane.
Materials and methods
Between November 2003 and October 2004, 50 rabbits of both sexes presented for elective neutering were included in the study. Rabbits aged less than 3 months or American Society of Anaesthesiologists (ASA) grade of greater than 2 were excluded from the study. ASA grading was assigned after clinical examination including measurement of rectal temperature with a digital thermometer. During the peri-operative period, water, commercial rabbit pellets and hay was available ad libitum.
Animals were assigned to one of two groups by block randomization: ketamine–midazolam (group KMZ) or ketamine–medetomidine (group KMT). The same anaesthetist (who was unaware of treatment groups) performed all anaesthetics (excluding injection of the induction agents), temperature measurements and subjective scoring.
A strip of hair approximately 1 cm wide was clipped over the marginal ear vein, on one ear on each rabbit and local anaesthetic cream (EMLA; AstraZeneca, Luton, UK) applied. The ear was then covered with an occlusive dressing (OpSite Flexigrid; Smith & Nephew, Hull, UK). Forty minutes later, a 24 SWG intravenous catheter (Abbocath; Abbot, Sligo, Eire) was placed in an auricular vein and secured in place. For rabbits in group KMZ, ketamine (Narketan-10; Vetoquinol, Bicester, UK) 15 mg kg−1 and midazolam (Hypnovel; Roche, Welwyn Garden City, UK) 3 mg kg−1 were administered by intramuscular (IM) injection. Ketamine 15 mg kg−1 and medetomidine (Domitor; Pfizer, Sandwich, Kent, UK) 0.25 mg kg−1 were administered IM to rabbits in group KMT. The two drugs in each case were mixed in the same syringe, and the volume was divided and injected into two sites: the left and right lumbar epaxial muscles.
Ten minutes after IM injection, endotracheal intubation was attempted using a blind technique with the rabbits in sternal recumbency. If endotracheal intubation were not successful, anaesthesia was maintained via a tight fitting mask.
Contingency for laryngospasm was application of a mask to the rabbit's face and application of intermittent positive pressure ventilation with 100% oxygen. If the rabbits were in group KMT, atipamezole (0.25 mg kg−1 IV and 0.25 mg kg−1 IM) was administered.
Anaesthesia was maintained with isoflurane (Isoflo; Abbot, Queensborough, Kent, UK) in 100% oxygen delivered with a Jackson Reese modified Ayre's T-piece. The fresh gas flow was set at 750 mL kg−1 minute−1. The same isoflurane vaporizer (Isotec 4; Datex Ohmeda, Chalfont St Giles, UK) was used throughout the study, and its calibration was checked with a Datascope Passport 2 monitor with a gas module 2 (at 20 °C) at the beginning, mid-part and end of the study period. The percentage vaporizer setting was recorded every 5 minutes. Carprofen (Rimadyl injection; Pfizer) 3 mg kg−1 and 0.18% saline with 4% glucose at room temperature (VetIvex 18; Ivex Pharmaceutical, Larne, UK) 5 mL kg−1 hour−1 were administered IV.
A temperature probe (Adult esophageal/ rectal temperature probe 401; YSI Precision temperature probes, Dayton, OH, USA) was inserted into the oesophagus to the level of the elbow, to measure body temperature in °C. A skin probe (400 series skin probe; YSI Precision temperature probes) was secured onto the unclipped skin of the noncatheterized pinna (sited at the middle of the ear width and two-thirds along the ear length) to measure skin temperature in °C. The probe was secured to the external surface of the ear using microporous tape (Micropore; 3M Health Care, Neuss, Germany). The temperature probes were connected to a monitor (Minimon 7136B; Kontron Instrument Ltd, Milton Keynes, UK) to display data. Calibration of these devices was checked against a thermocouple of known accuracy. A room thermometer and hygrometer, which was always situated in the same place in theatre (Pen-type Thermo hygrometer; Radiospares, Corby, Northamptonshire, UK) measured room temperature and humidity. Oesophageal temperature, skin temperature, room temperature and humidity were recorded every 5 minutes.
No insulating or heating techniques were used during anaesthesia, except the surgical drape and the operating lights consisting of three 50 W bulbs (Osram halogen photo optic lamp, Munich, Germany) approximately 80 cm from the rabbit. If the oesophageal temperature decreased to 36 °C, a heated mat under the rabbit was switched on.
Ovariohysterectomy or castration (both via a midline abdominal approach) were then performed by a final year veterinary student under the direct and constant supervision of a staff surgeon. The duration of anaesthesia was recorded [considered time from endotracheal intubation to discontinuation of volatile agent at the end of surgery (time = zero)]. The length of incision was measured and area of clipped skin was calculated after measurement of the width and length of the clip. Postoperative rectal temperature was measured by using a digital thermometer.
The rabbit was placed in sternal recumbency and observed during its recovery from anaesthesia. Tracheal extubation was performed when the rabbit started swallowing or making gross purposeful movements. A layer of bedding (Vet Bed; Jacksons, Shipley, UK) was placed underneath and on top of the animal during recovery.
At zero plus 30, 60 and 120 minutes, a mark was placed on a visual analogue scale (VAS) for postoperative activity of the rabbit. The scale was 10 cm in length and anchored with unrousable and normal pre-anaesthetic alertness. Rectal temperatures were measured at these time points with a digital thermometer. Rabbits in group KMT received atipamezole (Antisedan; Pfizer) 0.5 mg kg−1 IM at zero plus 30 minutes.
Statistics
A sample size calculation [two group t-test of equal means (nQuery Advisor)] using postoperative rectal temperature at time zero data from the first 10 rabbits indicated that a sample size of 25 in each group would have an 80% power to detect a 0.75 °C difference in means of the postoperative temperature between the two groups.
Mean and standard deviation values were calculated for each group at each time point for vaporizer setting, oesophageal and skin temperatures. Average room temperature(°C), room humidity (%) and vaporizer setting (%) over time were calculated. Rectal temperature decrease (°C) was calculated by subtracting postoperative rectal temperature from preoperative rectal temperature. Oesophageal temperature decrease (°C) was calculated by subtracting the first and last oesophageal temperature measurements for each rabbit. The oesophageal–skin difference (°C) was calculated by subtracting skin values from oesophageal temperatures, with the resultant figures averaged over time.
Data for age, weight, pre-anaesthetic rectal temperature, duration of anaesthesia, area of clipped skin, length of incision, averaged room humidity and temperature were compared between groups to verify the randomization process.
Frequency histograms for all data series were plotted to check for normal distribution. Normally distributed data (mass, averaged room temperature, averaged vaporizer setting, duration of anaesthesia, postoperative rectal temperature, area of clipped skin and length of incision) were compared using a two-tailed t-test (Minitab 14, Minitab Inc., State College, PA, USA). Results are presented as mean (standard deviation). Data which were not normally distributed (age, pre-anaesthetic rectal temperature, averaged room humidity, rectal temperature decrease, oesophageal temperature decrease and oesophageal–skin difference) were compared using a Kruskal–Wallis test (Minitab 14). Results are presented as median (total range). Pearson correlations were performed to investigate whether there were correlations between rectal temperature decrease and duration of anaesthesia, length of incision or area of clip. Statistical significance was taken at p < 0.05 for all tests.
The VAS for postoperative activity (1, 2) and rectal temperatures (3, 4) in recovery from anaesthesia were plotted against time.
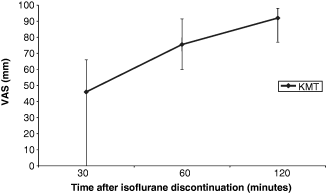
Median visual analogue scale (VAS) score for postoperative activity over time in group KMT. Error bars denote total range.
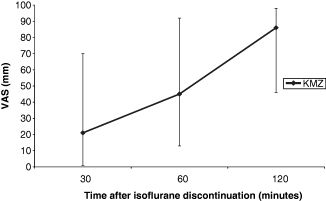
Median visual analogue scale (VAS) score for postoperative activity over time in Group KMZ. Error bars denote total range.
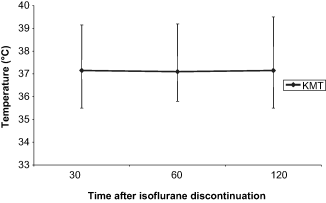
Change in median rectal temperature during recovery from anaesthesia in group KMT. Error bars denote total range.
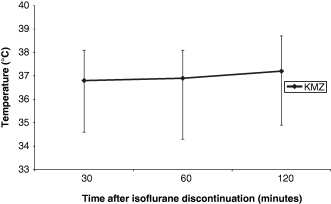
Change in median rectal temperature during recovery from anaesthesia in group KMZ. Error bars denote total range.
Results
The details of the rabbits in the two groups were similar, as was the distribution between ASA 1 and 2 (see Table 1). Pre-anaesthetic rectal temperatures were not significantly different between groups (KMZ median = 38.6 °C, KMT median = 38.6 °C, p = 0.831).
| KMT | KMZ | p | |
|---|---|---|---|
| Age, months [median (range)] | 10 (4–42) | 8 (3–32) | 0.402 |
| Mass, kg [mean (SD)] | 2.23 (0.568) | 2.26 (0.661) | 0.864 |
| ASA 1:ASA 2 | 10:15 | 11:14 | |
| Male:female | 13:12 | 12:13 |
Temperature data were collected from 46 rabbits. Four rabbits in groups KMT were withdrawn from the study because of laryngospasm at induction of anaesthesia. Anaesthesia of three animals in group KMZ was maintained via face mask.
The oesophageal temperature in one rabbit in the ketamine–midazolam group decreased below 36 °C at 35 minutes after endotracheal intubation. The heated mat was utilized from this time point; however, at the end of anaesthesia (60 minutes), the temperature had decreased further to 35.1 °C. The results from this rabbit were included in the statistical analysis.
There was no significant difference between groups with regard to area of clip, length of incision, duration of anaesthesia, averaged room temperature or averaged room humidity (see Table 2). However, averaged vaporizer setting (%) was significantly (p < 0.001) higher in the midazolam group [2.06 (0.555)] compared with the medetomidine group [1.22 (0.768)]. Mean and standard deviation values for vaporizer settings at all time points are presented in Table 3.
| KMT | KMZ | p | |
|---|---|---|---|
| Room temperature, °C [mean (SD)] | 24.6 (1.96) | 24.4 (1.79) | 0.789 |
| Room humidity, % [median (range)] | 44.8 (37.6–73.8) | 47.1 (29.9–73.5) | 0.798 |
| Duration of anaesthesia (minutes) [mean SD] | 55.3 (14.9) | 55.3 (12.4) | 0.999 |
| Area of clip, cm [mean (SD)] | 119.5 (49.0) | 121.1 (39.3) | 0.900 |
| Length of incision, cm [mean (SD)] | 3.3 (0.871) | 3.4 (1.12) | 0.691 |
| Time after endotracheal intubation (minutes) | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KMT – volatile agent % mean (SD) | 0.30 (0.55) | 0.48 (0.66) | 0.80 (0.91) | 1.09 (1.08) | 1.43 (1.40) | 1.39 (1.14) | 1.37 (1.03) | 1.68 (1.21) | 1.91 (0.97) | 1.73 (0.98) | 1.60 (0.74) | 1.93 (0.73) | 1.7 (0.74) | 1.69 (0.63) | 1.5 |
| Rabbits (n) | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 19 | 17 | 15 | 11 | 7 | 5 | 2 | 1 |
| KMZ – volatile agent % mean (SD) | 1.88 (0.74) | 1.85 (0.98) | 1.98 (0.77) | 2.06 (0.88) | 2.19 (0.75) | 2.22 (0.81) | 1.92 (0.76) | 2.04 (0.69) | 2.02 (0.70) | 1.94 (0.61) | 1.79 (0.63) | 1.75 (0.83) | 1.96 (0.59) | 1.7 (0.45) | 0.5 (0.87) |
| Rabbits (n) | 25 | 25 | 25 | 25 | 25 | 25 | 24 | 22 | 21 | 17 | 15 | 11 | 5 | 4 | 3 |
The averaged oesophageal–skin temperature difference was significantly greater in rabbits that had received medetomidine than those in the midazolam group (see Table 4). Postoperative rectal temperature and rectal temperature decrease were not significantly different between the two groups. However, oesophageal temperature decrease was significantly less in the medetomidine group than in the midazolam group (see Table 4). Mean and standard deviation values for oesophageal temperatures and skin temperature for each time point are presented in Tables 5 & 6 respectively.
| KMT | KMZ | p | |
|---|---|---|---|
| Pre-operative rectal temperature, °C [median (range)] | 38.60 (37.6–40.3) | 38.60 (37.6–39.7) | 0.831 |
| Postoperative rectal temperature, °C [mean (SD)] | 38.45 (0.75) | 37.60 (0.97) | 0.569 |
| Oesophageal–skin temperature difference, °C [median (range)] | 9.85 (6.42–13.85) | 4.38 (2.83–10.43) | <0.001* |
| Rectal temperature decrease, °C [median (range)] | 0.75 (−1 to +3.6) | 1.50 (−0.4 to +3.47) | 0.117 |
| Oesophageal temperature decrease, °C [median (range)] | 1.10 (−0.1 to +2.7) | 1.40 (+0.6 to +3.1) | 0.035* |
- *Significant.
| Time after endotracheal intubation (minutes) | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KMT – oesophageal temperature, °C [mean (SD)] | 39.4 (0.56) | 39.3 (0.53) | 39.2 (0.47) | 39.0 (0.50) | 38.9 (0.51) | 38.7 (0.50) | 38.6 (0.52) | 38.5 (0.59) | 38.4 (0.68) | 38.4 (0.74) | 38.2 (0.76) | 38.3 (0.72) | 38.1 (0.71) | 38.7 (1.13) | 37.6 |
| Rabbits (n) | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 19 | 17 | 15 | 13 | 7 | 5 | 2 | 1 |
| KMZ – oesophageal temperature, °C [mean (SD)] | 39.1 (0.49) | 38.9 (0.52) | 38.7 (0.56) | 38.5 (0.61) | 38.3 (0.69) | 38.1 (0.69) | 38.0 (0.74) | 37.9 (0.79) | 37.8 (0.82) | 37.7 (0.90) | 37.5 (0.94) | 37.3 (1.03) | 37.2 (0.68) | 37.0 (0.68) | 37.2 (0.75) |
| Rabbits (n) | 25 | 25 | 25 | 25 | 25 | 25 | 24 | 22 | 21 | 17 | 15 | 11 | 5 | 4 | 3 |
| Time after endotracheal intubation (minutes) | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 | 45 | 50 | 55 | 60 | 65 | 70 | 75 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KMT – skin temperature, °C [mean (SD)] | 29.8 (2.04) | 29.4 (2.64) | 28.8 (2.00) | 28.7 (2.07) | 29.1 (3.09) | 28.9 (2.19) | 29.0 (2.11) | 29.0 (2.05) | 29.3 (2.19) | 30.0 (2.28) | 29.1 (2.14) | 28.7 (2.25) | 29.0 (2.63) | 28.6 (3.61) | 29.3 |
| Rabbits (n) | 21 | 21 | 21 | 21 | 21 | 21 | 21 | 19 | 17 | 15 | 13 | 7 | 5 | 2 | 1 |
| KMZ – skin temperature, °C [mean (SD)] | 33.2 (2.28) | 33.4 (2.71) | 33.1 (2.88) | 33.0 (2.81) | 33.2 (2.15) | 33.5 (1.60) | 33.4 (1.78) | 33.3 (1.95) | 33.3 (2.21) | 33.7 (1.84) | 33.5 (1.87) | 33.5 (1.79) | 33.5 (1.69) | 33.6 (1.99) | 33.3 (1.95) |
| Rabbits (n) | 25 | 25 | 25 | 25 | 25 | 25 | 24 | 22 | 21 | 17 | 15 | 11 | 5 | 4 | 3 |
There was a significant correlation (p = 0.007, r = 0.390) between the fall in rectal temperature and duration of anaesthesia. Rectal temperature decrease was not correlated significantly with length of incision (p = 0.802, r = 0.038) or area of clip (p = 0.584, r = 0.082).
1, 2 show a general trend for higher activity scores in the rabbits recovering from ketamine/medetomidine/isoflurane anaesthesia, compared with ketamine/midazolam/isoflurane anaesthesia. The range of VAS scores is also noticeably narrower after administration of atipamezole in group KMT rabbits at zero plus 30 minutes. 3, 4 demonstrate that there is little change in postoperative rectal temperatures in rabbits from both groups during the initial recovery period.
Discussion
Rectal temperature has been investigated as a variable in studies comparing different anaesthetic protocols in rabbits (Peeters et al. 1988; Hellebrekers et al. 1997; Capner et al. 1999) but this does not represent the core temperature of an animal. Core temperature is the value of the deep body sites or hypothalamus, and can be performed using techniques such as oesophageal probes (Chen & White 2006). In this study, the oesophageal temperature decrease in rabbits with medetomidine included in the anaesthesia (group KMT) was significantly less than rabbits anaesthetized with midazolam combinations (group KMZ).
Core to periphery temperature difference, represented in this study by the oesophageal–skin temperature difference may provide information about the blood flow to the periphery, circulation and vasoconstriction (Murison 2001). Vasomotor tone is one of the major factors influencing core temperature in humans in the peri-anaesthetic period who are neither actively cooled nor warmed (Kurz et al. 1996). In awake patients, thermoregulatory vasoconstriction is able to protect core temperature by reducing cutaneous heat transfer and thus functionally isolating the peripheral and core thermal compartments (Kurz et al. 1996). One of the aims of this study was to observe the effect of two anaesthetic protocols of the oesophageal–skin temperature difference in rabbits. Rabbits that had received medetomidine had a significantly greater oesophageal–skin temperature difference than rabbits that had received midazolam.
Body temperature may decrease in small animals after administration of potent sedatives such as α2-adrenoreceptor agonists because of central nervous system depression and a reduction in muscular activity. Conversely, it may be better maintained due to the peripheral vasoconstriction which occurs after administration of α2-adrenoreceptor agonists, and central redistribution of blood, with a consequent reduction in cutaneous heat losses (Sinclair 2003). It has been shown that in dogs anaesthetized with isoflurane, the administration of medetomidine produces a significant increase in systemic vascular resistance (Keegan et al. 1995) because of vasoconstriction of the peripheral vasculature. Dexmedetomidine causes initial peripheral vasoconstriction in rabbits followed by long-lasting peripheral vasodilation (Xu et al. 1998).
Hellebrekers et al. (1997) suggested that the overall peripheral vasculature of rabbits has a limited sensitivity to the peripheral vasoconstrictive effects of α2-adrenergic agonists. The α-adrenoreceptors in the rabbit ear have been studied (Li et al. 1998); both α-1 and α-2 adrenoceptors contribute to the regulation of overall ear microcirculation, although α-1 influences the basal tone of the microvasculature more than α-2 adrenoreceptors. Arteriovenous anastamoses (AVAs) are more sensitive (compared with arterioles) to the constrictive effects of dexmedetomidine. Constriction of AVAs may prevent the shunting of large volumes of blood from the arterial to the venous circulation (which may be attempted to maintain the core temperature) (Abramson 1972). Medetomidine was the α2-agonist used in this study, compared with dexmedetomidine studied by Li et al. (1998) and Xu et al. (1998). The selectivity of the active isomer (dexmedetomidine) is greater for α-2 receptors than for α-1 when compared with the racemic mixture (medetomidine) (Aantaa et al. 1989) and therefore the response of the microcirculation in the rabbit ear to each drug may not be comparable.
In this study, the oesophageal–skin temperature differences were significantly greater in the medetomidine group. Factors other than medetomidine may affect vasomotor tone and influence the difference between groups. Rabbits in the medetomidine group required less isoflurane to maintain surgical anaesthesia. Isoflurane has peripheral vasodilatory effects and therefore the lower dose used may have caused less vasodilation than in the midazolam group. In addition, midazolam impairs tonic thermoregulatory vasoconstriction in human beings, allowing core to peripheral heat redistribution (Matsukawa et al. 1997).
Other factors which may potentially influence peri-anaesthetic and peri-operative heat loss include room temperature and humidity, surgical clip area, length of incision and duration of anaesthesia. Rabbits presented for both castration and ovariohysterectomy were included in this study as both procedures were undertaken using an abdominal approach. There was no significant difference between duration of anaesthesia, incision lengths and clipped areas between the two groups. Ambient temperature and humidity were also similar. Although no warming devices were used during anaesthesia, the rectal temperatures of several rabbits (in both groups) were higher at the end of surgery than pre-anaesthesia. This may have been due to the fact that the pre-anaesthetic temperatures were recorded whilst the rabbits were conscious, and movement of the rabbit at this time may have yielded a false reading. The heat radiating from the surgical lights, and the insulation from the surgical drape, may have also been sufficient to increase the rabbits’ rectal temperature during anaesthesia, although the drape and intensity of surgical lighting were maintained throughout the study period.
The rectal temperature of rabbits at the beginning of the recovery period was higher in the animals anaesthetized with the medetomidine combination, but during the 2 hours after isoflurane discontinuation, they increased very little in either group. Rabbits to which intravenous buprenorphine after fentanyl/fluanisone anaesthesia had been administered had significantly lower rectal temperatures during recovery from anaesthesia when compared with a saline control group (Capner et al. 1999). The authors postulated that this may have been due to a reduced activity in the recovery period. VAS scores for postoperative activity were greater at all time points in the medetomidine group than in the midazolam group, and this activity may have aided maintenance of body temperature.
In conclusion, this study demonstrated that the oesophageal–skin temperature difference is greater in rabbits anaesthetized with ketamine–medetomidine/isoflurane than ketamine–midazolam/isoflurane. As a result, the oesophageal temperatures of rabbits decrease less over the anaesthetic period when anaesthetized with ketamine/medetomidine and isoflurane compared with ketamine/midazolam and isoflurane.
Acknowledgements
The authors would like to acknowledge the surgeons, anaesthetists and students who assisted in this study. They would like to thank Ms Rachel Burt, Dr Louisa Slingsby and Mr Colin Capner for randomization and allocation of the drugs, and Mrs Rachel Garroway for her administration of the neutering clinic. They would also like to thank Dr Toby Knowles and Dr Gina Pinchbeck for assistance with statistical analysis.




