Ophthalmomyiasis interna anterior in a cat: surgical resolution
Abstract
A 4-year-old Domestic short-haired cat was presented for severe anterior uveitis in the right eye associated with a Cuterebra spp. larva in the anterior chamber. This report describes successful surgical removal of the parasite with preservation of vision.
INTRODUCTION
Ophthalmomyiasis is the presence of dipteran fly larvae in the orbit or adnexa (ophthalmomyiasis externa) or within the globe (ophthalmomyiasis interna). Ophthalmomyiasis interna has been uncommonly reported in humans,1–4 dogs,5,6 and cats.5,7–10 In the two cats that have been reported to date in which a larva was surgically removed from the anterior segment, blindness resulted from retinal degeneration in one cat8 and severe corneal edema and retinal degeneration in the other cat.9 This report describes the successful surgical removal of a Cuterebra spp. larva from the anterior chamber of a cat with the preservation of vision.
CASE REPORT
A 4-year-old female spayed, Domestic short-haired cat was presented to an emergency clinic with a 1-day history of blepharospasm and a serous ocular discharge in the right eye (OD). The referring veterinarian administered 1% atropine ointment (Fougera, Mellville, NY, USA), flurbiprofen (Bausch & Lomb, Tampa, FL, USA), and artificial tear ointment topically OD, and prednisone 5 mg PO q 12 h. The cat was referred the following day. A general physical examination revealed no significant abnormalities. On ophthalmic examination, the cat had a negative menace response OD, a positive dazzle reflex OD, and a positive menace response and dazzle reflex in the left eye (OS). The direct and consensual pupillary light reflexes (PLR) were absent OD as a result of pharmacologic blockage with atropine. Direct and consensual PLRs were positive OS as was the remainder of the examination in this eye. The right globe had periocular swelling and lagophthalmos, with an approximately 4 mm gap in the closure of the palpebral fissure. The third eyelid was elevated and the conjunctiva was moderately hyperemic. There was an approximately 3 mm hyperemic raised area on the dorso-lateral bulbar conjunctiva 2 mm posterior from the limbus. There were 2 superficial areas in the central cornea that retained fluorescein dye. Intraocular pressures (IOP) were normal bilaterally (OD = 12 mmHg, OS = 17 mmHg). An approximately 5-mm linear segmented structure, identified as a dipteran larva, was present in the anterior chamber (Fig. 1). The larva was adherent to the anterior surface of the iris and was immobile and presumed dead. Grade III/IV aqueous flare was present along with fibrin. The fundus in this eye could not be visualized because of the flare and fibrin.
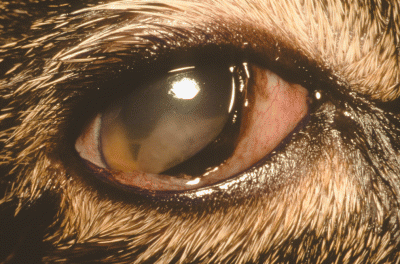
Photograph of the right eye of a 4-year-old Domestic short-haired cat at presentation for ophthalmomyiasis interna. Note the curved structure, identified as a Cuterebra larva, in the anterior chamber. Fibrin covers the larva.
A complete blood count (CBC), serum chemistry panel, feline leukemia virus, feline immunodeficiency virus, and feline heartworm antibody tests were performed. Significant abnormalities on the CBC and chemistry panel included a mild hyperproteinemia 7.9 g/dL (normal 5.5–7.1 g/dL), hyperglobulinemia 4.6 g/dL (normal 2.3–3.8 g/dL), decreased albumin to globulin ratio 0.7 (normal 0.8–1.5), and a marked eosinophilia 2.77 × 103/µL (normal 0.1–1.5 × 103/µL). The cat was negative for feline leukemia and feline immunodeficiency viruses, as well as for heartworm antibody.
The cat was initially treated with flurbiprofen q 4 h (Bausch & Lomb), ofloxacin q 4 h (Ocuflox, Allergan, Hormigueros, Puerto Rico, USA), bacitracin-neomycin-polymixin B ointment q 6 h (Akorn, Buffalo Grove, IL, USA), artificial tear ointment q 8 h (Puralube, Fougera), amoxicillin-clavulanic acid 62.5 mg PO q 12 h (Clavamox, SmithKline Beecham, Research Triangle Park, NC, USA), and prednisone 5 mg (1.5 mg/kg) PO q 12 h (Falcon, Ft. Worth, TX, USA). During the next 24 h, the periocular swelling and lagophthalmos resolved, although the anterior uveitis worsened. The larva was more difficult to visualize because of an increased amount of fibrin in the anterior chamber. The decision was made to surgically remove the larva from the anterior chamber because of the worsening anterior uveitis.
The cat was premedicated with acepromazine (0.08 mg/kg) (Phoenix Pharmaceutical, St. Joseph, MO, USA) and butorphanol (0.2 mg/kg) (Torbugesic, Fort Dodge, IA, USA) intramuscularly. General anesthesia was induced with intravenous pentothal (10 mg/kg) (Abbott Laboratories, Abbott Park, IL, USA) and maintained with isoflurane and oxygen. Immobilization of the eye was achieved with atracurium besylate (0.2 mg/kg) (Mayne Pharma, Paramus, NJ, USA), and ventilation was controlled with a mechanical ventilator. A 4-mm lateral canthotomy was performed to enhance exposure of the globe. Following a limbal entry to the eye at the 10 o’clock position, a viscoelastic substance (Hylartin V, Pharmacia & Upjohn, New York, NY, USA) was injected to maintain anterior chamber inflation. An iris hook was used to remove the larva and fibrin clot from the anterior chamber. A portion of the fibrin clot was adhered to the anterior iridal surface and could not be removed without causing damage to the iris. The corneal incision was closed with 9-0 polyglactin 910 (Vicryl, Ethicon Inc, Somerville, NJ, USA) and the lateral canthotomy was closed with 5-0 polyglactin 910 (Vicryl). An intracameral injection of 25 µg of tissue plasminogen activator (Activase, Genentech, Vacaville, CA, USA) was given to lyse the remaining fibrin in the anterior chamber. A sample of aqueous humor and fibrin was submitted for cytology and aerobic bacterial culture and sensitivity. Cytology revealed a severe pyogranulomatous and eosinophilic inflammation. There was no growth on the bacterial culture. The parasitology report identified the dipteran larva removed from the anterior chamber OD as a first instar Cuterebra spp. larva based on its characteristic bands of black spines separating each segment, spiracles, and a posterior adhesive organ (Fig. 2).
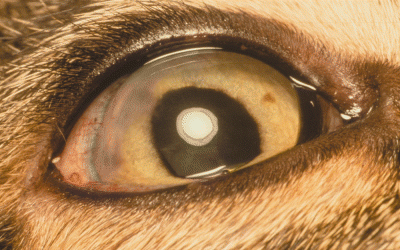
Photograph of the right eye 9 weeks following surgery. Mild conjunctival hyperemia remains and a corneal scar is present at the surgical site.
One day postoperatively, the cat was visual OU as assessed by menace and tracking tests. IOPs were normal (OD = 14 mmHg, OS = 14 mmHg). A small fibrin clot, grade III/IV aqueous flare, and iris congestion were present OD. The fundic exam was normal OU using indirect ophthalmoscopy, although the view OD was hazy. Following 20 min of dark adaptation, a scotopic, white single-flash electroretinogram (ERG) showed normal retinal responsiveness in both eyes, although the amplitude was lower OD than OS (284 µV and 558 µV). The cat was discharged on flurbiprofen q 6 h, ofloxacin q 6–8 h, bacitracin-neomycin-polymixin B ointment q 8 h, predisone 5 mg q 12 h PO, and amoxicillin-clavulanic acid 62.5 mg PO q 12 h.
One week postoperatively the cat appeared more comfortable in the right eye. On a general physical examination no significant abnormalities were detected. On ophthalmic examination the cat was visual OU as assessed by menace and tracking tests. Neither cornea retained fluorescein dye. IOPs were normal (OD = 13 mmHg, OS = 9 mmHg). Mild anisocoria was still present with the right pupil larger. Direct and consensual PLRs were positive OU. Mild rubeosis iridis was present as well as grade I/IV aqueous flare OD. A focal area of anterior synechia was noted at the incision site. An anterior subcapsular cortical cataract was present OD and fundic exam was normal OU. The cat was discharged with atropine 1% ointment q 48 h (Fougera) ofloxacin q 6 h, prednisone acetate 1% q 8 h (Falcon), amoxicillin-clavulanic acid 62.5 mg PO q 12 h, and prednisone 2.5 mg PO q 12 h.
Three weeks postoperatively, the cat was comfortable and vision was present OD. Neither cornea retained fluorescein dye, and IOP were normal. Anisocoria was still present resulting from atropinization OD. Trace aqueous flare was present and the anterior cortical cataract was unchanged. The fundic exam remained normal. Atropine was discontinued. Medications were changed to ofloxacin q 12 h, prednisolone acetate 1% q 12 h for 3 weeks then q 24 h, amoxicillin-clavulanic acid 62.5 mg PO q 12 h for 1 week, prednisone 2.5 mg q 48 h PO for 1 week and then discontinued.
Nine weeks postoperatively, the cat had vision OD (Fig. 3). IOP were normal (OD = 15 mmHg, OS = 14 mmHg), and the direct and consensual PLRs were positive OU, although the reflexes were incomplete OD. The fundic exam was normal OU and the cataract OD had not changed. Following 20 min of dark adaptation, a scotopic, white single-flash ERG showed normal retinal responsiveness OU, with similar amplitudes in each eye (317 µV OD, 310 µV OS). All medications were discontinued.
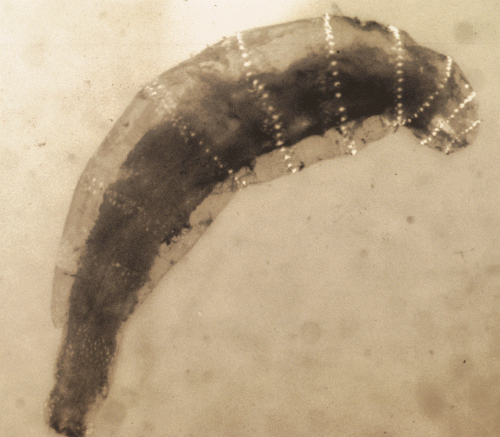
Photograph of the dipteran larva removed from the anterior chamber. The larva was identified as a first instar Cuterebra larva based on its characteristic bands of black spines separating each segment, spiracles, and a posterior adhesive organ.
The cat was examined again 6 months after surgery. At this time the right eye was unchanged from previous visits. The eye was visual and comfortable; there was no progression of the cataract and the fundus was normal.
DISCUSSION
Ophthalmomyiasis interna anterior occurs when a fly larva migrates into the anterior chamber, either from the posterior segment or from direct penetration of the cornea or sclera. Ophthalmomyiasis interna posterior involves invasion of the posterior segment of the globe. Characteristic lesions of ophthalmomyiasis interna posterior are visualization of the larva within the vitreous cavity, or wandering, intersecting, curvilinear tracts in the tapetal and nontapetal fundi with or without preretinal or retinal hemorrhages. In humans, the most common cause of ophthalmomyiasis externa is infection with the sheep nasal botfly, Oestrus ovis.11 In human cases of ophthalmomyiasis interna in which the larvae could be identified, the most common species was the cattle botfly Hypoderma bovis.12 However, human cases of ophthalmomyiasis interna caused by Cuterebra spp. have been reported.1,3,13,14 The most common cause of ophthalmomyiasis interna in the cat and dog, in which the larva was identified, was Cuterebra spp.8–10
Cuterebra spp. infect rabbits, squirrels, chipmunks, mice, cats, dogs, and occasionally man.16 The adult female Cuterebra fly lays up to 1000 eggs on the vegetation around lagomorph and rodent trails and nests. The eggs hatch into motile first stage larvae in approximately 1 week with the right temperature and moisture conditions. The larva usually enters the host through its natural body openings such as the nose, mouth, anus, or eyes.16–19 In rodents and lagomorphs, the larva migrates to the subcutis and cuts warble pores (respiratory holes) where it matures to second-stage larva. After several weeks of development, the third-stage larva leaves the host and burrows into the soil, and later emerges as an adult fly.19 A larva can enter the eye by penetrating the conjunctiva and sclera, the optic nerve, or an intraocular blood vessel.2,10,13,20–22 In the cat reported here, it is possible that the egg or larva was deposited on the conjunctival surface by the adult female fly, a vector such as a tick or mosquito, or the cat's contaminated extremities. The infective larva likely penetrated the sclera dorso-laterally where the conjunctival lesion was identified, then entered the anterior chamber.
Ophthalmic sequelae from ophthalmomyiasis interna can range from mild inflammation and minimal damage to the ocular structures to severe inflammation and vision loss.5,7–10,23 The clinical signs and prognosis may vary depending upon the viability of the larva and its location in the eye.7 It has been proposed that larvae within the vitreous incite less inflammation because the avascular vitreous may protect the eye from toxic or immunologic damage from the dead larvae.24,25 The inflammation may be caused by toxic or enzymatic materials that are produced by the larva14 or by an immunologic reaction.26 The inflammation may be more severe after death of the larva.14,24 It has been speculated that the subretinal space may provide better nutrition for the larva than the anterior chamber.15,27 As the larva migrates from the subretinal space into the anterior chamber, the oxygen tension is reduced and the larva may die.14 When the larva dies within the anterior chamber or the subretinal space, the larval antigens can be exposed to the immune system and an inflammatory response may occur.14
In the anterior segment, dipteran larvae can cause iridocyclitis, hypopyon, dislocation of the lens, and retinal degeneration.8,9,13,27 Two cases of feline ophthalmomyiasis interna anterior with Cuterebra spp. resulted in severe anterior uveitis, cataract, diffuse retinal degeneration, and loss of vision.8,9 In contrast, four other reported cases of feline ophthalmomyiasis interna posterior resulted in only minimal intraocular inflammation.5,7 In the most recent report, a cat with a dipteran larva in the vitreous presented with depression and abnormal mentation.10 Cerebrospinal fluid from this cat had a severe mixed inflammatory profile, although no histopathologic abnormalities were found in the central nervous system following euthanasia for a worsening condition. There was coagulation necrosis and hemorrhage of the optic nerve, retina, and choroid in the affected eye, as well as mild fibrinous and eosinophilic anterior uveitis. Different locations of the larvae in these cases, as well as possible different species of larvae, could account for the various degrees of inflammation and the different outcomes.
There are two reports in which a Cuterebra larva has been surgically removed from the anterior chamber of a cat.8,9 In these two cases, the larvae were removed 5 and 91 days after the onset of clinical signs. In one case, there were no signs of subretinal migration of the larva and the cat was visual 2 weeks following surgery.8 Six weeks following surgery, however, the cat was blind in the operated eye as a result of retinal degeneration. The authors theorized that toxic substances released from the dead larva may have affected the retina. Electroretinography was performed in the cat reported here on two occasions to document retinal function following surgery. In the other reported case, severe corneal edema persisted following surgery and an immature cataract developed.9 Examination of the fundus was difficult because of anterior segment opacity, but diffuse tapetal hyper-reflectivity was noted. Diffuse unilateral subacute neuroretinitis is a condition in humans that is characterized by severe vision loss because of toxic damage resulting from the aberrant subretinal migration of nematode larvae.28,29 Direct damage to the neurosensory retina from migrating parasites has also resulted in decreased vision.30
The management of ophthalmomyiasis depends on the location of the parasite and degree of inflammation. In human medicine it has been suggested that if the inflammation is minimal and can be controlled with topical corticosteroids and cycloplegics, the condition may not require surgical removal of the larvae.1,24,27,31 If the inflammation does not respond to treatment, surgical removal of the larva is recommended.13 Photocoagulation with an argon laser of live subretinal larvae causes only minimal inflammation in human patients. The minimal inflammation may be a result of the denaturing of the larval proteins, which are then less antigenic.25,32 The limited number of reported cases of ophthalmomyiasis interna in the veterinary literature makes any conclusions regarding the best therapy difficult.
In the case reported here the choice was made to remove the dead larva in an attempt to preserve vision and decrease intraocular inflammation, which was not responding adequately to medical therapy alone. The positive outcome of this case suggests that early surgical intervention for ophthalmomyiais interna anterior may result in the preservation of vision in some cases.




