Advances in molecular systematics of the vetigastropod superfamily Trochoidea
Abstract
Williams, S.T. (2012). Advances in molecular systematics of the vetigastropod superfamily Trochoidea. —Zoologica Scripta, 41, 571–595.
The gastropod superfamily Trochoidea Rafinesque, 1815 is comprised of a diverse range of species, including large and charismatic species of commercial value as well as many small or enigmatic taxa that are only recently being represented in molecular studies. This study includes the first sequences for rarely collected species from the genera Gaza Watson, 1879, Callogaza Dall, 1881, Antimargarita Powell, 1951 and Kaiparathina Laws, 1941. There is also greater taxon sampling of genera that have proved difficult to place in previous phylogenetic analyses, like Tectus Montfort, 1810, Tegula Lesson, 1832, Margarites Gray, 1847, Margarella Thiele, 1893 and trochoid skeneimorphs. There is also greater sampling of poorly represented families Solariellidae and Liotiidae. Bayesian analysis of combined gene data sets based on four (28S, 12S, 16S and COI) or five genes (plus 18S) suggests that there are eight, possibly nine families in Trochoidea including the families Margaritidae and Tegulidae, which are recognized for the first time at familial rank. Other trochoidean families confirmed are Calliostomatidae, Liotiidae, Skeneidae, Solariellidae, Trochidae and Turbinidae. A clade including Cittarium and the commercially important genera Rochia and Tectus may represent a possible ninth family, but this is not formally recognized or described here and awaits confirmation from further studies. Relationships among families were not generally well supported except in the 5-gene tree. In the 5-gene tree, Turbinidae, Liotiidae, Tegulidae, Cittarium, Rochia and Tectus form a well-supported clade consistent with the previous molecular and morphological studies linking these groups. This clade forms another well-supported clade with Margaritidae and Solariellidae. Trochidae is sister to Calliostomatidae with strong support. Subfamilial relationships within Trochidae are consistent with recent molecular studies, with the addition of one new subfamily, Kaiparathininae Marshall 1993 (previously a tribe). Only two subfamilies are recognized within Turbinidae, both with calcareous opercula: Prisogasterinae and Turbininae. Calliostomatidae includes a new subfamily Margarellinae. Its assignment to Calliostomatidae, although well supported by molecular evidence, is surprising considering morphological evidence.
Introduction
Recent estimates suggest there are approximately 3700 species in the Vetigastropoda, making Trochoidea Rafinesque, 1815, with more than 2000 species, by far the largest and most diverse of the vetigastropod superfamilies (Geiger et al. 2008). Members of this superfamily can be found in all oceans of the world, at all latitudes, from the high intertidal to the ocean abyss. Trochoideans have been the focus of many ecological, morphological, molecular and systematic studies over the last century, but one of the most important recent systematic studies was the monograph of Hickman & McLean (1990). These authors used detailed morphological data from anatomy, radulae and shells to develop a concept of Trochoidea that included three families (Turbinidae Rafinesque, 1815, Trochidae Rafinesque, 1815 and Skeneidae Clark, 1851). The first two they divided into subfamilies with some subfamilies further subdivided into tribes. Historically, Turbinidae have been distinguished from Trochidae and Skeneidae by the presence of a calcareous operculum, trochids and skeneids having a corneous operculum. However, Hickman & McLean (1990) separated Turbinidae from Trochidae on the basis of radular features (rachidian tooth with secondary cusp or attachment flap) and the presence of a long growing edge on the operculum, and from Skeneidae on the basis of shell size and pigmentation. Skeneidae is distinguished from Trochidae primarily by its small size and lack of nacre.
Since then, there have been several recent molecular studies focusing on systematics of Trochoidea or in some cases on Vetigastropoda, but including some trochoids (e.g. Geiger & Thacker 2005; Williams & Ozawa 2006; Heßet al. 2008; Kano 2008; Kunze et al. 2008; Williams et al. 2008, 2010; Kano et al. 2009; Aktipis & Giribet 2010, 2012; Donald et al. 2012). As a result of these new studies, there have been a number of changes to the taxon composition and arrangement of families of Trochoidea sensuHickman & McLean (1990) (see Table 1). In particular, several families have been removed from Trochoidea.
| Trochiform groups discussed in Hickman & McLean (1990) | New classification | Comment | References for change |
|---|---|---|---|
| Turbinidae Rafinesque. 1815 | |||
| Angariinae Thiele, 1921 | Angarioidea; Angariidae | Change to superfamily and status | Williams & Ozawa (2006); Williams et al. (2008) |
| Colloniinae Cossmann, 1916 | Phasianelloidea; Colloniidae; Colloniinae | Change to superfamily and status | Williams & Ozawa (2006); Williams et al. (2008) |
| Gabrieloninae Hickman & McLean 1990 | Phasianelloidea; Phasianellidae; Gabrieloninae | Change to superfamily and family | Bouchet et al. (2005); Williams & Ozawa (2006); Williams et al. (2008); |
| Liotiinae Adams and Adams, 1854 | Trochoidea; Liotiidae Cinysca and Arene are sister to Angariidae in a clade with familial status. Family awaits formal description. | Polyphyletic. Change to status (some taxa), change to superfamily (some taxa) and taxon composition | Williams & Ozawa (2006); Williams et al. (2008); this study |
| Moelleriinae Hickman & McLean 1990 | Phasianelloidea; Colloniidae; Moelleriinae | Change to superfamily and family | McLean & Kiel (2007); Williams et al. (2008) |
| Prisogasterinae Hickman & McLean 1990 | Trochoidea; Turbinidae; Prisogasterinae | No change | – |
| Turbininae Rafinesque. 1815 | Trochoidea; Turbinidae; Turbininae | No change | – |
| Tricoliinae Woodring, 1928 | Phasianelloidea; Phasianellidae; Tricoliinae | Change to superfamily and family | Bouchet et al. (2005); Williams & Ozawa (2006); Williams et al. (2008) |
| Phasianellinae Swainson, 1840 | Phasianelloidea; Phasianellidae; Phasianellinae | Change to superfamily and family | Bouchet et al. (2005); Williams & Ozawa (2006); Williams et al. (2008) |
| Trochidae Rafinesque. 1815 | |||
| Calliostomatinae Thiele, 1924 | Trochoidea; Calliostomatidae | Change to status | Marshall (1995); Williams et al. (2008) |
| Cataeginae McLean and Quinn, 1987 | Seguenzioidea; Cataegidae | Change to superfamily and status | Bouchet et al. (2005); Kano (2008) |
| Eucyclinae; Eucyclini Koken, 1897 | Seguenzioidea; Chilodontidae Seguenzioidea; Calliotropidae Seguenzioidea; Eucyclidae | Polyphyletic. Change to superfamily, status (some species), family (some species) and taxon composition | Kano (2008); Kano et al. 2009 |
| Eucyclinae; Chilodontini Wenz, 1938 | Seguenzioidea; Chilodontidae | Change to superfamily, status and taxon composition | Bouchet et al. (2005); Kano (2008); Kano et al. (2009) |
| Eucyclinae; Calliotropini Hickman & McLean 1990 | Seguenzioidea; Calliotropidae | Change to superfamily, status and taxon composition | Bouchet et al. (2005); Kano (2008); Kano et al. (2009) |
| Halistylinae Keen, 1958 | Trochoidea; Trochidae; Halistylinae | Not tested molecularly | – |
| Lirulariinae Hickman & McLean 1990 | Trochoidea; Trochidae; Umboniinae | Invalid | Williams et al. (2010) |
| Margaritinae Stoliczka, 1868 | Trochoidea; Margaritidae | Change to status | This study |
| Margaritinae; Gazini Hickman & McLean 1990 | Trochoidea; Margaritidae | Invalid | This study |
| Margaritinae; Margaritini Stoliczka, 1868 | Trochoidea; Margaritidae | Invalid | This study |
| Trochinae; Cantharidini Cotton, 1959 | Trochoidea; Trochidae; Cantharidinae | Change to status and taxon composition | Williams et al. (2008, 2010) |
| Trochinae; Gibbulini Stoliczka, 1868 | Trochoidea; Trochidae; Monodontinae | Change to name, status and taxon composition | Williams et al. (2008, 2010) |
| Trochinae; Trochini Rafinesque. 1815 | Trochoidea; Trochidae; Trochinae | Change to status and taxon composition | Williams et al. (2008, 2010) |
| Solariellinae Powell, 1951 | Trochoidea; Solariellidae | Change to status | Williams et al. (2008) |
| Stomatellinae Gray, 1840 | Trochoidea; Trochidae; Stomatellinae | No change | – |
| Tegulinae Kuroda, Habe and Oyama, 1971 | Trochoidea; Tegulidae | Change to status | Williams et al. (2008); This study |
| Thysanodontinae Marshall, 1988 | Trochoidea; Calliostomatidae; Thysanodontinae | Change to family | Marshall (1995); Williams et al. (2008) |
| Trochaclidinae Thiele, 1928 | Vetigastropoda; superfamily unassigned; Trochaclidae? | Not currently assigned to Trochoidea, but possibly related to Calliostomatidae? Family name uncertain | Bouchet et al. (2005); Gründel (2008) |
| Umboniinae Adams and Adams, 1854 | Trochoidea; Trochidae; Umboniinae | No change | – |
| Umboniinae; Bankiviini Hickman & McLean 1990 | Trochoidea; Trochidae; Umboniinae | Invalid. Not tested molecularly, but tribes not currently recognized | Williams et al. (2010) |
| Umboniinae; Monileini Hickman & McLean 1990 | Trochoidea; Trochidae; Umboniinae | Invalid. Talopiini Finlay, 1928 (=Monileini) is polyphyletic. | Williams et al. (2008) |
| Umboniinae; Umboniini Adams and Adams, 1854 | Trochoidea; Trochidae; Umboniinae | Invalid. Tribes not currently recognized | Williams et al. (2010) |
| Skeneidae Clark, 1851 | |||
| ‘North European group sensu Fretter and Graham, 1977’ (includes only Skenea) | Trochoidea; Skeneidae | Nominotypical genus of Skeneidae s.s. Skenea not yet included in any molecular tree | – |
| ‘Australo-Neozealandic deep-water group sensu Marshall 1988’ (includes Dillwynella, Leptogyra, Leptogyropsis, Xyloskenea, Bathyxylophila Hyalogyra Hyalogyrina Xyleptogyra) | Polyphyletic group | Dillwynella currently assigned to Skeneidae, but most other genera listed reassigned to other superfamilies (see text) | See references in text |
| ‘Parviturbo-Happlocochlias group’ | ? | Unranked group. Genera listed currently assigned to Skeneidae. Not tested molecularly | – |
| ‘Austral microliotiform group’ (includes Lodderena, Liotella, Calomphala) | ? | One COI sequence for Lodderena suggests that this genus does not belong in Skeneidae | This study. Kano (2008) has Lodderena clustering in Turbinidae |
| ‘Crosseola group’ (includes Crossea, Crosseola, Dolicrossea, Conjectura) | ? | Unranked group. Genera listed currently assigned to Skeneidae. Not tested molecularly | – |
| ‘Deep-water group’ (includes Lissospira, Ganesa) | ? | Unranked group. Genera listed currently assigned to Skeneidae. Not tested molecularly | – |
- COI, cytochrome oxidase subunit I.
Molecular phylogenetic studies have shown unambiguously that ‘trochiform’ gastropods belonging to the families Angariidae Thiele, 1921, Phasianellidae Swainson, 1840, Colloniidae Cossmann, 1917, Chilodontidae Wenz, 1938, Calliotropidae Hickman & McLean 1990; Cataegidae McLean and Quinn, 1987 and Eucyclidae Koken, 1897 are not trochoideans (Williams & Ozawa 2006; Kano 2008; Williams et al. 2008; Kano et al. 2009; Aktipis & Giribet 2012). Angariidae is reassigned to Angarioidea Thiele, 1921 (Williams et al. 2008), Phasianellidae and Colloniidae to Phasianelloidea Williams et al. 2008 (Williams et al. 2008) Chilodontidae, Calliotropidae, Eucyclidae and Cataegidae to Seguenzioidea Verrill, 1884 (Bouchet et al. 2005; Kano 2008). The families Solariellidae Powell, 1951 and Calliostomatidae Thiele, 1924 are retained with Trochoidea but are now accorded familial rank (Marshall 1995; Bouchet et al. 2005; Williams et al. 2008).
In total, six phylogenetic groups are currently recognized in the superfamily Seguenzioidea: Seguenziidae Verrill, 1884, Chilodontidae, Calliotropidae, Cataegidae, Spinicalliotropis Poppe, Tagaro and Dekker, 2006 and several ‘skeneimorph seguenzioids’ (Kano et al. 2009). All these taxa have at sometime been assigned to Trochoidea. The subfamily Eucyclinae sensuHickman & McLean (1990) is now divided into three groups at family level within Seguenzioidea: Eucyclidae (which has no extant species, Bouchet et al. 2005), Calliotropidae and Chilodontidae (Warén et al. 2003; Bouchet et al. 2005; Kano 2008; Williams et al. 2008; Kano et al. 2009). Molecular results are supported by radular and conchological similarities among some taxa (Kano 2008; Kano et al. 2009).
Phasianellids and colloniids both have a calcareous operculum, a character they share with Turbininae and Prisogasterinae Hickman & McLean 1990; and it was for this reason that many early authors included them in Turbinidae s.l. However, a calcified operculum or operculum-like device has evolved independently at least 13 times in gastropods (Vermeij & Williams 2007), and Williams & Ozawa (2006) showed that a calcareous operculum likely arose in at least two independent events within Vetigastropoda. The independent radiation of these taxa is supported by morphological differences in the structure of their opercula (Vovelle 1969; Hickman & McLean 1990). Although the operculum has not been studied in detail in colloniids, it differs from that of Turbininae in that the calcareous layer is much thinner. Vovelle (1969) commented in a published abstract that although the operculum of turbinine Bolma rugosa (Linnaeus, 1767) (as Astralium rugosum) is superficially ‘identical’ in structure and manner of formation to that of phasianellid Tricolia pullus (Linnaeus, 1758), closer examination showed that the operculum of the Bolma was more complex and differed in the presence of ‘a mucopolysaccharidic component, on the inner side of the hornlike operculum’.
Phasianellids also differ from Turbinidae in sperm morphology (Hodgson & Foster 1992; Hodgson 1995), shell microstructure (Böggild 1930; Hedegaard 1990, 1997), number of shell muscles (Haszprunar 1985, 1988; Robertson 1985), kidney structure (Haszprunar 1985) and the loss of nacre (Hedegaard 1997). Molecular evidence suggests that phasianellids are sister to colloniids (Williams & Ozawa 2006; Kano 2008; Williams et al. 2008; Aktipis & Giribet 2012), many of which also lack nacre. I follow McLean & Kiel (2007) in treating Moelleriinae Hickman & McLean 1990 as a subfamily of Colloniidae and as such, Moelleria Jeffreys, 1865 does not belong to Trochoidea (Williams et al. 2008), although this has not been tested from a molecular perspective.
The Angariidae have historically been treated as a distinct family within Trochoidea or placed with Trochidae s.l. However, Marshall (1979) suggested on the basis of shell and radular characters that the family was ‘an atypical, perhaps archaic’ member of the Turbinidae. Hickman & McLean (1990) agreed, treating Angariidae as a subfamily of Turbinidae. Molecular data on the other hand show that this family is sister to Cinysca and Arene. Together, these taxa are more closely allied to Phasianelloidea than Trochoidea, either as sister clades (Williams & Ozawa 2006; Aktipis et al. 2011) or as sister to most of the Vetigastropoda (excluding Pleurotomarioidea; Williams & Ozawa 2006; Williams et al. 2008).
Other systematic changes involve the reassignment of trochoidean taxa to new families. Some genera are no longer assigned to Trochidae and have been tentatively reassigned to Turbinidae, but their phylogenetic placement needs further testing. In particular, the removal of the commercially important genus Tectus Montfort, 1810 from Trochidae is particularly surprising given its conchological similarity to the nominotypical genus Trochus Linnaeus, 1758 (Williams et al. 2008). Subfamilies tentatively assigned to Turbinidae by Williams et al. (2008) include Tegulinae Kuroda, Habe and Oyama, 1971 (possibly including some Tectus species) and Margaritinae Stoliczka, 1868. ‘True’ skeneids were assigned to Turbinidae by Bouchet et al. (2005) based on similarities in the radulae (Hickman & McLean 1990; Warén 1992; Bouchet et al. 2005), and their decision was followed by Williams et al. (2008) after confirmation with molecular data (Kano 2008; Williams et al. 2008).
Molecular support for monophyly of Trochoidea sensuWilliams et al. (2008) is robust in phylogenetic studies with large taxon sampling (four gene tree, Williams & Ozawa 2006; three gene tree, Williams et al. 2008). Even in morphological studies (Sasaki 1998) and studies with fewer taxa, the clade is regularly recovered, albeit usually with lower support (e.g. three gene tree, Kano 2008; five gene tree, Aktipis et al. 2011). In Aktipis & Giribet (2010, 2012), Trochoidea was not recovered as monophyletic because the trochid Pseudostomatella erythrocoma clusters with Colloniidae, however, as noted by the authors (in the 2012 paper), some sequences for this trochid are likely contaminated. Ignoring this species, Trochoidea was monophyletic in a five-gene tree in Aktipis & Giribet (2012), but not in five- or seven-gene trees in Aktipis & Giribet (2010), which have fewer trochoid taxa. This suggests that taxon sampling is as important, if not more so, as the number of loci for resolving relationships at the familial level and below.
Trochoideans have been distinguished from other vetigastropods by the following characters: (i) shells lacking a slit or hole, (ii) undeveloped right gill, (iii) epipodium differentiated into inhalant and exhalant neck lobes, (iv) sensory papillae on cephalic and epipodial tentacles, (v) lateromarginal radular plates and (vi) shafts of marginal radular teeth with a curved profile forming a food-collecting groove (Haszprunar, 1987Hickman & McLean 1990; Warén 1992; Warén & Bouchet, 1993; Künz & Haszprunar 2001; Geiger et al. 2008). None of these characters is unique to the clade as it is currently defined, in part because the current concept of Trochoidea sensuWilliams et al. (2008) has seen the removal of taxa from Trochoidea sensuHickman & McLean (1990) (and ongoing removal of skeneimorph taxa; e.g. Kano 2008; Kunze et al. 2008; Heßet al. 2008; Kano et al. 2009). However, despite the ongoing removal of taxa and rearrangement of taxa within the superfamily, these characters remain important, because no new families have been moved to Trochoidea since Hickman & McLean (1990).
In this paper, I produce a new molecular phylogeny for Trochoidea based on five genes including exemplars from all five families recognized by Williams et al. (2008) (Trochidae, Turbinidae, Liotiidae, Calliostomatidae and Solariellidae) with an especial focus on ‘problematic’ genera like Tectus, Tegula Lesson, 1832, Cittarium Philippi, 1847, Margarites Gray, 1847, Margarella Thiele, 1893 and including the first sequences for rarely collected species from related genera, Gaza Watson, 1879, Callogaza Dall, 1881, Antimargarita Powell, 1951 and Kaiparathina Laws, 1941. I also include new trochoid skeneimorph sequences and greater taxon sampling than in the previous studies for Solariellidae and Liotiidae.
Materials and methods
Samples
This study includes representatives from the five currently recognized families in Trochoidea (sensuWilliams et al. 2008) and one fissurellid and seven seguenzioid species as outgroup taxa. That fissurellids and seguenzioids are both distinct from trochoids has been confirmed by multiple molecular studies (Williams & Ozawa 2006; Kano 2008; Williams et al. 2008; Kano et al. 2009; Aktipis et al. 2011; Aktipis & Giribet 2012). Sequences include both new and the previously published data. A total of 211 new sequences were obtained from 63 specimens representing 55 species. Published sequences are primarily from Williams & Ozawa (2006), Williams (2007), Williams et al. (2008, 2010) and Williams et al. (2011) although a small number of published sequences from GenBank are also included (mostly from Hellberg 1998 and Kano 2008).
Photos of specimens used in Williams et al. (2010) are available on MorphoBank (http://www.morphobank.org/): Project: (P223) Molecular systematics of the marine gastropod families Trochidae and Calliostomatidae (Mollusca: Superfamily Trochoidea). Photos of selected new specimens used in this study (actual specimen sequenced, or in a few cases, a specimen from the same museum lot) are shown in 1, 2. Not all new samples are figured. Photos of seguenzioids are available from the author.
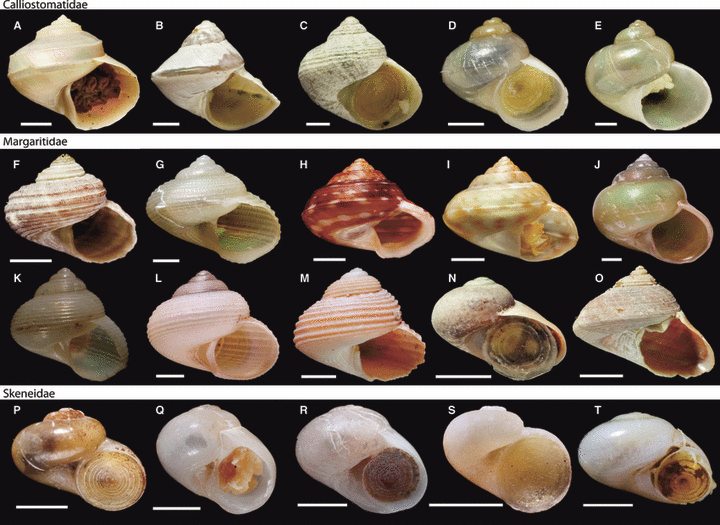
Photos of selected specimens, from which DNA has been extracted and new sequences are used in this study. Not all samples have been shown. —A–E. Calliostomatidae; —F–O. Margaritidae; —P–T. Skeneidae. —A. New genus and species MNHN 18543. Scale bar = 5 mm. —B. Margarella biconica NHMUK 20110457. Scale bar = 1 mm. —C. Margarella crebrilirulata NHMUK 20110458. Scale bar = 1 mm. —D. Margarella refulgens NHMUK 20110459. Scale bar = 1 mm. —E. Margarella sp. NHMUK 20110460. Scale bar = 1 mm. —F. Antimargarita dulcina NHMUK 20110462. Scale bar = 2 mm. —G. Antimargarita powelli NHMUK 20110463. Scale bar = 2 mm. —H. Callogaza sericata (Morph 1) MNHN 20098862. Scale bar = 3 mm. —I. Callogaza sericata (Morph 2) MNHN 200915165. Scale bar = 3 mm. —J. Gaza daedala MNHN 18293. Scale bar = 5 mm. —K. Gaza huloti SMHN 103190. Shell height 23 mm. —L. Gaza cf polychoronos MNHN 20098872. Scale bar = 3 mm. —M. Margarites groenlandicus NHMUK 20110447. Scale bar = 2 mm. —N. Margarites sp. 1 NHMUK 20110449. Scale bar = 2 mm. —O. Margarites vorticiferus NHMUK 20110451. Scale bar = 5 mm. —P. Cirsonella extrema NHMUK 20110464. Scale bar = 1 mm. —Q. Cirsonella sp. MNHN 18415. Scale bar = 1 mm. —R. Dillwynella vitrea MNHN 18550. Scale bar = 1 mm. —S. Protolira thorvaldssoni SMNH 82617. Scale bar = 1 mm. —T. Skeneid sp. MNHN 18385. Scale bar = 1 mm.
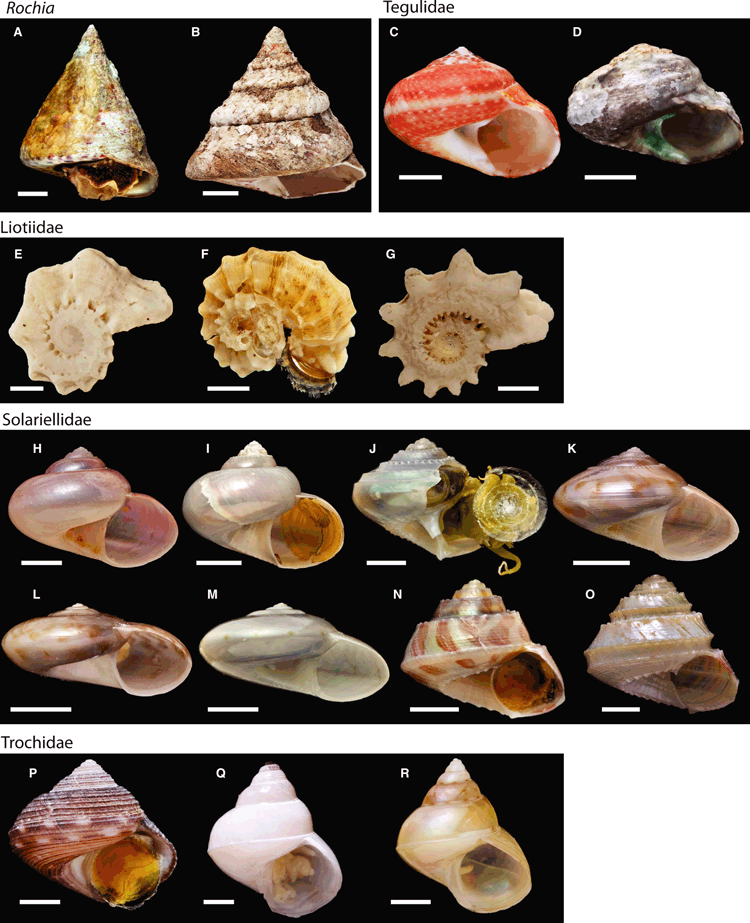
Photos of selected specimens, from which DNA has been extracted and new sequences are used in this study. Not all samples have been shown. —A–B. Rochia; —C–D. Tegulidae; —E–G. Liotiidae; —H–O. Solariellidae; —P–R Trochidae. —A. Rochia conus MNHN 18387. Scale bar = 10 mm. —B. Rochia maximus NHMUK 20050475. Scale bar = 10 mm. —C. Tegula fasciata NHMUK 20110453. Scale bar = 3 mm. —D. Tegula felipiensis NHMUK 20110454. Scale bar = 3 mm. —E. Liotina sp.1 – no voucher. Scale bar = 1 mm. —F. Liotina sp. 2 – no voucher. Scale bar = 1 mm. —G. Pseudoliotina springsteeni– no voucher. Scale bar = 2 mm. —H. Solariellid sp. 1 MNHN 20098861. Scale bar = 4 mm. —I. Solariellid sp. 2 MNHN 20098739. Scale bar = 4 mm. —J. Bathymophila sp. MNHN 18323. Scale bar = 2 mm. —K. Ilanga biradiatula MNHN 20098740. Scale bar = 4 mm. —L. Ilanga discus MNHN 20098761. Scale bar = 3 mm. —M. Ilanga sp. MNHN 18328. Scale bar = 3 mm. —N. Spectamen philippensis NHMUK 20110452. Scale bar = 2 mm. —O. Spectamen sp. MNHN 18288. Scale bar = 1 mm. —P. Pseudotalopia sakurai MNHN 18226. Scale bar = 3 mm. —Q. Kaiparathina boucheti MNHN 20098864. Scale bar = 1 mm. —R. Kaiparathina coriolis MNHN 18329. Scale bar = 2 mm.
DNA extraction, amplification and sequencing
The DNA extraction and amplification protocols described by Williams & Ozawa (2006) and Williams et al. (2010) were used to amplify the portions of four genes for all specimens: the nuclear 28S rRNA gene (28S: approximately 1500 bp) and three mitochondrial genes: cytochrome oxidase subunit I (COI: 709 bp), 16S rRNA (16S: approximately 610 bp) and 12S rRNA (12S: approximately 685 bp). A fifth gene, the slowly evolving 18S rRNA gene (18S: approximately 1000 bp), was also amplified for a subset of samples. Sequence reactions were performed directly on purified PCR products using a BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems) and run on an Applied Biosystems 3730 DNA Analyser automated capillary sequencer (Applied Biosystems, Foster City, California, USA). Sequencing and PCR primers are listed in Table S1.
Sequence analysis
Sequences were edited using Sequencher (v. 4.6 or 4.8; Gene Codes Corporation, Ann Arbor, MI, USA). All new sequences have been deposited in EMBL (accession numbers in the Appendix S1). Alignment of COI sequences was unambiguous and was made by eye in MacClade (v 4.08 OSX; Maddison & Maddison 2003). Alignment of ribosomal genes was more complicated, and these sequences were aligned using MAFFT (Multiple Alignment using Fast Fourier Transform); a high-speed multiple sequence alignment program (v 6.0; Katoh et al. 2002; online: http://mafft.cbrc.jp/alignment/server/). This program has been shown to produce better alignments than many more traditionally used programs (e.g. Katoh et al. 2002, 2005; Wilm et al. 2006). The G-INS-i option was used to align ribosomal gene sequences, as this is recommended for sequences of similar lengths with global homology (Katoh et al. 2005); the gap-opening penalty was set to 1.0, and the offset value was set at 0.1, as long gaps were not expected. Further minor modifications were made by eye in MacClade. Poorly aligned sites in mitochondrial rRNA alignments were identified using Gblocks Server (0.91b, Castresana 2000; http://molevol.cmima.csic.es/castresana/Gblocks_server.html) and removed from analyses. Parameters used in Gblocks allowed for smaller final blocks, gap positions within the final blocks and less strict flanking positions. A chi-square test was used to test for significant deviations from homogeneity of base frequencies across taxa for each gene data set in PAUP* (v 4.0b10; Swofford 2000). Saturation at COI was tested using the test of Xia et al. (2003) as implemented in DAMBE (Xia & Xie 2001).
Phylogeny reconstruction
Species trees using individual genes and concatenated sequences from four genes for the large taxon set (108 individuals with 28S and at least two mitochondrial gene sequences) and for five genes for a reduced taxon set (50 individuals with 18S, 28S and at least two mitochondrial gene sequences) were produced using Bayesian inference as implemented in MrBayes (v. 3.1.2, Huelsenbeck & Ronquist 2001). Nucleotide substitution models were those suggested by MrModelTest (v 2.1, J. Nylander, http://www.ebc.uu.se/systzoo/staff/nylander.html) (GTR +G + I in all cases, except 18S, which was SYM + I + G). Analyses were run for 15 500 000 generations (12 500 00 generations for 16S) with a sample frequency of 1000. The first ten per cent of trees sampled were discarded. The data sets were analysed in two independent runs, and the final tree was computed from the combination of accepted trees from each run. Convergence between the two runs was tested by examining values for the potential scale reduction factors (PSRF), standard deviation of split frequencies, minimum effective sample sizes (ESS) of all parameters in the combined runs in Tracer and by visual examination of traces in .p files in Tracer (v. 1.5; available from http://beast.bio.ed.ac.uk/Tracer).
ESS values remained below 200 for TL (all) (sum of all branch lengths) in the four-gene tree, even after 15 500 000 runs, so the analysis was run again using MrBayes (Beta version 3.2) with the temperature lowered to 0.15 to encourage swapping among chains, and the propset command was used to increase the proposal probability of the topology parameter ExtTBR(Tau{all},V{all}) from 5 to 10%.
Results
Sequence analyses
Examining the individual gene data sets used in the MrBayes analyses, alignment lengths after removal of ambiguously aligned sites were 478 base pairs (bp) for 16S, 524 bp for 12S, 1422 bp for 28S and 980 bp for 18S (corresponding to 68% of the original 16S alignment of 699 bp, 60% of the 12S alignment of 872 bp, 92% of the 28S alignment of 1533 bp and 96% of the 18S alignment of 1019 bp). No sites were removed from the COI data set as the alignment was unambiguous. Most COI sequences were 658 bp, but the liotiid sequences were 664 bp, and two insertions each of a single amino acid were required to align the sequences of liotiid species with other vetigastropod sequences (length difference previously noted by Kano 2008).
Mitochondrial genes were the most variable, with COI and 12S more variable than 16S. The 12S fragment used in this study was particularly useful for recovering families and subfamilies. A total of 372 bp were variable in COI (344 phylogenetically informative, PI), 278 bp were variable in 16S (250 bp PI), 385 bp were variable in 12S (347 bp PI), 328 bp were variable in 28S (225 bp PI) and 146 bp were variable in 18S (87 bp PI). No significant deviations from of homogeneity of base frequencies across taxa were observed for individual gene data sets. Little saturation was observed for COI, with the index of substitution saturation (ISS) values significantly lower than the critical index of substitution saturation (Iss.c) values, suggesting that the use of all codon positions in phylogenetic analyses was warranted.
Convergence between the two independent Bayesian runs for each data set was confirmed: average standard deviation of split frequencies approached zero in all analyses (COI: 0.007433; 16S: 0.005945; 12S: 0.005630; 28S: 0.022459; 18S: 0.005503; four genes: 0.004756; five genes: 0.001545); average PSRF for all parameter values were ≤1.001 in all analyses; minimum ESS values in combined runs exceeded 200 for all parameters in all analyses. Convergence was reached very quickly for the four-gene tree (all ESS values >200 after about 3 million generations) in the analysis where the proposal probability and temperature were changed to improve the run.
Trees
All gene trees are shown (3, 4), because for some species, sequences of only one or two genes were available (especially for some taxa represented only by GenBank sequences). Family names used in this section are those used in Table 2 and in the Discussion.
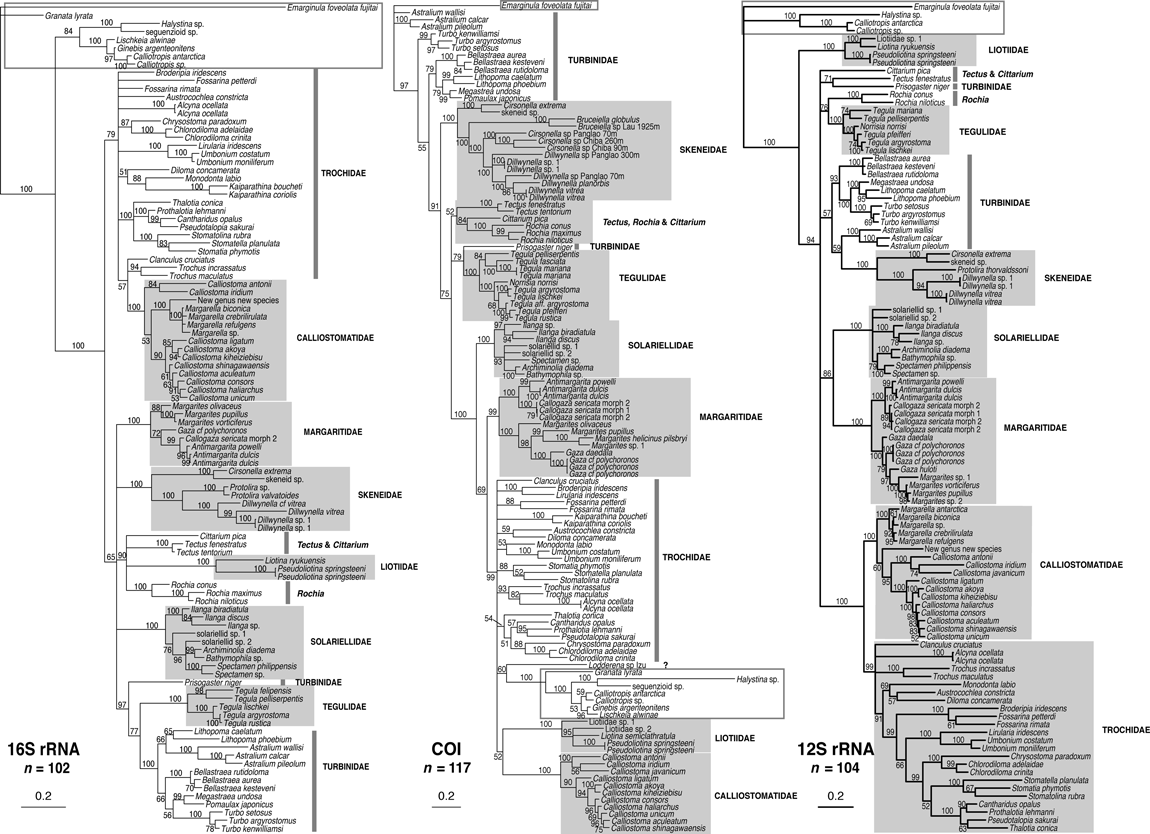
Single-gene trees for Trochoidea based on mitochondrial genes (16S, COI and 12S), with outgroups from Seguenzioidea and Fissurellidae, based on Bayesian inference using MrBayes. Support values are posterior probabilities (PP); branches with PP < 50% were collapsed. See Appendix S1 for sample details. Monophyletic families are indicated with a grey box, non-monophyletic groups with a grey line. Outgroup taxa are marked with a grey outline box.
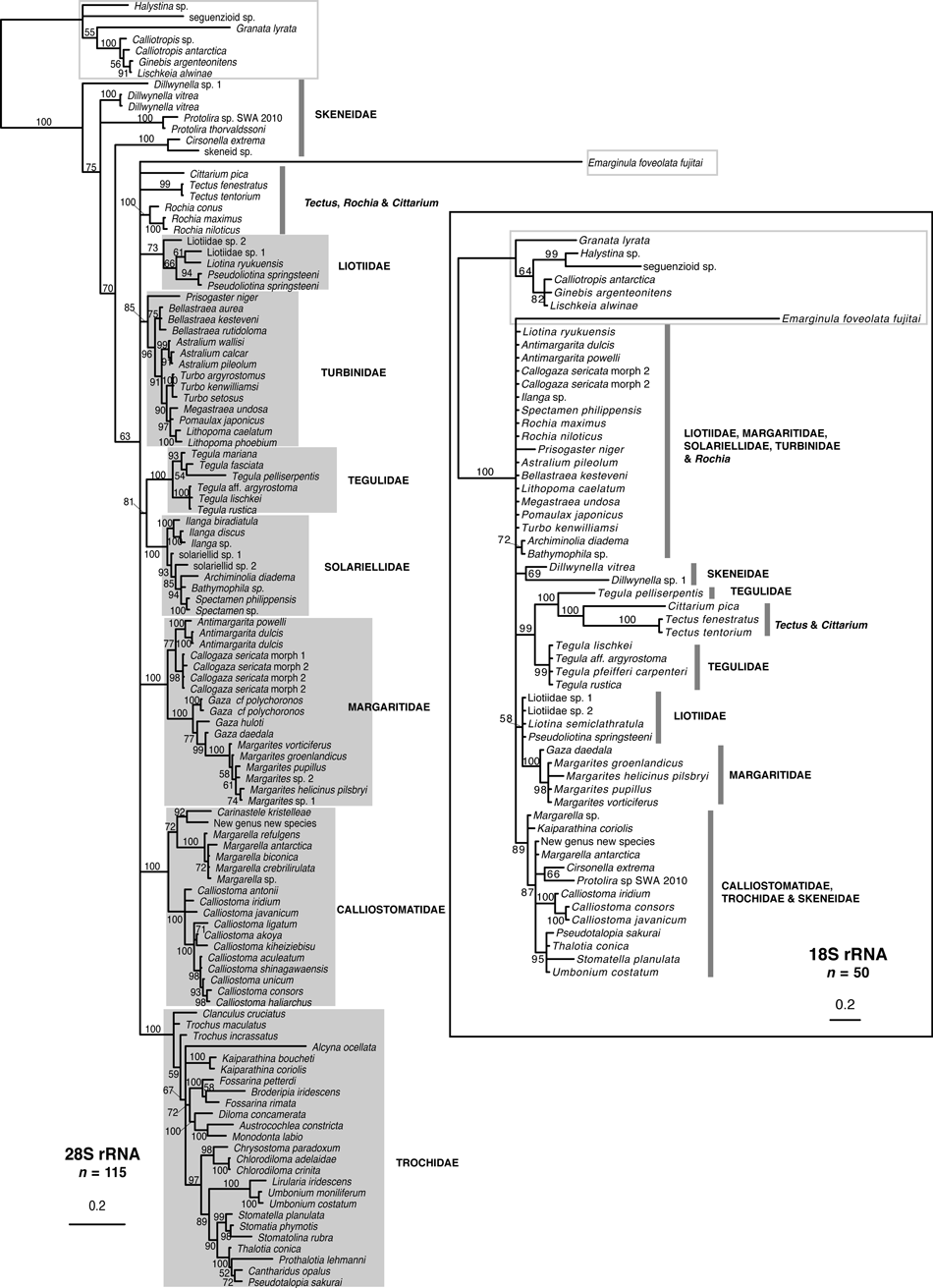
Single-gene trees for Trochoidea based on nuclear genes (28S and 18S), with outgroups from Seguenzioidea and Fissurellidae, based on Bayesian inference using MrBayes. Support values are posterior probabilities (PP); branches with PP < 50% were collapsed. See Appendix S1 for sample details. Monophyletic families are indicated with a grey box, non-monophyletic groups with a grey line. Outgroup taxa are marked with a grey outline box.
| Classification | Exemplar genera and comments |
|---|---|
| Family Calliostomatidae Thiele, 1924 | |
| Subfamily Calliostomatinae Thiele, 1924 | See Marshall (1995) |
| Subfamily Margarellinae Williams, new | Margarella |
| Subfamily Thysanodontinae Marshall 1988 | See Marshall (1995) |
| Family Liotiidae Adams and Adams, 1854 | |
| No subfamilies currently recognized | Liotia Other generic names are in a state of flux. James McLean is preparing a monograph on this family |
| Family Margaritidae Stoliczka, 1868 | |
| No subfamilies currently recognized | Margarites, Gaza, Callogaza, Antimargarites. New rank |
| Family Skeneidae Clark, 1851 | |
| No subfamilies currently recognized | Skenea, Bruceiella, Cirsonella, Dillwynella, Dikoleps, Lissospira, Protoliria, Skeneoides and others. This group is in desperate need of revision. Yasunori Kano is preparing a molecular study of this family, and Carole Hickman and Thomas Kunze are working on monographs |
| Family Solariellidae Powell, 1951 | |
| No subfamilies currently recognized | Solariella, Archiminolia, Bathymophila, Hazuregyra, Ilanga, Microgaza, Minolia, Minolops, Spectamen, Suavotrochus, Zeminolia, Zetela and others. A molecular phylogeny of this group is in preparation (STW and others) |
| Family Tegulidae Kuroda, Habe and Oyama, 1971 | |
| No subfamilies currently recognized | Tegula, Norrisia. New rank |
| Family Trochidae Rafinesque, 1815 | |
| Subfamily Alcyninae Williams et al. 2010 | Alcyna |
| Subfamily Cantharidinae Cotton, 1959 | See Williams et al. (2010) |
| Subfamily Chrysostomatinae Williams et al. 2010 | Chrysostoma, Chlorodiloma |
| Subfamily Fossarininae Bandel, 2009 | Fossarina, Broderipia, Clydonochilus, Minopa, Synaptocochlea, ‘Roya’. (See Williams et al. 2010 for discussion) |
| Subfamily Halistylinae Keen, 1958* | Botelloides, Charisma, Halistylus |
| Subfamily Kaiparathininae Marshall 1993 | Kaiparathina. See Marshall (1993) |
| Subfamily Monodontinae Gray, 1857 | See Williams et al. (2010) |
| Subfamily Stomatellinae Gray, 1840 | See Hickman & McLean (1990) |
| Subfamily Trochinae Rafinesque. 1815 | See Williams et al. (2010) |
| Subfamily Umboniinae Adams and Adams, 1854 | See Williams et al. (2010) |
| Family Turbinidae Rafinesque, 1815 | |
| Subfamily Prisogasterinae Hickman & McLean 1990 | Prisogaster |
| Subfamily Turbininae Rafinesque. 1815 | Turbo, Astraea, Astralium, Bolma, Bellastraea, Cookia, Guildfordia, Lithopoma, Megastraea, Modelia, Pomaulax. A molecular phylogeny for this subfamily was published in Williams (2007) |
| Unassigned to family | |
| Tectus, Rochia, Cittarium | Williams et al. (2008); this study |
- *Not yet included in any molecular phylogeny.
Clades corresponding to Calliostomatidae, Margaritidae, Solariellidae and Tegulidae were recovered with high support in all gene trees except the 18S tree (all PP ≥ 99%). Liotiidae and Skeneidae were recovered in all mtDNA gene trees with high support (PP = 100%) and Liotiidae were recovered with poor support in the 28S tree (PP = 73%). Monophyletic Trochidae were recovered in the 12S and 28S trees (PP ≥ 99%). Turbinidae were recovered with modest support in the 28S trees (PP = 85%). Tectus and Cittarium formed a poorly supported clade in the COI tree (PP = 52%). No families were monophyletic in the 18S tree. Despite good support for families (and subfamilies in some trees), relationships among families were poorly resolved in individual gene trees.
In the combined gene trees (5, 6), Calliostomatidae, Liotiidae, Margaritidae, Skeneidae, Solariellidae, Tegulidae and Trochidae were all recovered with full support (PP = 100%). Turbinidae were recovered with high support (PP ≥ 98%). A clade with Tectus and Cittarium was recovered with modest support in the 5-gene tree (PP = 90%) and low support in the 4-gene tree (PP = 80%).
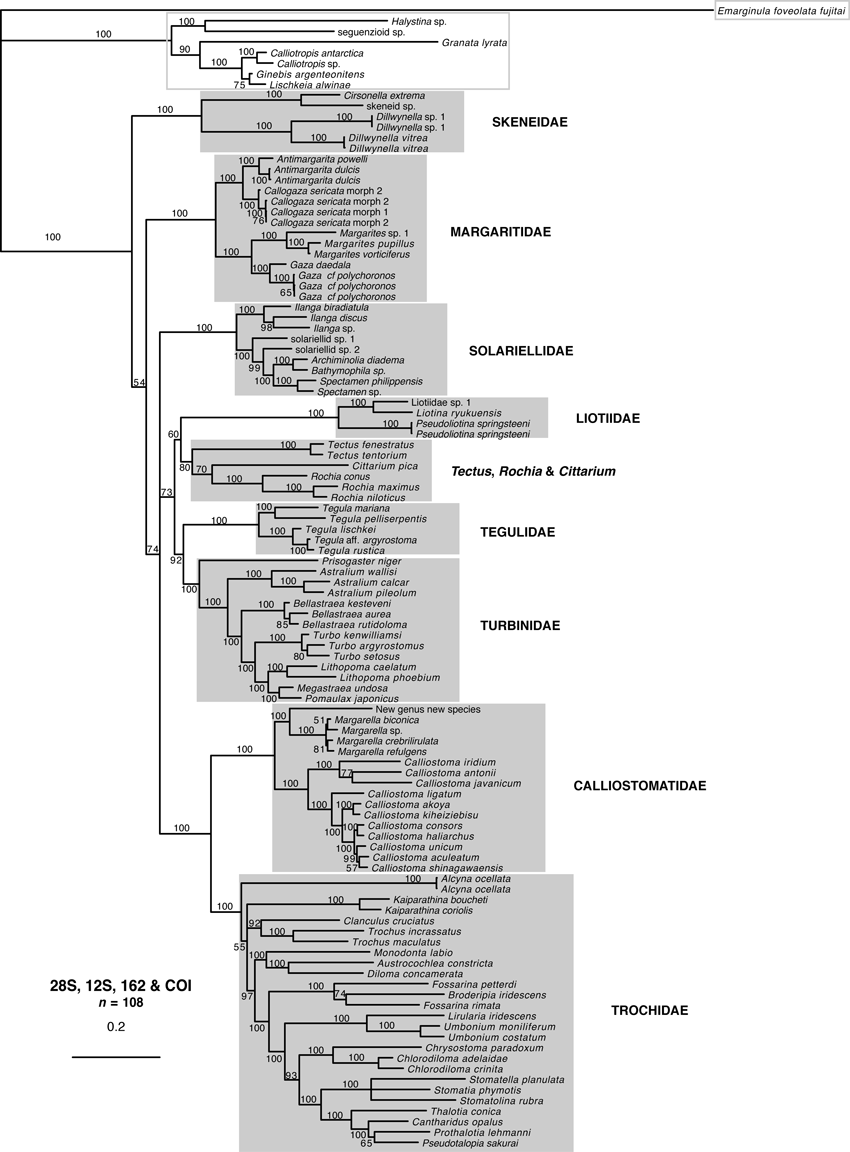
Combined gene tree for Trochoidea based on four genes (28S, 16S, 12S and COI), with outgroups from Seguenzioidea and Fissurellidae, based on Bayesian inference using MrBayes. Support values are posterior probabilities (PP); branches with PP < 50% were collapsed. See Appendix S1 for sample details. Monophyletic families are indicated with a grey box. Outgroup taxa are marked with a grey outline box.
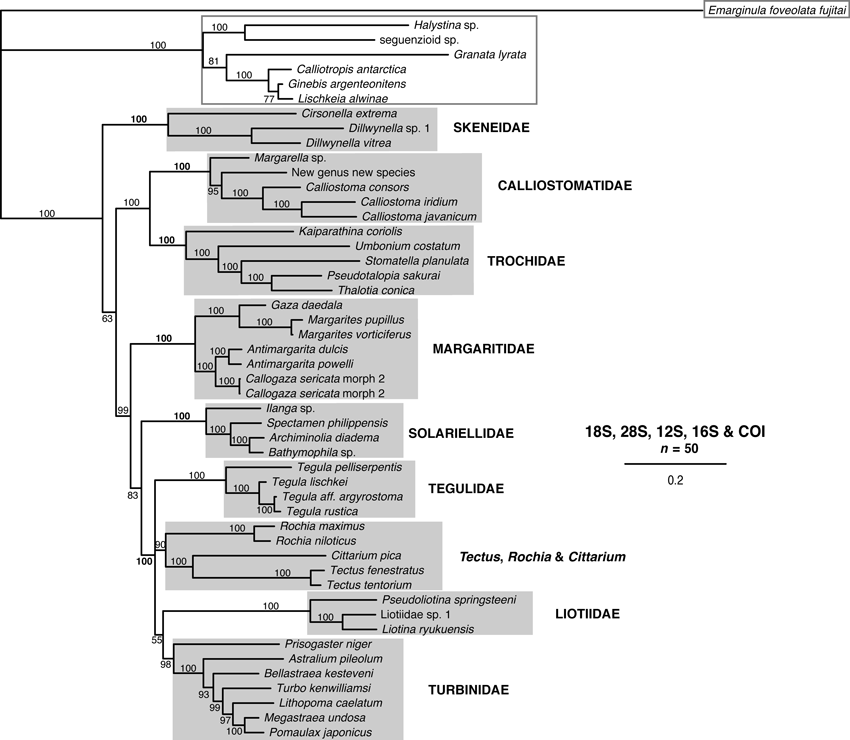
Combined gene tree for Trochoidea based on five genes (18S, 28S, 16S, 12S and COI), with outgroups from Seguenzioidea and Fissurellidae, based on Bayesian inference using MrBayes. Support values are posterior probabilities (PP); branches with PP < 50% were collapsed. See Appendix S1 for sample details. Monophyletic families are indicated with a grey box. Outgroup taxa are marked with a grey outline box.
Relationships among families were not fully resolved even in the combined gene trees, but there was strong support for Trochidae as sister to Calliostomatidae in both combined gene trees (PP = 100%). Tegulidae, Liotiidae and Turbinidae formed a clade with Tectus and Cittarium with high support in the 5-gene tree (PP = 100%) and weak support in the four-gene tree (PP = 73%). These formed a clade with Margaritidae and Solariellidae with high support in the five-gene tree (PP = 99%).
Discussion
Obtaining a systematic framework that accurately reflects the evolutionary history of a group is a large undertaking when the group is diverse as Trochoidea. Recent molecular studies have made many changes to traditional systematic concepts based purely on morphology, and as a result of increased taxon sampling and the use of a greater number of genes, this study suggests yet further changes.
A total of eight, possibly nine, trochoidean families were recovered in this study. The familial status of Calliostomatidae, Liotiidae, Solariellidae, Trochidae and Turbinidae was confirmed, but three clades tentatively treated as subfamilies of Turbinidae by Williams et al. (2008) are here given familial rank. Skeneidae, Margaritidae and Tegulidae are shown to be distinct clades with robust support. A ninth clade, which includes both Cittarium and the commercially important genus Tectus, is recognized but not formally described as a family. All families can be distinguished by a combination of shell, radular and anatomical characters discussed below.
The new classification proposed here is summarized in Table 2. Except where otherwise indicated, the topology and support of the four-gene tree (Fig. 5) are discussed as this combines the greatest taxon sampling with the most sequence data.
Family Calliostomatidae
Three clades were recovered within Calliostomatidae in the 28S tree (only 28S sequence was available for Carinastele kristelleaeMarshall 1988b). The three clades correspond to Calliostomatinae Thiele, 1924 (represented by 11 species of Calliostoma Swainson, 1840; PP = 100%), a clade including five species of Margarella (PP = 100%) and a third clade including New Zealand C. kristelleae and an unnamed tropical deep-sea species (PP = 92%).
The new, as yet unnamed deep-water (897–1057 m) tropical species (Fig. 1A) is sister to C. kristelleae, a member of the subfamily Thysanodontinae Marshall, 1988. It is not, however, a member of this subfamily. Thysanodontinae are typified by their unusual radulae, which are ‘exceedingly slender’ with a series of backwardly inclined barbs along their length (Marshall 1988b, 1995). The new species has a more typically calliostomatid radula with a central field of thin, highly flexible teeth, an enlarged innermost marginal tooth with a heavy cusp and thin, marginal teeth. Unlike other calliostomatids, there are no serrations on any teeth, and all cutting edges are smooth. The smooth shell of the new species is somewhat similar to Venustatrochus georgianus Powell, 1951, but preliminary sequence data from that species show that they are clearly different species. The protoconch is large (approximately 480 μm) but corroded, and no sculpture is evident. The unique radula in combination with its position in the phylogeny might suggest it belongs to a new subfamily, but this awaits further study with the inclusion of more taxa. Additional, undescribed species that may belong to the same subfamily are also known from New Zealand, Australia, New Caledonia (B. Marshall, pers. comm.) and from deep-water off Brazil (A. Warén, pers. comm).
The specific identification, generic assignment and relationships of Magellanic and Antarctic species previously referred to Margarella or Margarites are extremely confused (Reid & Osorio 2000; Linse 2002). Williams et al. (2010) showed that the current concept of Margarella is polyphyletic, with one species (and likely others) from New Zealand falling out in the trochid subfamily Cantharidinae Gray, 1857 and one, Margarella antarctica (Lamy, 1905) recovered within the Calliostomatidae as sister either to Calliostomatinae or Thysanodontinae. This study confirms that finding with an additional four Antarctic species of Margarella forming a clade with M. antarctica within the Calliostomatidae. These new results in combination with Williams et al. (2010) confirm that there are at least two ‘Margarella-like’ groups: a New Zealand radiation of cantharadine trochids and an Antarctic clade of calliostomatids.
Previously, many southern hemisphere ‘trochid’ species were referred to Margarites, but Zelaya (2004) reassigned the Antarctic and Subantarctic species to Margarella. The type species of Margarella is Margarella expansa (Sowerby I, 1838), but de Deambrosi (1969) transferred it to Margarites on the basis of radular characters. This would mean that Margarella is a synonym of Margarites (Dell 1990). Margarella antarctica, Margarella bouvetia Powell, 1951, Margarella refulgens and Margarella crebrilirulata share the same characters and were also transferred to Margarites (de Deambrosi 1969; Arnaud 1972a,b; also Dell 1990; although see Linse 2002). Dell (1990) suggested that the remaining species usually assigned to Margarella including Margarella achilles, Margarella violacea, Margarella tropidophoroides and Margarella obsoleta should be referred to Promargarita Strebel 1908 (type species Promargarita tropidophoroides). However, in this study, Margarella does not cluster with Margarites in the Margaritidae, but rather with Calliostomatidae, and therefore, the genus is still considered valid (albeit assuming that the species used in this study are representative of M. expansa). No species from Promargarita were sampled.
The Margarella clade deserves to be treated as a new subfamily. Although the type species, M. expansa, was not included in this (or any other) molecular study, several authors have noted similarities with M. antarctica, which was included. On this basis, Williams et al. (2008) suggested that this species represented Margarella s.s. Following the same logic, I recognize this clade as a new calliostomatid subfamily, Margarellinae, with confirmation of the name pending further studies. Note that the unnamed species from Bouvet Island is not M. bouvetia Powell, 1951. The relationship between Promargarita and Margarella needs to be tested in further studies.
Hickman & McLean (1990) suggested that Margarella was closely allied to Margaritidae (as Margaritini). On the other hand, Numanami (1996) suggested that Margarella biconica (included in this study) was most closely allied to Calliostoma based on shell characters, but tentatively assigned it to Margarites because it did not have a typical calliostomatid radula. He also mentioned that Hain (1990) assigned this species (as Margarella sp. 1) to Margarella, but disagreed with this assignment, saying that the radula is quite different from typical Margarella radulae. The ‘extremities of rhachidian and lateral teeth [of M. biconica] are sharply pointed and the serrations of these lateral cups are course’, whereas the rhachidian of a Margarella radula has an ‘oval base’. However, M. biconica groups with the other ‘typical’Margarella species in this study, suggesting that radular characters (or their current interpretation) might not be informative at this level. Some Antarctic species currently assigned to Margarella have also been assigned to Photinula Adams and Adams, 1854, which is a calliostomatid genus (Zelaya 2004) not included in this study.
The assignment of Margarellinae to Calliostomatidae receives strong support in the molecular phylogenies of this study; however, it is morphologically quite distinct from Calliostomatinae and Thysanodontinae. Where Margarella radulae have been investigated, they are not similar to typical calliostomatids (Numanami 1996; photographs in Hain 1990 and Linse 2002). Protoconchs of Calliostomatinae and Thysanodontinae are characterized by a reticulate, honeycomb pattern (Hickman & McLean 1990). Unfortunately, protoconchs of all new calliostomatid specimens used in this study were badly corroded, leaving little or no original sculpture. Very small patches of protoconch where corrosion seemed less intense (close to sutures) did not appear to have this sculpture. Numanami (1996) described the protoconch of M. biconica as having an ‘uneven surface’. However, Falsimargarita, which is assigned to Calliostomatidae, also lacks a reticulate pattern on the protoconch, and some species have a finely nodulose surface (Warén & Bouchet 2001).
Zelaya (2004) described Margarella as a primarily intertidal or shallow subtidal radiation, but new specimens used in this study were from deeper water (82–668 m), although there are numerous reports of the same species occurring in much shallower water. Either there is some confusion over species identifications, or like many calliostomatids, species in this group have very wide depth ranges.
Calliostomatidae as currently defined includes three subfamilies: Calliostomatinae, Thysanodontinae and Margarellinae. The relationship among these subfamilies needs more study, and phylogenetic inference for the family would benefit from increased taxon sampling of Antarctic and deep-water lineages. It would also be interesting to include samples of the family Trochaclidae Thiele, 1928, which are currently classified as vetigastropods, but not assigned to superfamily (Hickman & McLean 1990; Bouchet et al. 2005; as Ataphridae Cossmann, 1915; Grundel, 2008). Hickman & McLean (1990) suggested that they might be closely related to calliostomatids on the basis of oral disc papillae and their association with sponges (e.g. Marshall, 1983, 1995).
Previous diagnoses for Calliostomatidae do not encompass the range of morphologies seen in the new subfamilies. One character that might unite all three subfamilies is their feeding habit. Where diet is known, calliostomatids are carnivores of sessile invertebrates (Marshall 1995). Although species belonging to the genus Margarella are not generally thought to be carnivorous, M. antarctica is commonly found in association with sponges (Gutt & Schickan 1998). Further work is needed.
Family Liotiidae
Hickman & McLean (1990) treated Liotiidae Adams and Adams, 1854 as a subfamily of Turbinidae. However, their concept of Liotiinae has not proven to be a monophyletic entity in molecular studies, with some species forming a sister clade to Angariidae (Williams & Ozawa 2006; Williams et al. 2008; Aktipis et al. 2011). This clade is often referred to as ‘Areneidae’, but this family has not yet been formally described, and as such, the name is not officially recognized (P. Bouchet, pers. comm). I do not describe the family here, but rather wait for a monograph of the family that is currently in preparation (by James McLean). The clade includes the Caribbean genus Arene Adams and Adams, 1854 and South African genus Cinysca Kilburn, 1970 (Williams & Ozawa 2006; Williams et al. 2008; Aktipis & Giribet 2012) (a grouping noticed by Leal 1991) and likely other East Pacific and Caribbean genera. It also includes at least one highly coloured unnamed species that occurs in the Philippines (unpubl. data).
This division between the trochoidean and non-trochoidean groups is supported by shell microstructure, which splits the ‘liotiid’ taxa into three groups, one (including Liotina Munier-Chalmas, 1885) more similar to Turbininae than the others (including Arene and Cinysca) (Hedegaard 1990; Williams & Ozawa 2006). It is also supported by statistical tests comparing alternative molecular tree topologies. Both the Shimodaira-Hasegawa test (Shimodaira and Hasegawa 1999) and the AU test (Shimodaira 2002) showed that an unconstrained Bayesian tree based on three genes was significantly better than trees where Arene was constrained to be sister to Liotina and belong to Trochoidea (Williams et al. 2008). Equally, there were large differences in Bayes Factors between constrained and unconstrained gene trees for 28S and COI (Williams et al. 2008). There was no difference in 18S trees because of lower resolution of this gene (Williams et al. 2008). Despite morphological similarities, the molecular data strongly suggest that these two groups are not sister taxa and that Arene and related genera are not trochoideans.
True trochoidean liotiids have been treated as a subfamily of Turbinidae (Hickman & McLean 1990; Williams & Ozawa 2006) or as a distinct family (Bouchet et al. 2005; Williams et al. 2008). The topology of the combined gene tree in this study suggests that trochoidean liottids are a family closely associated with Tegulidae, Turbinidae and Tectus and Cittarium. This is consistent with groupings discussed in recent studies (Bouchet et al. 2005; Williams et al. 2008).
The family includes small species with planispiral shells that are usually white or light brown and not usually highly coloured. Species are typified by a corneous operculum covered with calcareous beads and edged with a fringe. Molecular evidence has shown that this feature is not unique to the family, as it also occurs in the non-trochoidean clade (including Cynisca and Arene) that is sister to Angariidae (Williams & Ozawa 2006; Williams et al. 2008; Aktipis 2012). A monograph of the two families, describing many new species, is being prepared by Jim McLean.
Family Margaritidae (new rank)
The following genera have been confirmed as members of this family: Gaza, Callogaza, Margarites and Antimargarita. Together, these genera form a well-supported clade (PP = 100%). Hickman & McLean (1990) treated Margaritidae as a subfamily of Trochidae, which they divided into two tribes Margaritini (for Margarites, and subgenera Cantharidoscops Galkin, 1955 and Valvatella Gray, 1857) and Gazini (for Gaza and subgenus Callogaza).
Williams et al. (2008) removed Margarites from Trochidae on the basis of a molecular phylogeny based on three genes and suggested that it was either a basal turbinid or a separate clade more closely related to Turbinidae than Trochidae. In this study, with greater taxon sampling and more genes, Margaritidae is in a clade with Turbinidae, Tegulidae, Solariellidae and Tectus and Cittarium as in Williams et al. (2008), with the addition of Liotiidae, which was sister to Calliostomatidae in Williams et al. (2008).
Recently, Hickman (2012) raised Gazini to familial rank. This study shows that neither Gazini (or Gazidae) nor Margaritini are monophyletic as Margarites is sister to Gaza and Antimargarita sister to Callogaza. The name Margaritidae has precedence over Gazidae.
A close relationship between Margaritidae and some Antarctic and Subantarctic species was postulated by Hickman & McLean (1990), and they thought Margarella closely allied with Margaritini. The molecular phylogeny in this study suggests that Margarella is better assigned to Calliostomatidae, but other Antarctic species from the genus Antimargarita do indeed belong to Margaritidae.
The type species of Gaza, G. daedala, has been collected only rarely, but was included in this study. Having the type species confirms that Margarites huloti Vilvens & Sellanes (2006) (Fig. 1K) should be reassigned to Gaza and confirms the generic assignment of a new species (Gaza cf polychoronosVilvens 2012; Fig. 1L). Warén et al. (2011) noted that Gaza huloti was difficult to assign to genus on shell characters alone but that its radula was similar to Gazini sensuHickman & McLean (1990). Although the type species of Callogaza, C. watsoni Dall, 1881, was not included, the phylogeny is consistent with the generic status of Callogaza, as used by Simone & Cunha (2006).
Two morphs of Callogaza sericata from New Caledonia (Figs 1H,I) were included. These morphs were collected from similar locations (Isle of Pines, New Caledonia) and depths, and were genetically indistinguishable but differed in colour pattern. The lighter specimens lack a septum over the umbilicus and do not have a retroflected apertural lip (Fig. 1I), suggesting they may not be fully grown (Simone & Cunha 2006). As genetic differences are so small, I conservatively retain these as one species. Equally, the specimens identified as Gaza cf polychoronos may not be fully grown because, unlike the holotype, they do not have a callus over the umbilicus. If so, the species range is extended beyond the original description (Vilvens 2012).
As defined here, Margaritidae are a small family including only four genera and <100 species. The most diverse genus, Margarites, is found in high, northern latitudes in cold temperate shallow waters (intertidal to 50 m). Gaza and Callogaza occur in tropical deep waters (see Simone & Cunha 2006; Hickman 2012 for a revision of these genera), and Antimargarita occurs around Antarctica in cold temperate deep waters (146–1497 m in this study) (see Aldea et al. 2009 for a discussion of this genus). Shell characters of the Margaritidae have not changed from the diagnosis in Hickman & McLean (1990). Shells vary from smooth to predominantly spiral ornament, shells of about equal height and width, a roundish and oblique aperture and an interrupted peristome. The umbilicus may be open or closed. These characters, while typical, are not diagnostic for the group, occurring also in other trochoideans.
Family Skeneidae
Many authors have suggested that Skeneidae Clark, 1851 is in fact a polyphyletic group, and as such, these species are usually referred to as ‘skeneimorph’ gastropods (e.g. Hickman 1984, 1998; Marshall 1988a; Hickman & McLean 1990; Warén 1991, 1992; Kano 2008; Kano et al. 2009). Skeneimorphs have often been treated as a family (e.g. Marshall 1988a; Hickman & McLean 1990), and Marshall (1988a) suggested they might have undergone ‘an independent radiation parallel with Turbinidae and Trochidae’. This suggestion and their familial status are supported by the results here, although the phylogeny of the skeneids would benefit greatly from further study.
Marshall (1988a) recognized five major groups in the skeneimorphs; four of these are no longer considered true skeneids. For instance, Bathyxylophila Marshall, 1988 is not a trochoidean genus, being more closely related to Scissurellidae Gray, 1847 (Kano 2008; Kunze et al. 2008; Aktipis & Giribet 2012). Xyloskenea Marshall, 1988, VentsiaWarén and Bouchet, 1993 and Adeuomphalus Seguenza, 1876 have been reassigned to Seguenzioidea based on both morphological and molecular studies (Kano 2008; Kunze et al. 2008; Kano et al. 2009). Kano (2008) also suggested that Benthobrookula Clarke, 1961, Trenchia Knudsen, 1964 and Vetulonia Dall, 1913 should be assigned to Seguenzioidea based on shell morphology and that another eleven genera are also likely to belong in this superfamily (Aequispirella Finlay, 1924, AkritogyraWarén 1992; Anekes Bouchet and Warén, 1979, Brookula Iredale, 1912, Granigyra Dall, 1889, Lissotesta Iredale, 1915, Moelleriopsis Bush, 1897, PalazziaWarén 1991; RetigyraWarén, 1989). Munditiella ammonoceras (Adams, 1863) is removed from Trochoidea based on molecular studies, but remains within the Vetigastropoda, although as yet unassigned to a family or superfamily (Williams et al. 2008; Kano et al. 2009; Aktipis & Giribet 2012).
Still other skeneimorph taxa have been removed from Vetigastropoda entirely. Leptogyra Bush, 1897, Xyleptogyra Marshall, 1988 and Leptogyropsis Marshall, 1988 are now assigned to the clade Neomphalina based on morphological and histological studies (Hickman 1984; Warén & Bouchet, 1989; Haszprunar 1989; Beck et al. 2007; Speimann et al. 2007; Kunze et al. 2008; Heßet al. 2008), shell structure (Haszprunar & Kiel 2005) and molecular studies (Heßet al. 2008; Kano 2008). Yet, others (including Hyalogyra Marshall, 1988 and Hyalogyrina Marshall, 1988) are assigned to the Caenogastropoda or Heterobranchia (e.g. Moore 1972; Robertson 1973; Warén 1992; Gofas et al. 2001; Heßet al. 2008; Kano 2008; Kunze et al. 2008; Kano et al. 2009; Spencer et al. 2009).
However, it is thought that the nominotypical genus Skenea Fleming, 1825 belongs to Trochoidea based on morphological evidence (Marshall 1988a; Hickman & McLean 1990; Sasaki 1998; Kano 2008; Kunze et al. 2008) although this has not been tested using molecular data. Morphologically similar genera, Dikoleps Hoisaeter, 1968, Lissospira Bush, 1897, SkeneoidesWarén 1992; FucariaWarén & Bouchet, 1993 and Iheyaspira Okutani, Sasaki & Tsuchida, 2000 are also thought to belong to the same family or subfamily as Skenea (Kunze et al. 2008; Sasaki et al. 2010).
Previously, the molecular study with the greatest taxon sampling to date was Kano (2008), although only one gene (COI) can be compared with this study. Kano’s (2008) sequences (available on GenBank) were included in this study and these, together with new data for five species show that Dillwynella, Cirsonella, Bruceiella and two additional species form a well-supported clade within Trochoidea in the COI tree (PP = 100%). Skeneidae was also recovered as monophyletic in both combined gene trees and in the mitochondrial gene trees including Protolira in the 16S and 12S trees. This is important, as species of Protolira and Skenea both have a propodial penis suggesting that Protolira is representative of the nominotypical genus (A. Warén, pers. comm.).
Other molecular studies have also shown that these genera belong to Trochoidea (Warén et al. 2003; Kano 2008; Williams et al. 2008; Aktipis & Giribet 2012). Most recently, these genera were assigned to a subfamily of Turbinidae based both on morphology and preliminary molecular results (Bouchet et al. 2005; Williams et al. 2008). Many species with turbinid-like radulae have been noted (Hickman & McLean 1990; Warén 1992; Kano 2008). Checa & Jiménez-Jiménez (1998) also noted that the rigiclaudent nature of their operculum was similar to Turbinidae. However, this study suggests that the group deserves familial rank.
As currently identified, Cirsonella is not monophyletic in the COI tree. Three species from a previous study (Kano 2008) cluster together, whereas the Antarctic species Cirsonella extrema is sister to an unidentified species (probably new) from deep-water Philippines. The Antarctic species C. extrema has shell characters consistent with Cirsonella, having a complete periostome, a circular aperture and thick apertural rim (Marshall 1988a). Either C. extrema or the other three species need to be assigned to another genus. Which group corresponds to true Cirsonella would need to be confirmed by including the type species Cirsonella australis Angas, 1877; unfortunately, it has never been collected alive (C. Hickman, pers. comm.). Linse (2002) suggested that a new genus might be needed for C. extrema, but C. Hickman (pers. comm.) suggests that morphologically this species is most similar to the type species and that it represents true Cirsonella.
Only one skeneimorph species, Lodderena sp. (a sequence from GenBank; Kano 2008), was not recovered in Skeneidae in the COI tree; instead, it is sister to Segenzioidea with poor support (PP = 60%). The radula of Lodderena is noted to be of a ‘typically turbinid plan’, having characters more in common with other turbinids than any skeneimorph examined (Hickman & McLean 1990). Sequences from additional genes would be helpful for confirming whether this species is a true member of Skeneidae.
Kunze et al. (2008) most recently defined the family as species with small-sized shells, lacking nacre, having a single left gill, a heart lying more on the right-hand side of the mantle cavity, hermaphroditic genital system and a penis on the right propodium. Skeneids also have fewer epipodial tentacles than other trochoideans and lack cephalic lappets (Marshall 1988a). Many of these characters have been noted to be typical of small species (Marshall 1988a).
Family Solariellidae
This is the first molecular phylogenetic analysis of solariellids that includes more than two specimens and a more comprehensive study is in preparation (by the author and others). Bouchet et al. (2005) expressed some doubt as to whether Solariellidae belonged to Trochoidea, suggesting that they might also belong to Seguenzioidea on the basis of reductions in the number of lateral and marginal teeth in the radula. However, molecular studies (albeit with only a few samples) have shown that solariellid species belonged in Trochoidea (Kano 2008; Williams et al. 2008; Aktipis & Giribet 2012). In this analysis, the solariellids are recovered as a well-supported clade within Trochoidea (PP = 100%). This study also shows that the genus Pseudotalopia Habe, 1961, sometimes assigned to Solariellidae, is in fact a member of the trochid subfamily Cantharidinae, as suggested by Williams et al. (2010). Many new solariellid species have been recently discovered (e.g. Vilvens 2009), and a new genus is currently being described based on morphological characters (Vilvens & Williams 2012).
Species belonging to this family are characterized by small, nacreous shells and by the presence of a ring of digitate papillae around snout. They have a short radula with 20–30 transverse rows of teeth. The radula is unusual in that it is straight and not coiled, probably because it is so short (Hickman & McLean 1990). The anterior end of the foot is bilobed. Cephalic tentacles are often long and thick. Eye-stalks are generally much shorter than cephalic tentacles, and eyes are often unpigmented, especially in deeper water species. Sensory papillae on cephalic tentacles are much reduced compared with other trochoideans (Hickman & McLean 1990). Solariellids have often been confused with margaritids and umboniine trochids; however, these three groups can be distinguished by protoconch morphology (Herbert 1987) and in some cases habitat. Solariellids are rare in tropical or warm-temperate shallow water, and umboniine trochids are rare in deep water.
Tectus, Rochia and Cittarium
Four Tectus species form a clade with Cittarium pica in the COI and both combined gene trees, although only with low to moderate support (PP = 52–90%). Previous molecular studies with less species also showed a sister relationship between Cittarium and Tectus (Williams et al. 2008). Although these authors suggested that Tectus and Cittarium might be assigned to Tegulidae (treated as a turbinid subfamily in that study), they did not officially assign them to any subfamily. This study suggests they might be better treated as a separate clade and given familial rank. However, owing to the modest level of support for the clade and the long branches evident in the 18S tree, I do not yet formally recognize a new taxon and await further studies with additional taxa.
Tectus species form two clades: one including Tectus conus Gmelin, 1791, the type species of subgenus Rochia Gray, 1857 and Tectus niloticus (Linnaeus, 1767) and Tectus maximus (Koch in Philippi, 1844), which are usually assigned to Tectus s.s. The second clade includes Tectus fenestratus and Tectus tentorium, which are usually assigned to subgenera Cardinalia Gray, 1847 and Tectus, respectively. The great genetic difference between these two clades and the sister relationship between C. pica and two Tectus species suggest that Tectus as currently recognized is not monophyletic. The first clade is treated here as Rochia at generic rank as the molecular split is also supported by morphological differences. Thus, Rochia includes the commercially important species Rochia niloticus and Rochia maximus (R. maximus is often thought to be a synonym, but is genetically distinct; Williams et al. 2008). Tectus has flat-sided whorls and a single-columellar twist, whereas Rochia has a more unmodified columellar, sometimes with a tooth (Knight et al. 1960; J. McLean, pers. comm.). This split is supported by unpublished COI sequence data for Tectus pyramis, which suggests that it clusters with T. fenestratus and T. tentorium.
Shells in this clade are relatively thick, strongly nacreous and have an incomplete peristome (Hickman & McLean 1990; Robertson 2003). Shells may have a small tooth on the columella (e.g. Rochia conus, Knight et al. 1960; Oligocene fossil Cittarium maestratiiLozouet 2002). Where known, members of Tectus, Rochia and Cittarium are broadcast spawners, with free-swimming trochophores (Eisawy, 1971; Heslinga, 1981; Herbert 1993; Robertson 2003), in contrast to at least some Trochus species, which deposit gelatinous egg masses (e.g. Tr. erithreus; Herbert 1993). The morphology, behaviour, ecology, habitat, fossil record and general natural history of Cittarium were comprehensively reviewed by Robertson (2003).
Family Tegulidae (new rank)
Tegulidae was treated as subfamily of Trochidae by Hickman & McLean (1990) based on plesiomorphic radular and ctenidial characters, although they noted that the group could also be a primitive member of the Turbinidae that lost the ability to form a calcareous operculum. Hickman (1996) suggested that the Tegulidae represent an enigmatic group located somewhere between the Trochidae and Turbinidae. More recently, it has been treated as a subfamily of Turbinidae by both Bouchet et al. (2005; based on radulae and 16S sequences) and Williams et al. (2008; based on molecular analysis of three genes). High support for the grouping of Turbinidae with Tegulidae and Skeneinae was also noted in a Bayesian analysis of three genes by Kano (2008).
The family Tegulidae as defined here includes Tegula (and associated subgenera) and Norissia Bayle, 1880, but excludes Cittarium Philippi, 1847. This coincides with Hickman & McLean’s (1990) concept of Tegulidae (as the trochid subfamily Tegulinae) and their diagnosis stands.
Family Trochidae
Trochidae were the subject of a recent detailed molecular phylogeny, where nine subfamilies were recognized (Williams et al. 2010). Eight were recovered in a four-gene tree, and the ninth, Halistylinae, was accepted as a trochid subfamily following the decision of Hickman & McLean (1990), although this has yet to be tested with molecular data (Williams et al. 2010).
This study recovered the same eight subfamilies plus one additional trochid subfamily; the new subfamily Kaiparathininae (Halistylinae has still not been sampled). Beu (1973) suggested that ‘the shallow sinus in the outer lip, the lightly angled whorls, and the weak surface sculpture indicate that Kaiparathina should be referred to the Trochidae, and probably to the subfamily Margaritinae’. Marshall (1993) concurred with this decision, referring the genus to a tribe, Kaiparathini Marshall 1993; in the trochid subfamily Margaritinae s.l. Hickman (2012), on the other hand, removed Kaiparathina from the trochid subfamily Margaritinae s.l. and suggests that it is ‘similar to Gazidae in having features in common with both Trochoidea and Seguenzioidea’. Phylogenetic analysis shows that Kaiparathina does not cluster with Margarites or Gaza (now both belonging to the family Margaritidae), but rather with Trochidae. Here, I accord the tribe subfamilial status within Trochidae. The subfamily includes only the nominotypical genus, so the diagnosis is the same as for the genus Kaiparathina.
Williams et al. (2010) defined the family Trochidae sensuWilliams et al. (2008) as a radiation of primarily shallow-water species living intertidally or subtidally, with few species found in deeper water and fewer still in bathyal depths. The redefinition here of Trochidae as excluding Margaritidae means that trochid species do not occur in either Antarctica or the Arctic. Trochids (and turbinids) are particularly abundant and diverse in the tropical Indo-West Pacific particularly in the intertidal zone. This may be because the trochoid shell morphology reduces predation by shell-crushing predators. The shell lacks a slit, which has been suggested to make the shell more susceptible to breakage (Geiger et al. 2008), and in intertidal species, the shell is often thick, sometimes with labral teeth (e.g. Clanculus), spines or a heavily calcified operculum (in turbinids), characteristics thought to offer protection against predation (Vermeij 1993).
Family Turbinidae
The family Turbinidae has been the most difficult to resolve using either morphological or molecular data, and as such, systematics of the group has been in a state of flux. Hickman & McLean (1990) included Angariinae, Liotiinae, Moelleriinae, Colloniinae, Prisogasterinae, Turbininae, Phasianellinae, Gabrieloninae and Tricoliinae as subfamilies of Turbinidae. However, all these clades, other than Liotiidae, Prisogasterinae and Turbininae, do not belong to Turbinidae and are not even assigned to Trochoidea (see Introduction and Table 1 for details).
On the basis of more recent molecular and morphological studies, Turbinidae was thought to include Turbininae, Prisogasterinae and Liotiinae (Williams & Ozawa 2006) or Turbininae, Prisogasterinae, Tegulinae, Margaritinae, Skeneinae and Tectus and Cittarium (the latter two possibly as members of Tegulinae) (Bouchet et al. 2005; Williams et al. 2008). However, molecular support for the inclusion of the last four groups was not particularly strong, and Williams et al. (2008) suggested that Skeneinae, Margaritinae, Tegulinae, Tectus and Cittarium could be considered as a group distinct from both Trochidae and Turbinidae, but more closely related to Turbinidae. However, they were treated in that study as subfamilies of Turbinidae based on the inclusion of Tegulinae and Skeneinae in Turbinidae by other studies (Bouchet et al. 2005). The results of this study do not support that decision.
In this study, as in previous molecular and morphological studies, Turbininae and Prisogasterinae form a well-supported clade (PP = 100%) (Hickman & McLean 1990; Williams et al. 2008). This group forms a clade with Tegulidae, Liotiidae, Tectus, Rochia and Cittarium which is well supported in the five-gene tree (Fig. 6; PP = 100%) and poorly supported in the four-gene tree. This suggests a close relationship among these groups as has been found in the previous studies. The five clades could be assigned familial rank (Turbinidae including Turbininae and Prisogasterinae, Tegulidae, Liotiidae and a clade with Tectus, Rochia and Cittarium), or all four clades could be considered to belong to Turbinidae. Because all four clades are morphologically distinct, I have chosen to assign familial rank to each clade. Turbinidae as defined here reverts to a more traditional concept of those species with robust, nacreous shells and thick, calcareous opercula.
Skeneidae and Margaritidae, which were also assigned to Turbinidae by Williams et al. (2008) and Bouchet et al. (2005; Skeneinae only) are not sister taxa to Turbinidae in any tree. The difference in the molecular results between the two studies is probably due to the difference in taxon sampling for these groups.
Relationships among families
Relationships among families are generally not well resolved, except for strong support for a sister relationship between Trochidae and Calliostomatidae and strong support in the 5-gene tree and weak support in the four-gene tree for a clade including Liotiidae, Tegulidae, Turbinidae, Tectus, Rochia and Cittarium (5, 6). The five-gene tree also clusters Liotiidae, Tegulidae, Turbinidae, Tectus, Rochia and Cittarium with Solariellidae and Margaritidae with high support.
These groupings are consistent with morphological as well as previous molecular studies discussed above. Further morphological support can be found in the fact that Tegula spermatozoa share more characters with turbinids than trochids (especially the length of the head and acrosome, and the acrosome length as percentage of total head length; Collado et al. 2008). Hickman & McLean (1990) also noted that the radula and neck lobes of Prisogaster are similar to the members of the Tegulidae. Moreover, further morphological similarities are the unusual ‘opercular fringe’ that is found in Liotiidae, which also occurs in the tegulid Norrisia (Hickman & McLean 1990).
Although not previously noted, T. pyramis also shares some sperm characteristics with turbinids; in that, they are larger and have a subacrosomal space (see fig. 3 in Collado et al. 2008). Other authors have suggested Cittarium is related to Tegula based on morphological characters (Celestino & Cáceres de Talarico 1984). Hickman & McLean (1990) considered it most closely related to their concept of Trochinae, which included Tectus. Beck (1995) considered it closely related to Trochus, Tectus and RubritrochusBeck 1995 based on shell, anatomical and radular characters.
Fossil record
Fossil records of trochiform molluscs (vetigastropods with shells lacking a slit or hole) are thought to have originated in the Ordovician; however, this is likely not a monophyletic group (Geiger et al. 2008). Fossils that can be unambiguously assigned to living trochoidean families extend no further back than the Triassic (Knight et al. 1960), with the oldest fossils for living genera found no earlier than Cretaceous or Jurassic (Hickman & McLean 1990).
Earlier suggestions that liotiids may have originated in the Permian have since been revised (McLean & Kiel 2007). Some of the earliest fossil genera originally assigned to Liotiidae are now assigned to Colloniidae (treated as a subfamily of Turbinidae by McLean & Kiel 2007). The earliest fossil record for liotiids is now thought to be from the Cretaceous (Pseudoliotina Cossmann, 1925, from the Campanian of northern Spain; McLean & Kiel 2007). Earlier records cited in the same paper are likely not trochoidean liotiids, but instead refer to the clade sister to Angariidae because the authors consider ‘Metriomphalus’segregatus Hebert and Eudes-Delongchamps, 1860 from the Middle Jurassic congeneric with Arene truncatosphaera Sohl, 1998, from the late Cretaceous of Puerto Rico (McLean & Kiel 2007).
Its long fossil record, wide distribution and species-rich families highlight the evolutionary success of Trochoidea s.s. Further study of extinct stem group taxa is needed.
Brooding
Following the new definition of Trochoidea as defined in this paper, brooding of young occurs in four trochoidean families. Two types of brooding have been observed in solariellids. Females have been observed to brood up to 15 developing larvae in their mantle cavity in two South African species of Solariellidae [Spectamen multistriatum (Thiele, 1925) and S. gerula (Herbert 1987)] (Herbert 1987) and New Zealand solariellids Solariella plicatula (Murdoch & Suter, 1906), S. luteola (Powell, 1937) and possibly S. vera (Powell, 1937) are umbilical brooders (Marshall 1999). Eggs and developing young are brooded in the umbilicus in three families: Solariellidae (as above), the new calliostomatid subfamily Margarellinae (e.g. M. refulgens; Arnaud 1972a) and females of some Margarites (belonging to the family Margaritidae). The sexes show such disparity of morphology in some species of Margarites that they were originally described as different species [e.g. Margarites vorticiferus (Dall, 1873); Lindberg & Dobberteen 1981; Lindberg 1985]. An unusual and rare form of brooding occurs in one trochid species. Larvae are brooded on the base of the shell in grooves between spiral cords in Clanculus bertheloti (d’Orbigny, 1840) (Thorson 1967).
Current records of brooding in Liotiidae are not correct. Liotiid-like genera known to brood eggs and juveniles in the umbilical cavity (e.g. Arene,Shasky 1968; STW pers. obs.; Cinysca, Leal 1991) are not members of Trochoidea.
This suggests that brooding has likely evolved independently on at least four occasions in Trochoidea. It does not appear to be correlated with habitat as species that brood occur in both deep-sea and shallow-water habitats, although most live in temperate water.
Further work
Detailed molecular phylogenetic studies have been published for the largest trochoidean family Trochidae (Williams et al. 2010), and the current concepts of Turbinidae (Williams 2007) and Tegulidae (Hellberg 1998), and phylogenetic studies of Solariellidae and Calliostomatidae are in preparation (by the author and others). However, other families, in particular Skeneidae would also benefit from detailed morphological and molecular studies. Several studies are underway (by Y. Kano, T. Kunze, A. Warén and other authors) including a generic revision by C. Hickman, and these are likely to be extremely helpful for resolving the relationships within families. A morphological monograph is also in preparation for Liotiidae, describing many new species (by J. McLean).
In a few years, there should be an almost complete molecular phylogeny for Recent Trochoidea, including many type species, which is particularly important for resolving generic assignments where non-monophyly is suspected. An almost complete phylogeny for the group will surely prove to be a considerable asset for testing evolutionary and biogeographic hypotheses on a much larger scale than is currently possible. Such a large-scale phylogeny with a much larger taxon set could also help confirm or refute systematic decisions based on molecular data that are not currently supported by morphology, such as the assignment of Margarellinae to Calliostomatidae.
Ackowledgements
I am especially grateful to L. Smith, P. Dyal, K. Hopkins, J. Llewellyn-Hughes and C. Griffin for their expert help in the molecular laboratory. I also thank D. Herbert, B. Marshall, A. Warén, P. Bouchet, C. Hickman, J. McLean, Y. Kano and one anonymous reviewer for expert, constructive comments that helped improve the manuscript. I am also grateful to C. Hickman for access to an early version of her manuscript prior to publication, D. Reid for radula dissection of the new calliostomatid species, J. McLean for identifying liotiid species, A. Warén for a photo of Gaza huloti, and as always to H. Taylor for beautiful photos of all other taxa. I am very much grateful to the many people who have provided samples for my studies. New samples first used in this study were kindly provided by K. Linse (all Antarctic samples), P. Kuklinski (Svalbaard and Aleutian Islands), A. Warén (Gaza huloti and Protolira thorvaldssoni) P. Marko and A. Marko (Friday Harbour) and G. Williams (Hong Kong). I am especially grateful to P. Bouchet, who provided most of the remaining samples and to B. Buge, P. Maestrati, N. Puillandre, V. Heros and P. Lozouet for co-ordinating the loans. The MNHN material used in this study was collected during the following expeditions: PANGLAO 2004 Marine Biodiversity Project [a joint project between University of San Carlos, Cebu City (co-PI D. Largo) and MNHN (co-PI P. Bouchet], funded by the Total Foundation and the French Ministry of Foreign Affairs); PANGLAO 2005 deep-sea expedition [a collaboration between the MNHN (PI, P. Bouchet) and the Philippines Bureau of Fisheries and Aquatic Resources (PI, L. Labe) and was supported by the Total Foundation for Biodiversity and the Sea, French Ministry of Foreign Affairs, the National University of Singapore and the University of San Carlos; the AURORA 2007 cruise [M/V DA-BFAR associated the National Museum of the Philippines (co-PI M. Manuel), MNHN (co-PI P. Bouchet) and BFAR, funded through a grant from the Lounsbery Foundation]; the MNHN-IRD-PNI Santo 2006 expedition (funded by grants from, among others, the Total Foundation and the Stavros Niarchos Foundation); EBISCO, SALOMON2 and TERRASSES cruises [R/V Alis deployed from Nouméa by the Institut de Recherche pour le Développement (IRD)]; TAIWAN 2001 [from chartered commercial trawler off Dashi, PI, T.Y. Chan] and MAINBAZA and MIRIKY expeditions to Mozambique and Madagascar in 2009-2010, funded by the Total Foundation, Prince Albert II of Monaco Foundation and Stavros Niarchos Foundation, and conducted by MNHN and Pro-Natura International (PNI). I am also grateful to the following people for help in the field: B. Richer de Forges (IRD, Noumea) M. Pandolfi (Service de l’environnement marin, Noumea), J.-J. Cassan (Department of Economic Development and Environment, Noumea) and M. Hendrickx (Unidad Academica Mazatlan, Mexico).
Appendix
Superfamily Trochoidea Rafinesque, 1815
Family Calliostomatidae Thiele, 1924
Subfamily Margarellinae Williams, new
Type genus: Margarella Thiele, 1893
Description: Calliostomatids with small shells (usually < 1 cm), of fewer than four whorls, a highly nacreous interior surface, and a glossy or dull external surface lacking sculpture or with spiral sculpture (lines, ribs or keels). The columella is thick and lacks denticles. Shells are not patterned, nor usually highly coloured; several species are white or pale shades of brown, rose or green. The umbilicus may be open or closed. Protoconchs do not have the reticulate, honeycomb pattern typical of many calliostomatids. The operculum is corneous and mulitispiral. When retracted it withdraws beyond the lip of the shell. The first marginal plate of the radula forms a protolateromarginal plate in most species. The foot has two horn-like projections. [Correction added on 21 August 2012, after first online publication: The above Appendix section has been added].




