Mhp182 (P102) binds fibronectin and contributes to the recruitment of plasmin(ogen) to the Mycoplasma hyopneumoniae cell surface
Summary
Mycoplasma hyopneumoniae is a major, economically damaging respiratory pathogen. Although M. hyopneumoniae cells bind plasminogen, the identification of plasminogen-binding surface proteins and the biological ramifications of acquiring plasminogen requires further investigation. mhp182 encodes a highly expressed 102 kDa protein (P102) that undergoes proteolytic processing to generate surface-located N-terminal 60 kDa (P60) and C-terminal 42 kDa (P42) proteins of unknown function. We show that recombinant P102 (rP102) binds plasminogen at physiologically relevant concentrations (KD ∼ 76 nM) increasing the susceptibility of plasmin(ogen) to activation by tissue-specific plasminogen activator (tPA). Recombinant proteins constructed to mimic P60 (rP60) and P42 (rP42) also bound plasminogen at physiologically significant levels. M. hyopneumoniae surface-bound plasminogen was activated by tPA and is able to degrade fibrinogen, demonstrating the biological functionality of M. hyopneumoniae-bound plasmin(ogen) upon activation. Plasmin(ogen) was readily detected in porcine ciliated airways and plasmin levels were consistently higher in bronchoalveolar lavage fluid from M. hyopneumoniae-infected animals. Additionally, rP102 and rP42 bind fibronectin with KDs of 26 and 33 nM respectively and recombinant P102 proteins promote adherence to porcine kidney epithelial-like cells. The multifunctional binding ability of P102 and activation of M. hyopneumoniae-sequestered plasmin(ogen) by an exogenous activator suggests P102 plays an important role in virulence.
Introduction
Mycoplasma hyopneumoniae is one of the most economically significant pathogens impacting swine production worldwide. M. hyopneumoniae is the causative agent of porcine enzootic pneumonia (PEP), a chronic non-fatal respiratory disease. M. hyopneumoniae is considered to be an extracellular pathogen. Affected airways show M. hyopneumoniae predominantly attached to ciliated epithelial surfaces with colonies adhering at the tips and along the length of cilia, and in contact with microvilli (Mebus and Underdahl, 1977; Tajima and Yagihashi, 1982; Blanchard et al., 1992; Jacques et al., 1992). These events compromise the mucociliary clearance mechanism by inducing ciliostasis, cilial shedding and epithelial cell death, exposing subepithelial sites (Mebus and Underdahl, 1977; Blanchard et al., 1992; Jacques et al., 1992). Epithelial cell regeneration underpins repair of the mucociliary clearance system, which is likely to stimulate the expression of fibronectin and its receptor α5β1 (Herard et al., 1996). An inflammatory response, ranging from limited accumulation of polymorphonuclear cells surrounding the airways in the early stages of disease, to extensive bronchiolar and lymphoreticular hyperplasia with accumulation of monocytes in peribronchiolar and perivascular sites surrounding infection, is typical of infections caused by M. hyopneumoniae (Baskerville and Wright, 1973; Blanchard et al., 1992).
Adhesins belonging to two paralogue families sharing sequence similarity with P97 (Mhp183) and P102(Mhp182) (Minion et al., 2004; Vasconcelos et al., 2005) have been described in M. hyopneumoniae. These high-molecular-weight proteins (> 100 kDa) are often highly expressed and contain a single N-terminal transmembrane domain that is not removed during secretion to the cell surface. They are targets of endoproteolytic cleavage events that generate smaller products displayed on the M. hyopneumoniae cell surface (Djordjevic et al., 2004; Burnett et al., 2006; Wilton et al., 2009; Seymour et al., 2010). These proteolytic fragments are known to bind epithelial cilia, extracellular matrix components and other key host circulatory molecules (Burnett et al., 2006; Jenkins et al., 2006; Wilton et al., 2009; Deutscher et al., 2010; Seymour et al., 2010; Seymour et al., 2011).
Mycoplasma hyopneumoniae cells bind plasminogen in a dose-dependent and saturable manner (Seymour et al., 2010). Plasminogen is a proenzyme of the serine protease plasmin, which is involved in the degradation of fibrin clots and migration of cells via degradation of extracellular matrix proteins (Plow et al., 1995; Lähteenmäki et al., 2001). The ability to recruit plasminogen to the cell surface promotes the virulence of a number of bacterial species, often enabling the degradation of extracellular matrix and basement membranes and thereby facilitating host invasion (Coleman and Benach, 1999; Lähteenmäki et al., 2001; Yavlovich et al., 2001; Bower et al., 2003; Bergmann et al., 2004; Walker et al., 2005). The capacity of M. hyopneumoniae to bind plasminogen therefore warrants further investigation.
Fibronectin is a highly abundant multi-functional glycoprotein that circulates in body fluids (soluble or plasma fibronectin), is deposited on cell surfaces and is an extracellular matrix component (McDonald, 1988). Fibronectin is also important in wound repair of respiratory epithelial cells (Coraux et al., 2008). The capacity to bind fibronectin is widespread among bacteria including M. hyopneumoniae, several other Mycoplasma species, viruses, fungi and protozoa (Giron et al., 1996; Joh et al., 1999; Grundmeier et al., 2004; May et al., 2006; Balasubramanian et al., 2008; Deutscher et al., 2010; Henderson et al., 2010; Seymour et al., 2010) and facilitates bacterial adherence and invasion of a variety of host cells (Henderson et al., 2010).
mhp182 encodes P102 (GenBank Accession No. AE017332) and is located in the same operon as mhp183, which encodes the archetype cilium adhesin P97 (Hsu and Minion, 1998b; Adams et al., 2005). Transcription of these genes occurs during disease (Adams et al., 2005). P97 is a highly expressed, proteolytically processed protein that binds porcine epithelial cilia and a range of glycosaminoglycans (Zhang et al., 1995; Wilton et al., 1998; Hsu and Minion, 1998a; Djordjevic et al., 2004; Jenkins et al., 2006). While P102 is the archetype of the P102 family of M. hyopneumoniae surface molecules, its function remains unknown. The M. hyopneumoniae J strain (GenBank Accession No. NC_007295) P102 protein is known to be cleaved at amino acid 556 (Djordjevic et al., 2004). The resulting C-terminal 349-residue fragment has a predicted mass of ∼40 kDa (P42), a pI of 9.3 and the sequence 556AEEAKG561 as the N-terminus (Djordjevic et al., 2004). This sequence is also conserved in P102 (Mhp182) from strains 232 and 7448 (MHP_0199) (Vasconcelos et al., 2005), suggesting that cleavage occurs at this site in these M. hyopneumoniae strains. Here we investigate the ability of P102 and its naturally occurring cleavage fragments to bind plasminogen and fibronectin. The ability of M. hyopneumoniae-bound plasminogen to be activated and become biologically functional as the serine protease plasmin is also examined.
Results
Proteomic and molecular analyses of P102 and P102 cleavage fragments
P102 is 904 amino acids in length and contains a signal peptide sequence (Adams et al., 2005). Analysis of the P102 sequence using the TmPred algorithm (http://www.ch.embnet.org/software/TMPRED_form.html) identified a single putative transmembrane domain between amino acids 11–34 (score 2095). Bioinformatic analyses failed to identify any known binding motifs within P102, but the region from amino acid 7–359 of P102 displays a level of similarity with the LppT protein from Mycoplasma conjunctivae (identity 24%, positive 44%). LppT contains a RGD motif involved in host cell adherence (Zimmermann et al., 2010).
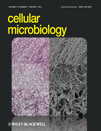
Immunoblot analysis of a M. hyopneumoniae strain 232 whole-cell lysate probed with antisera raised to purified rP102 identified three proteins with masses of ∼100 kDa (P102 pre-protein), ∼72 kDa (P72) and ∼42 kDa (P42) (Djordjevic et al., 2004). The N-terminal cleavage fragment (P72) has not been identified previously by mass spectrometry (MS) and the cellular location of these cleavage fragments has not been determined in M. hyopneumoniae. To elucidate the location of P72 and P42, Triton X-114-fractionated proteins of M. hyopneumoniae were resolved by SDS-PAGE followed by characterization using liquid chromatography (LC)-MS/MS. Tryptic peptides that specifically aligned to the 60 kDa N-terminal and 42 kDa C-terminal portion of P102 were generated from proteins that resolved with masses of ∼55–70 kDa and ∼40–55 kDa respectively (Fig. 1A and B). As expected, peptide matches partitioned either side of the amino acid 556 cleavage site identified by Djordjevic et al. (2004). These data indicate that the original mass of 72 kDa given to the larger of the P102 cleavage fragments was over estimated and we have reassigned this fragment as P60. This is the first time P60 has been definitively characterized by LC-MS/MS, while the identity of P42 was reconfirmed by LC-MS/MS in this study. The band at ∼102 kDa (P102 pre-protein) appears inconsistently in immunoblot experiments and we were unable to confirm the presence of P102 by performing LC-MS/MS on proteins with masses of 90–150 kDa excised from an SDS-PAGE gel.
Proteomic analysis confirms that P102 is cleaved to produce P60 and P42. A. SDS-PAGE of Mycoplasma hyopneumoniae strain J whole-cell lysate. The gel was cut into 16 equal slices, in-gel trypsin digested and analysed by LC-MS/MS. Sizes of molecular mass markers are in kDa. Indicated are regions where P102 cleavage fragments P60 and P42 are located. B. Tryptic peptide matches to P60 (bold) and P42 (bold, underlined) fragments of P102 from slice 7 and slice 8 (numbered sequentially from the top, A) respectively were identified by LC-MS/MS. The sequence determined by Edman degradation that delineates the N-terminus of P42 (Djordjevic et al., 2004) is boxed.
Cell surface location of P102, P60 and P42
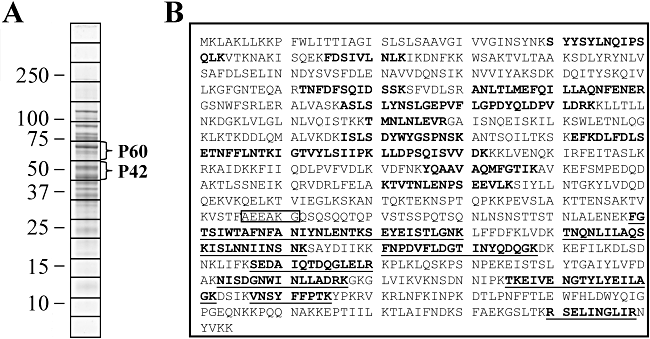
Immunogold labelling experiments of M. hyopneumoniae cells derived from broth culture showed proteins recognized by anti-P102 antisera were not cell-associated but were found clustered in an extracellular matrix (Djordjevic et al., 2004). In similar experiments conducted on respiratory tissues from M. hyopneumoniae-infected pigs, anti-P102 antisera labelled with gold particles were observed within and on the cell surface of mycoplasmas and on the surface of cilia, with gold particles often clustered at high concentration at each of these sites (Adams et al., 2005). These data indicate that P102 or its cleavage fragments P60 and P42 are present on the surface of M. hyopneumoniae, but it is not clear precisely which of these proteins are present on the cell surface. In this study, enzymatic cell surface shaving experiments were used to determine if P102, P60 and P42 reside on the cell surface of M. hyopneumoniae. Freshly cultured, washed cells were exposed (5 min) to trypsin (50 µg ml−1) and the shaved products were harvested for further analyses. LC-MS/MS of tryptic digests of these products identified nine unique peptides spanning amino acids 60–806 in P102 (Fig. 2A). To provide further evidence that P102 and/or its cleavage fragments P60 and P42 are present on the surface of M. hyopneumoniae, immunoblot studies of trypsin-shaved M. hyopneumoniae cells were performed (Fig. 2B and C). P60 and P42 were found to be digested by concentrations of trypsin of 50 µg ml−1 and above, while L7/L12, a control cytosolic protein (Burnett et al., 2006), remained intact with treatment of trypsin at 300 µg ml−1. These findings suggest both P60 and P42 are located on the cell surface.
P102 proteins are located on the surface of the M. hyopneumoniae cell. A. Peptide matches (bold, underlined) to P60 and P42 fragments of P102 from enzymatic cell surface shaving experiments were identified by LC-MS/MS. Boxed is the N-terminal sequence of P42. B and C. Immunoblots of M. hyopneumoniae cells treated with trypsin before preparation as whole-cell lysates. Cells were treated with 0 (lane 1), 1 (lane 2), 5 (lane 3), 10 (lane 4), 50 (lane 5) and 300 µg ml−1 trypsin (lane 6). Proteins were separated by SDS-PAGE and transferred onto PVDF membrane. Blots were probed with polyclonal rabbit sera raised against rP102 (B) or L7/L12, a cytosolic protein, to act as a control for cell lysis (C). D. M. hyopneumoniae proteins with observed sizes comparable to P102 and P42 bind plasminogen. Ligand blots of M. hyopneumoniae Triton X-114 detergent phase proteins separated by SDS-PAGE and transferred onto PVDF membrane. Lane 1 was probed with polyclonal rabbit serum raised against rP60. Lane 2 depicts the blot shown in lane 1, re-probed with polyclonal rabbit serum raised against rP42. Lane 3 shows a ligand blot incubated with biotinylated porcine plasminogen and reacted with ExtrAvidin® peroxidase conjugate. E. SDS-PAGE of purified recombinant rP102 (lane 1), rP60 (lane 2) and rP42 (lane 3) proteins stained with Coomassie blue. Sizes of molecular mass markers are in kDa. F. Graphical representation of M. hyopneumoniae protein Mhp182, with a transmembrane domain and signal peptide sequence represented as an unfilled region. Shown are the recombinant proteins constructed for this study with amino acid positions noted.
Frequently a C-terminal lysine residue contributes to plasminogen binding sites (Plow et al., 1995; Sanderson-Smith et al., 2007). Two lysine residues are located at the C-terminus of P102 and this prompted our investigation of the potential of P102 to bind plasminogen. Ligand blots of the Triton X-114 detergent phase of the M. hyopneumoniae proteome detected plasminogen-binding proteins which correspond in size to P102 and P42 (Fig. 2D). Recombinant polyhistidine tagged proteins of P102 (rP102) and the known cleavage products P60 and P42 (rP60 and rP42) were expressed and purified (Fig. 2E and F) to enable further investigation of the potential role of P102 as a plasminogen-binding protein.
P102 domains bind plasminogen increasing the susceptibility of plasmin(ogen) to activation by tPA
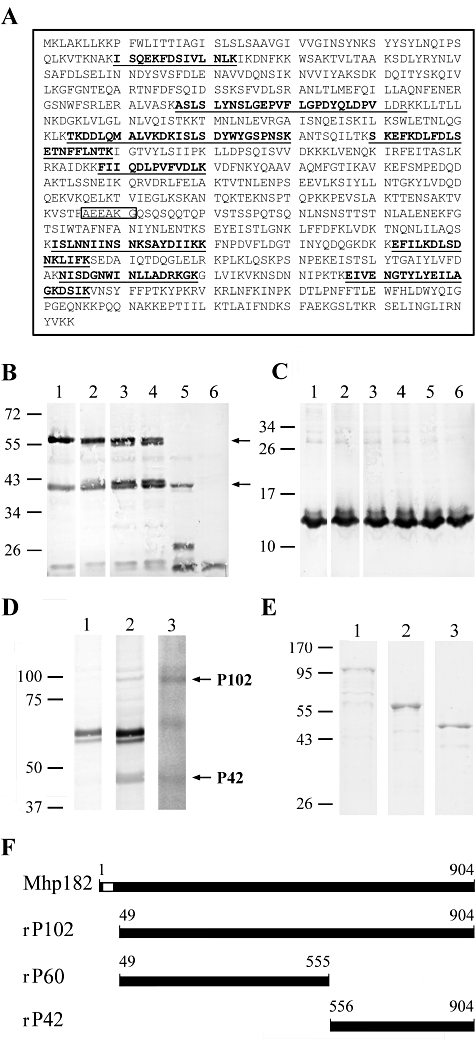
To determine if P102 and its cleavage products bind plasminogen we utilized surface plasmon resonance to characterize real-time interactions of these molecules with plasminogen. rP102 bound plasminogen in a dose-dependant manner at physiologically relevant concentrations with a KD of 76 ± 19 nM; ka = (1.0 ± 1.0) × 105 M−1 s−1 (Fig. 3A–E). Deletion of the tandem C-terminal lysine residues from rP102 (rP102ΔKK) did not abolish plasminogen binding, and although the KD did not change significantly (84 ± 8 nM), the level of binding was noticeably reduced. rP60 does not possess a C-terminal lysine, but an affinity for plasminogen with low binding levels was still observed [KD 109 ± 47 nM; ka = (4.8 ± 6.3) × 105 M−1 s−1]. The C-terminal P42 fragment still contains the two C-terminal lysine residues of P102. rP42 bound plasminogen in a dose-dependant manner at physiologically relevant concentrations [KD 37 ± 15 nM; ka = (7.4 ± 6.7) × 104 M−1 s−1]. Deletion of the C-terminal lysine residues from rP42 (rP42ΔKK) abolished the majority of observed binding, although binding was still physiologically relevant [KD 88 ± 7 nM; ka = (2.0 ± 1.7) × 105 M−1 s−1]. Binding of plasmin(ogen) by lysine residues alters the conformation of plasminogen rendering it more susceptible to activation (Lahteenmaki et al., 2005). C-terminal lysine residues appear to contribute to P102 binding plasminogen. To determine if binding by P102 increases the susceptibility of plasminogen to activation we investigated the proteolytic activity of P102-bound plasmin(ogen). Plasminogen was mixed with a tissue-specific plasminogen activator (tPA) following pre-incubation with rP102 and plasmin activity was determined with a plasmin-specific chromogenic substrate. Porcine plasmin(ogen) was more readily activated in the presence of rP102 compared with the tPA control (Fig. 3F) suggesting that plasminogen undergoes a conformation change upon binding by P102.
P102 binds plasminogen enhancing activation by tissue-specific plasminogen activator (tPA) with C-terminal lysine residues contributing to plasminogen binding. A–E. The sensorgrams depict binding of immobilized plasminogen by recombinant proteins rP102 (A), rP102ΔKK (B), rP60 (C), rP42 (D) and rP42ΔKK (E). Analyte was injected at 10 (bottom), 50, 100 and 200 (top) nM for 600 s at 10 µl min−1 over plasminogen. Arrows indicate the end of the injection period at which point dissociation of the analyte from plasminogen is observed. F. Porcine plasminogen was pre-incubated with rP102 prior to the addition of tPA. rP102 was pre-incubated with plasminogen at a molar ratio of 0.5:1 (▴), 1:1 (●) and 4:1 ( ). Plasminogen only (Δ) and plasminogen with the addition of tPA (□) were used as controls. Activation of plasmin(ogen) was measured by the addition of a plasmin-specific chromogenic substrate, Spectrozyme PL. Data represent the mean values ± standard error from a representative assay performed in duplicate and corrected to readings taken immediately after the addition of the Spectrozyme PL.
). Plasminogen only (Δ) and plasminogen with the addition of tPA (□) were used as controls. Activation of plasmin(ogen) was measured by the addition of a plasmin-specific chromogenic substrate, Spectrozyme PL. Data represent the mean values ± standard error from a representative assay performed in duplicate and corrected to readings taken immediately after the addition of the Spectrozyme PL.
Activation of plasminogen bound by M. hyopneumoniae
Although M. hyopneumoniae binds plasminogen to its cell surface (Seymour et al., 2010), the potential for activation of this bound plasminogen has not been previously investigated. Activation of the bound zymogen to the serine protease plasmin is required for M. hyopneumoniae to utilize plasmin(ogen) as a potential mechanism for proteolysis of fibrin matrices, evasion of the innate immune system or to enable degradation and invasion of tissue barriers (Lähteenmäki et al., 2001; Degen et al., 2007). We have shown that activation of plasminogen by tPA is accelerated when plasminogen is pre-incubated with rP102. To determine whether M. hyopneumoniae surface-bound plasminogen can be converted to plasmin, M. hyopneumoniae cells were incubated with plasminogen and tPA and proteolytic activity was measured using a plasmin-specific chromogenic substrate. Human and porcine plasminogen were cleaved to form plasmin by human tPA (Fig. 4A). Porcine plasminogen was not activated as readily as human plasminogen, but it has previously been shown that human plasminogen is more readily activated than porcine plasminogen irrespective of the origin of the activator (Nieuwenhuizen and Keyser, 1985; Flight et al., 2006). No substrate cleavage was detected when tPA was excluded from the assay, indicating that M. hyopneumoniae does not produce its own activator. Minimal activation was seen when the bacteria were incubated with tPA only, which is most likely the result of their plasminogen recruitment (porcine and equine) from serum, since this is an essential component of the Friis medium routinely used for the culture of M. hyopneumoniae. Western blot analysis of M. hyopneumoniae whole-cell lysates with anti-porcine plasminogen antibodies illustrated that M. hyopneumoniae cells are able to recruit plasmin from Friis medium (Fig. 4B and C).
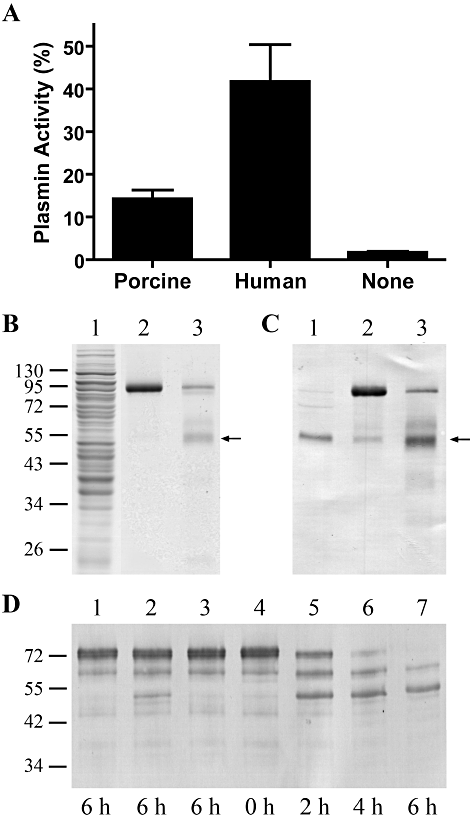
M. hyopneumoniae-bound plasminogen is activated by tPA to biologically active plasmin. A. Activation of M. hyopneumoniae surface-bound plasmin(ogen). M. hyopneumoniae cells were coated onto microtitre plates and incubated with 5 µg of human or porcine plasminogen, followed by the addition of a saturating amount of tPA. Activation of surface-bound plasminogen was measured by the addition of a plasmin-specific chromogenic substrate, Spectrozyme PL. Results are expressed as percentage of total plasmin activity after 5 h incubation with Spectrozyme PL. Cells incubated with no plasminogen were used as a negative control. Data represent the mean values ± standard error of two assays performed in triplicate. B and C. Plasmin is present in the whole-cell lysate of washed M. hyopneumoniae 232 cells. Proteins in M. hyopneumoniae whole-cell lysate (lane 1), porcine plasminogen (lane 2) and tPA-activated porcine plasminogen (lane 3), were separated by 12% SDS-PAGE and stained with Coomassie blue (B). Proteins in a duplicate gel were transferred onto PVDF membrane and probed with anti-porcine plasminogen antibodies (C). The arrows indicate the presence of plasmin. Molecular mass markers with sizes in kDa are on the left. D. M. hyopneumoniae cells coated with plasminogen can degrade fibrinogen in the presence of tPA. Cells were incubated with or without plasminogen, in the presence or absence of tPA, at 37°C before incubation with fibrinogen. M. hyopneumoniae cells only (lane 1), M. hyopneumoniae cells in the presence of tPA (lane 2), M. hyopneumoniae cells coated with plasminogen in the absence of tPA (lane 3), M. hyopneumoniae cells coated with plasminogen in the presence of tPA (lanes 4–7). The length of the incubation period with fibrinogen is indicated in hours below each lane. The reaction mixtures were separated by SDS-PAGE, transferred onto PVDF membrane and probed with anti-human fibrinogen antiserum. Molecular mass markers with sizes in kDa are on the left.
To demonstrate that M. hyopneumoniae surface-bound plasmin(ogen) possesses biological activity, the ability of plasminogen-coated M. hyopneumoniae cells to degrade fibrinogen in the presence and absence of tPA was examined. Fibrinogen degradation was illustrated by immunoblots probed with anti-fibrinogen antibodies (Fig. 4D). Plasminogen-coated M. hyopneumoniae cells were able to degrade full-length fibrinogen in the presence of tPA within 6 h. No fibrinogen degradation was observed in the absence of an activator and very little degradation occurred when tPA was added to M. hyopneumoniae cells incubated without porcine plasminogen. These data demonstrate that M. hyopneumoniae-bound plasminogen is accessible to activators and can be converted into the active enzyme plasmin.
Plasmin(ogen) is present in bronchoalveolar lavage fluid and lung tissue of both uninfected and M. hyopneumoniae-infected pigs
Previous studies with mice indicate that plasminogen is distributed throughout various tissues, including the lungs (Zhang et al., 2002; Swaisgood et al., 2007). To determine whether plasminogen is present in the airways of porcine lungs, a dot blot of bronchoalveolar lavage (BAL) fluid collected from both uninfected and M. hyopneumoniae-infected pigs was performed (Fig. 5A). Plasminogen was detected in all pigs pre-challenge, and in unchallenged pigs at day 18, indicating that plasminogen is present in the airways of healthy pigs. Plasminogen was also detected in M. hyopneumoniae-infected pigs at 18 days post challenge. Plasminogen levels in challenged and unchallenged pigs did not appear to vary. We also compared plasmin levels in the BAL fluid of pre-challenge and M. hyopneumoniae-infected pigs. In the majority of pigs plasmin activity was significantly higher in the BAL fluid following challenge with M. hyopneumoniae (Fig. 5B). These findings provides further evidence that M. hyopneumoniae interacts with the plasmin(ogen) system during colonization.
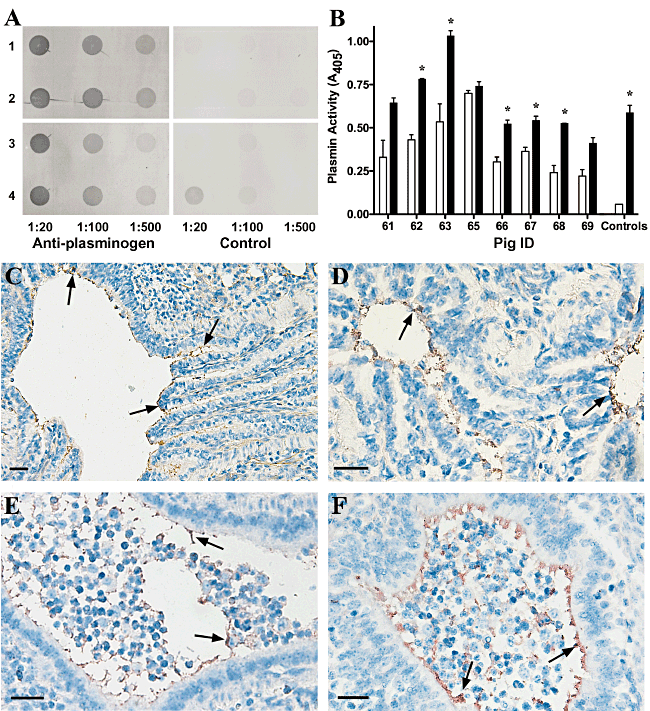
Plasmin(ogen) is present in the porcine lung. A. Detection of plasminogen in bronchoalveolar lavage (BAL) fluid of uninfected and M. hyopneumoniae-infected pigs. Dot blots were performed using BAL fluid from unchallenged (rows 1–2) and M. hyopneumoniae-infected (rows 3–4) pigs pre-challenge (rows 1 and 3) and at 18 days post challenge (rows 2 and 4). BAL fluid was applied to the membrane at various dilutions as indicated. Blots were probed with anti-porcine plasminogen antibodies as the primary antiserum, control blots were probed with secondary antiserum only. B. Plasmin activity in BAL fluid of pre-challenge and M. hyopneumoniae-infected pigs. Spectrozyme PL was incubated with BAL fluid collected from pre-challenge and M. hyopneumoniae-infected pigs and plasmin activity was measured. Unfilled bars represent data from pre-challenge pigs and filled bars represent data from M. hyopneumoniae-infected pigs. Spectrozyme PL incubated with plasminogen with and without the addition of tPA was used as a positive and negative control respectively. Data represent the mean values ± standard error of a representative assay performed in duplicate. The asterisk (*) indicates significantly different plasmin activity in BAL fluid from the M. hyopneumoniae-infected pig when compared with the pre-challenge BAL fluid (P < 0.001) as determined by a two-tailed t-test. C–F. Plasminogen is present along the ciliary borders of the bronchioles in porcine lungs. Immunohistochemical staining of lung sections from unchallenged (C–D) and M. hyopneumoniae-challenged (E–F) pigs was performed with anti-porcine plasminogen antibodies. The presence of red/brown stain highlights the location of plasminogen; some areas containing plasminogen are indicated with arrows. A large number of inflammatory cells can be observed in the airways of the M. hyopneumoniae-challenged lung sections. Scale bars represent 100 µm.
Sections of lung from unchallenged and M. hyopneumoniae-challenged pigs were subjected to immunohistochemical staining to determine the distribution of plasminogen in the tissue. Sections were initially stained with a M. hyopneumoniae-specific antibody to confirm that challenged pigs were infected with M. hyopneumoniae and that unchallenged pigs were uninfected. Samples from pigs 18 days post challenge with M. hyopneumoniae stained positive for this organism, while all other samples were negative (data not shown). All samples, from both infected and uninfected animals, stained positive for plasminogen along the ciliary borders of the bronchioles (Fig. 5C–F). As expected, staining was also observed in the blood vessels perfusing the lung tissue (data not shown). The distribution of staining in the bronchioles suggests that plasminogen is present in the extracellular fluid lining the airways. Staining in uninfected samples did not appear substantially different from that observed in infected samples, suggesting that plasminogen is available at the site of colonization both before and during M. hyopneumoniae infection.
P102 domains bind fibronectin
Although the function of P102 is unknown, the close genetic linkage of P102 to P97 in a single operon implies that this protein is involved in adherence to the host. Recently it has been discovered that M. hyopneumoniae is a fibronectin-binding pathogen with surface located fibronectin binding adhesins Mhp271, Mhp107 and P116 (Mhp108) (Deutscher et al., 2010; Seymour et al., 2010; Seymour et al., 2011). Using surface plasmon resonance, real-time interactions between rP102 and fibronectin were observed (Fig. 6A–C). rP102 binds fibronectin in a dose-dependant and physiologically relevant manner [KD 26 ± 2 nM; ka = (2.7 ± 7.8) × 104 M−1 s−1]. The recombinant P102 cleavage fragment rP42 also bound fibronectin in a dose-dependant manner with a KD of 33 ± 2 nM [corresponding ka of (5.2 ± 16.1) × 104 M−1 s−1]. rP60 also appeared to bind fibronectin but the rate constants of specific binding were not able to be calculated.
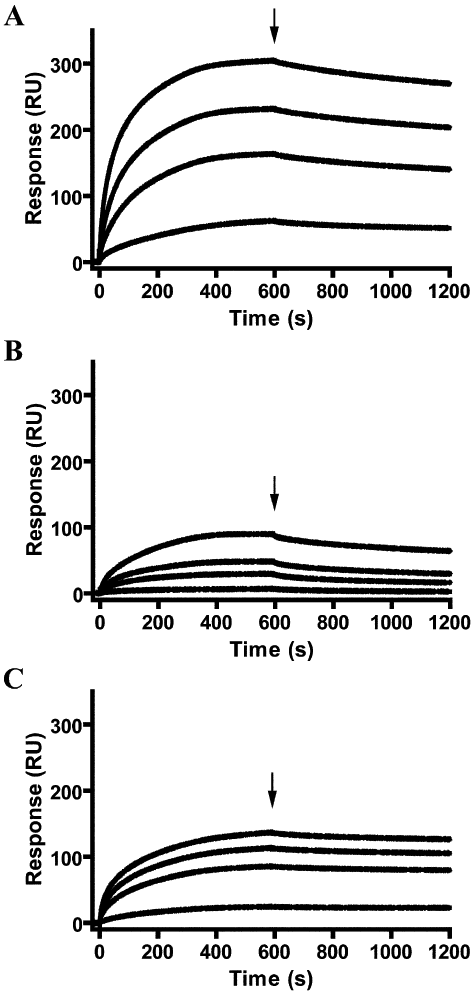
P102 is a fibronectin-binding protein. Sensorgrams depict binding of immobilized fibronectin by recombinant proteins rP102 (A), rP60 (B) and rP42 (C). Analyte was injected at 10 (bottom), 50, 100 and 200 (top) nM for 600 s at 10 min−1 over fibronectin. Arrows indicate the end of the injection period at which point dissociation of the analyte from fibronectin is observed.
P102 domains promote binding to porcine kidney epithelial-like (PK15) cells
The observation that antibodies specific to P102 were localized to porcine cilia in M. hyopneumoniae-infected lungs (Adams et al., 2005) prompted us to investigate if recombinant P102 proteins promote adherence to PK15 cells. Zielinski et al. (1990) developed an in vitro model for adherence of M. hyopneumoniae using PK15 cells, since then this model has been used to elucidate adherence mechanisms employed by M. hyopneumoniae (Burnett et al., 2006; Wilton et al., 2009; Seymour et al., 2010; Seymour et al., 2011). Using PK15 cells as an in vitro model we found that rP102 and rP60 were able to promote adherence of microspheres to PK15 cells (Fig. 7).
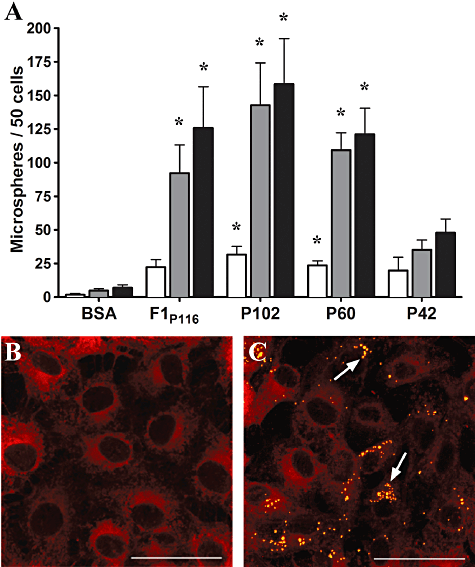
P102 promotes adherence to PK15 cell monolayers. Microspheres coated with recombinant P102 proteins were incubated with PK15 cells and adherent microspheres were visualized using confocal microscopy. F1P116 coated microspheres were used as a positive control, while BSA coated microspheres acted as a negative control. A. Graphical overview of adherent microspheres observed on PK15 cell monolayers following a 1 h (unfilled bars), 4 h (grey bars) and 7 h (filled bars) incubation. Microspheres were counted from duplicate coverslips at each timepoint and the results represent the mean from experiments performed in triplicate. The asterisk (*) denotes results differing significantly from BSA when a one-way anova is performed (P < 0.05). B and C. Confocal microscopy images of protein coated microspheres adhered to PK15 cells. Adherent BSA (B) and rP102 (C) coated microspheres following a 7 h incubation. PK15 cells are stained with Vybrant® Dil cell-labelling solution (red), microspheres appear yellow and are indicated with arrows. Scale bar is 50 µm.
Discussion
Mycoplasma hyopneumoniae cells are able to bind plasminogen in a dose-dependent and saturable manner (Seymour et al., 2010). Plasmin(ogen) bound on the surface of bacterial pathogens often undergoes conformational changes that enhance its susceptibility to activation by plasminogen activators (Lahteenmaki et al., 2005). Retaining surface-bound plasmin is advantageous to bacteria as they acquire an active host protease that may enable evasion of the innate immune system or degradation and penetration of host tissue (Lähteenmäki et al., 2001; Lahteenmaki et al., 2005; Degen et al., 2007). In this study we have investigated the relevance of the interactions of the M. hyopneumoniae protein P102 and to a larger extent M. hyopneumoniae with the plasmin(ogen) system. Manipulation of the mammalian plasminogen system by pathogens is widely accepted as a virulence strategy.
Recently, we demonstrated that P116 (Mhp108), a paralogue of P102, is a proteolytically processed multifunctional adhesin that binds plasminogen and fibronectin (Seymour et al., 2010). Analysis of in vitro grown M. hyopneumoniae cell lysates indicate that P102 (Mhp182) is a highly expressed member of the P102 paralogue family (M.P. Padula and S.P. Djordjevic, unpubl. results). Here we identified the P102 cleavage fragments P60 and P42 in cell lysates of M. hyopneumoniae. Trypsin shaving studies show that both P60 and P42 are accessible on the surface of M. hyopneumoniae, consistent with the presence of gold labelled anti-P102 antibodies on the cell surface in earlier studies (Djordjevic et al., 2004; Adams et al., 2005). Previous studies also show gold labelled anti-P102 antibodies localize at sites distal from the membrane (Djordjevic et al., 2004). P102 cleavage fragments are the first reported proteins that appear to be secreted to sites distal from the surface of M. hyopneumoniae (Adams et al., 2005).
C-terminal lysine residues are often responsible for binding plasminogen, although histidine and arginine residues may also be involved (Plow et al., 1995; Sanderson-Smith et al., 2007; Seymour et al., 2010). Furthermore binding to plasmin(ogen) via the lysine-binding kringle domains protects against the main physiological inhibitor of plasmin, α2-antiplasmin, which also binds to the kringle domains of plasmin(ogen) (Lähteenmäki et al., 2001). The two C-terminal residues of P102 are lysines. Using surface plasmon resonance we demonstrate that rP102, rP60 and rP42 all bind plasminogen in a dose-dependant and physiologically relevant manner. Both rP102 and rP42 show a large decrease in the amount of plasminogen that is bound with deletion of the two C-terminal lysine residues, suggesting that the C-terminal lysine residues contribute to the plasminogen-binding ability. However, binding ability is not abolished by deletion of C-terminal lysines or in the presence of a lysine analogue ε-aminocaproic acid (data not shown), demonstrating other residues such as histidine and arginine must be involved in plasminogen binding. The group A streptococcal plasminogen-binding proteins SEN and Prp both contain plasminogen-binding motifs (Sanderson-Smith et al., 2007; Cork et al., 2009).
Once activated, plasmin(ogen) is able to cause fibrinolysis and degradation of fibrin clots, extracellular matrix components, basement membranes and immune system components including complement factors and immunoglobulins (Coleman and Benach, 1999). Here we show that the rate of activation of porcine plasmin(ogen) is enhanced by the presence of P102 and that M. hyopneumoniae-bound plasmin(ogen) is activated in the presence of tPA. Additionally, broth-cultured M. hyopneumoniae recruits plasminogen/plasmin onto the cell surface. The biological functionality of M. hyopneumoniae-bound plasmin was demonstrated with freshly cultured cells, which when exposed to porcine plasminogen and tPA, degraded fibrinogen.
As plasminogen is a component of the fibrinolytic system involved in the maintenance of healthy lung tissue through tissue repair and inflammatory responses (Matsuoka et al., 2006; Degen et al., 2007), it may be available to porcine respiratory pathogens. The plasmin(ogen) system is active and tightly regulated in the respiratory tract of humans and mice (Wattiez et al., 1999; Swaisgood et al., 2007), but it has not previously been demonstrated that plasminogen/plasmin is available to M. hyopneumoniae in the lung environment of swine. Dot blots of BAL fluid and immunohistochemical sections show that plasmin(ogen) is present in both healthy pigs and after challenge with M. hyopneumoniae. These data indicate that M. hyopneumoniae attached to respiratory airway cilia have access to porcine plasmin(ogen). Following challenge with M. hyopneumoniae, an increase in plasmin activity is observed in BAL fluid providing further evidence that M. hyopneumoniae interacts with the plasmin(ogen) system during colonization. Degradation of the extracellular matrix of epithelial cells by plasmin(ogen) exposes underlying extracellular matrix components, such as fibronectin, thereby increasing the opportunity for the pathogen to adhere to these exposed components (Pancholi et al., 2003). Thus, the recruitment of plasmin(ogen) may contribute to the pathogenesis of M. hyopneumoniae and perhaps to the damage to ciliated cells that occurs during M. hyopneumoniae disease (Blanchard et al., 1992; DeBey and Ross, 1994).
Since an increased concentration of fibronectin is observed during repair of cell damage, such as damage inflicted by plasmin, cell damage may effectively present fibronectin as a target ligand for M. hyopneumoniae adherence (Coraux et al., 2008; Seymour et al., 2010). M. hyopneumoniae is a fibronectin-binding pathogen, which expresses fibronectin-binding proteins (Deutscher et al., 2010; Seymour et al., 2010; Seymour et al., 2011). Here we describe the ability of rP102 and the P102 cleavage fragment, rP42, to bind fibronectin with KD values comparable to adhesins of the respiratory pathogen Haemophilus influenzae (∼15 nM) (Fink et al., 2002) and the porcine pathogen Streptococcus suis (∼21 nM) (Esgleas et al., 2008). Some invasive bacteria are able to bind fibronectin to trigger cytoskeletal rearrangements, thereby promoting uptake of the pathogen by the host cell (Froman et al., 1984; Chaussee et al., 2000; Mongodin et al., 2002; Grundmeier et al., 2004). Fibronectin-binding adhesins of M. hyopneumoniae potentially contribute to colonization by promoting adherence or the events leading to M. hyopneumoniae becoming located at sites distal to the respiratory tract.
We have demonstrated that P102 is a multifunctional protein with a high affinity for plasminogen and fibronectin and able to promote adherence to PK15 cells. P102 is likely to play a key role in the recruitment of plasminogen to the surface of M. hyopneumoniae and potentially onto epithelial cilia in the lungs of swine. We cannot conclude precisely how M. hyopneumoniae benefits from the recruitment of plasmin(ogen) and fibronectin to the cell surface, but these observations raise an important question: does plasmin capture and interactions with fibronectin provide M. hyopneumoniae with opportunities to invade subepithelial sites? Further investigation on the effect of M. hyopneumoniae acquired plasmin activity and interactions with fibronectin on the ability of M. hyopneumoniae to adhere to and invade cell monolayers may provide additional insight. Although M. hyopneumoniae is widely considered an extracellular pathogen localized to the epithelial surfaces of the respiratory tract, it has also been isolated from the pericardium, liver, kidney and spleen of experimentally infected swine and naive cohorts (Buttenschon et al., 1997; Le Carrou et al., 2006; Marois et al., 2007). These findings indicate that M. hyopneumoniae possesses mechanisms for tissue invasion, migration and colonization of distal tissue sites.
Experimental procedures
Bacterial strains and culture conditions
Mycoplasma hyopneumoniae strains 232, J and Hillcrest (isolated in NSW, Australia) were grown in Friis medium and cultured as described previously (Bereiter et al., 1990; Scarman et al., 1997). Escherichia coli TOP10 and BL21 star (DE3) (Invitrogen, USA) were grown at 37°C on Luria–Bertani (LB) agar plates or cultured in LB medium with shaking at 200 r.p.m., supplemented with 100 µg ml−1 ampicillin where appropriate.
Plasmid constructs, protein expression and purification
PfuUltra™ High-Fidelity II DNA Polymerase (Stratagene, USA) was used in polymerase chain reactions. Primer sequences are listed in Table S1. M. hyopneumoniae 232 chromosomal DNA and pMHP182 (provided by Prof. F. Chris Minion, Iowa State University, USA) were used as the template for amplification of mhp182. pMHP182 contains a truncated mhp182 gene with in frame TGA codons converted to TGG, as TGA encodes tryptophan in M. hyopneumoniae. The overlap extension mutagenesis method was used to amplify mhp182 to alter a remaining TGA codon to TGG (Ho et al., 1989). Subsequent cloning of mhp182 used the pET100/D-TOPO® cloning kit (Invitrogen) according to the manufacturer's instructions. Segments of the pET100:mhp182 construct were amplified and cloned using the pET151/D-TOPO® kit, resulting in the recombinant polyhistidine fusion proteins used in this study (rP102, rP102ΔKK, rP60, rP42 and rP42ΔKK). Nucleotide sequencing of all plasmids ensured conformity of the sequences to mhp182. Plasmid constructs were transformed into E. coli BL21 star (DE3) for expression of recombinant proteins; when cell growth reached an OD600 of 0.8, protein expression was induced by the addition of 1 mM IPTG. Purification of proteins under native conditions used Ni-NTA agarose in accordance with the manufacturer's instructions (Qiagen, USA). Purification of rP42 and rP42ΔKK was performed under denaturing conditions as attempts to extract and purify the proteins under native conditions were unsuccessful. Purified proteins were dialysed into phosphate-buffered saline (PBS; 10 mM sodium phosphate, 137 mM sodium chloride, 2 mM potassium phosphate, 2.7 mM potassium chloride, pH 7.4). Polyclonal rabbit antisera were generated against recombinant proteins in New Zealand white rabbits using previously described methods (Jenkins et al., 2006).
Proteomics
A 0.1 g pellet of M. hyopneumoniae strain J cells was resuspended in 1 ml of solubilization buffer [7 M urea, 2 M thiourea, 40 mM Tris, 1% (w/v) C7Bz0] and disrupted by sonication at 10 W for 30 s. Proteins were reduced and alkylated with 20 mM acrylamide monomers, 5 mM tributylphosphine for 90 min. Proteins were precipitated in five volumes of ice-cold acetone for 30 min; the pellet was air dried and resuspended in SDS-PAGE sample buffer [15 mM Tris pH 6.8, 0.25% (w/v) SDS, 0.25% (v/v) β-mercaptoethanol, 2.5% (v/v) glycerol and 0.0025% (w/v) bromophenol blue]. Proteins were separated by SDS-PAGE and gel lanes cut into 16 equal slices for analysis. Following in-gel trypsin digestion, identification of proteins was achieved using LC-MS/MS as described previously (Deutscher et al., 2010).
To investigate the subcellular location of P102, P60 and P42, intact M. hyopneumoniae strain J cells were incubated in the presence and absence of trypsin and the supernatants were further digested and analysed by 2D-LC-MS/MS to detect peptides from proteins enzymatically released from the cell surface as previously described (Deutscher et al., 2010). Trypsin treatment of intact M. hyopneumoniae strain 232 cells was also performed and analysed with immunoblots using the method described by Wilton et al. (1998). Immunoblots were probed with pooled polyclonal rabbit sera raised against rP102 or against a cytosolic protein, L7/L12 (Burnett et al., 2006) as an intracellular control.
To perform immunoblots to investigate the ability of M. hyopneumoniae proteins to bind plasminogen, a 0.1 g pellet of M. hyopneumoniae cells was resuspended in 1 ml of 1% Triton buffer (1% Triton X-114, 10 mM Tris pH 8.0, 150 mM sodium chloride, 1 mM EDTA). Proteins were extracted as previously described (Wise and Kim, 1987). The protein pellet from the Triton X-114 detergent phase was resuspended in 0.5 ml MSS buffer [5 M Urea, 65 mM DTT, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 0.8% ampholytes, 40 mM Tris, 2 M thiourea, 2% sulfobetaine]. The detergent phase sample was sonicated at 10 W for 15 s, centrifuged and the supernatant collected. SDS-PAGE sample buffer was added to an aliquot of detergent phase sample and boiled for 5 min. The samples were separated by SDS-PAGE and visualized by staining with either Flamingo Fluorescent Gel Stain (Bio-Rad, USA) or Coomassie blue G-250. Alternatively protein was transferred to PVDF using a semi-dry transfer method as described previously (Kyhse-Andersen, 1984). Membranes were blocked with 5% (w/v) skim milk powder in PBS with 0.1% Tween 20 (v/v) (PBST) at room temperature for 1 h. Detection of P102 proteins was with polyclonal rabbit sera raised against purified rP60 and against rP42 diluted 1:100 in PBST for 1 h and a peroxidase-conjugated anti-rabbit IgG antibody, diluted 1:1000 for 1 h. Between incubations, the membrane was washed in three changes of PBST. The membrane was developed with SIGMAFAST™ 3,3′-diaminobenzidine tablets (Sigma-Aldrich, USA) as per manufacturer's instructions.
For detection of plasminogen-binding proteins, following blocking, the membrane was probed with purified biotinylated porcine plasminogen diluted 1:250 in PBST for 1 h. Porcine plasminogen was purified from porcine plasma as previously described (Andronicos et al., 1997; Sanderson-Smith et al., 2006), followed by gel filtration and ultrafiltration (Seymour et al., 2010). Spectrozyme PL (American Diagnostica, USA) was used to test the purity and activity of the purified plasminogen, while biotinylation was performed as described (Seymour et al., 2010). Membranes were then probed with ExtrAvidin® peroxidase conjugate (Sigma-Aldrich) diluted 1:20 000 in PBST for 1 h. Between incubations, the membrane was washed in three changes of PBST. The membrane was developed with Amersham™ ECL Advance™ Western blotting detection kit (GE Healthcare, UK) and detected with a Chemi-doc XRS imaging system (Bio-Rad) for improved sensitivity of detection.
Surface plasmon resonance analyses
The ability of P102 protein fragments to bind plasminogen and fibronectin were investigated on a Biacore T100 instrument (Biacore AB, Sweden). The preparation of analyte proteins, ligand immobilization and kinetics assays have been described previously (Seymour et al., 2010). Plasminogen and fibronectin were immobilized as ligands for surface plasmon resonance assays; binding of recombinant P102 proteins was performed at 20°C with a concentration range of 0–200 nM. The 1:1 Langmuir binding model with BiaEvaluation software 3.1 (Biacore AB) was used to analyse plasminogen association kinetics. Fibronectin association kinetics were analysed with the sum of two exponentials for a heterogeneous surface (Deutscher et al., 2010).
Collection of porcine lung tissue and BAL fluid
Pigs at 9–10 weeks of age, sourced from a high health status herd, were anaesthetized and subjected to BAL using 4 ml of sterile PBS. The fluid was collected and stored at −80°C until required. At this time a number of pigs were inoculated intratracheally with 10 ml broth cultures of M. hyopneumoniae strain Hillcrest, while the remaining two pigs were not inoculated and served as uninfected controls. At 2.5 weeks post infection, all pigs were anaesthetized and subjected to BAL as described above. At 3 weeks post infection the pigs were euthanized and necropsies performed. Samples of lung tissue including any obvious lesions typical of M. hyopneumoniae infection, were collected into neutral-buffered formalin and fixed for 24 h.
Activation of plasmin(ogen) and plasmin activity in BAL fluid
Porcine plasminogen and rP102 in 50 mM Tris pH 7.4 were mixed in various molar ratios from 1:0.5 to 1:10 to investigate the effect of P102 on the activation of plasmin(ogen). Samples were incubated at 37°C for 1 h. Two hundred units of tPA (Calbiochem, Germany) in 50 mM Tris (pH 7.4) were added and the plate was incubated at 37°C for 15 min with shaking prior to the addition of 20 µl of 2.5 mM Spectrozyme PL. The absorbance at 405 nm was measured using a Multiskan Ascent plate reader (Thermo Labsystems, USA). Absorbance reads were taken every 15 min for up to 2 h 30 min. Between reads the plate was incubated at 37°C with shaking. Wells containing rP102 only or no plasminogen were employed as a negative control, while wells containing plasminogen and tPA only were used as a positive control. Absorbance measured in wells immediately after the addition of Spectrozyme PL was subtracted from the absorbance measured during subsequent readings.
To investigate the activation of M. hyopneumoniae surface-bound plasminogen, freshly cultured M. hyopneumoniae strain 232 cells were adhered to a Linbro/Titertek microtitre plate (MP Biomedicals, USA) and washed according to Seymour et al. (2010). Each well was blocked with 2% (w/v) BSA in PBS for 1 h. Five micrograms human (Sigma-Aldrich, USA) or porcine plasminogen in 1% (w/v) BSA in PBS was added to each well and incubated at 37°C for 1 h with shaking. Unbound plasminogen was removed by washing the plate three times with PBS containing 0.5% (v/v) Tween 20 and once with 50 mM Tris (pH 7.4). tPA was added and incubated as described for the activation of plasminogen in the presence of P102. Absorbance readings were taken every hour for the first 5 h, then again from 20 h until the absorbance for wells containing human plasminogen reached saturation. The average of the maximum absorbance for human plasminogen (time > 5 h) was set to 100% plasmin activity. Absorbance measured in wells without cells was subtracted from the absorbance of wells with cells. Wells containing cells incubated without plasminogen were employed as a negative control.
Bronchoalveolar lavage fluid from pre-challenge and M. hyopneumoniae-challenged pigs was tested for plasmin activity. Pre-warmed BAL fluid was incubated with 20 µl of 2.5 mM Spectrozyme PL at 37°C. Absorbance readings at 405 nm were taken over 22 h. Wells containing 1 µg of porcine plasminogen were employed as a negative control, while wells containing 1 µg of plasminogen and 200 units of tPA only were used as a positive control. Data analysis was performed using GraphPad Prism version 5.04.
M. hyopneumoniae cells recruit plasminogen from serum in Friis media
To assess whether M. hyopneumoniae cells recruit plasmin(ogen) from the serum in Friis media, a Western blot was performed using anti-porcine plasminogen antibodies (AbD Serotec, USA). Non-tPA-activated and tPA-activated porcine plasminogen were included as references. Porcine plasminogen (10 µg) was incubated in the presence or absence of 250 units of tPA in PBS for 23.5 h at 37°C. SDS-PAGE sample buffer was added to each sample and to PBS washed cultured M. hyopneumoniae strain 232 cells. All samples were boiled for 5 min prior to loading onto an SDS-PAGE gel. Proteins were either stained with GelCode Blue stain reagent (Pierce Biotechnology, USA) or transferred onto PVDF membrane. The membrane was blocked with 5% (w/v) non-fat dry milk in Tris-buffered saline (TBS) (10 mM Tris, 150 mM sodium chloride, pH 7.4) at room temperature for 1 h. Detection of plasmin(ogen) was with anti-porcine plasminogen antibodies diluted 1:2000 in TBS for 1.5 h and a peroxidase-conjugated sheep anti-rabbit IgG antibody (Chemicon, Australia) diluted 1:1000 in TBS for 1 h. Between incubations the membrane was washed with TBS containing 0.05% (v/v) Tween 20 twice for 5 min, followed by one 5 min wash in TBS. After equilibration with 100 mM Tris (pH 7.6) the membrane was developed with DAB substrate (0.05% 3,3′-diaminobenzidene, 0.03% hydrogen peroxide in 100 mM Tris, pH 7.6).
M. hyopneumoniae surface-bound plasmin(ogen) degradation of fibrinogen
Mycoplasma hyopneumoniae strain 232 cells were grown to log phase, washed and resuspended to an OD600 of 0.9. M. hyopneumoniae cells (50 µl) were pre-incubated with 10 µg of porcine plasminogen in PBS for 30 min at 37°C. The cells were then washed three times with PBS prior to incubation with 5 µg of plasminogen-depleted human fibrinogen (Calbiochem) for 30 min at 37°C. Degradation of fibrinogen occurred in the presence of 225 units of tPA at 37°C. An aliquot of the sample was taken, the bacteria pelleted by centrifugation and the supernatant solubilized by boiling in SDS-PAGE sample buffer for 5 min. The samples were separated by SDS-PAGE, followed by transfer of the proteins onto PVDF membrane. The membrane was blocked with 5% (w/v) non-fat dry milk in TBS at room temperature for 1 h. Detection of fibrinogen peptides was with goat anti-human fibrinogen antiserum (Sigma-Aldrich) diluted 1:10 000 in TBS for 1.5 h and a peroxidase-conjugated anti-goat IgG antibody (Sigma-Aldrich) diluted 1:7500 in TBS for 1 h. Between incubations the membrane was washed with TBS containing 0.05% (v/v) Tween 20 twice for 5 min, followed by one 5 min wash in TBS. After equilibration with 100 mM Tris (pH 7.6) the membrane was developed with DAB substrate.
Anti-plasminogen dot blot
Bronchoalveolar lavage fluid collected from both unchallenged and M. hyopneumoniae-challenged pigs at time zero and 2.5 weeks post infection were thawed and diluted 1:20, 1:100 and 1:500 in PBS. Samples were then spotted in 100 µl volumes onto a pre-wetted PVDF membrane using a dot blot manifold. The membrane was blocked for 1 h in 2% BSA in PBS and then reacted with an anti-porcine plasminogen antibody diluted 1:500 in PBS. The membrane was washed 3 × 5 min in PBST and then incubated for 1 h in a 1:1500 dilution of horseradish peroxidase-conjugated sheep anti-rabbit antibody. The membrane was washed 3 × 5 min with PBST, equilibrated in 100 mM Tris (pH 7.6) and detected with DAB substrate.
Immunohistochemistry
Formalin-fixed tissues were placed into histology cassettes and embedded in paraffin. Sections of 4 µm thickness were cut from the paraffin blocks and mounted onto Superfrost slides. Sections were processed for immunohistochemistry by dewaxing the sections 2 × 3 min in xylene, rehydrating in an ethanol series and blocking with 1.8% peroxide in methanol for 10 min. Antigens were unmasked using 0.2% trypsin-0.1% CaCl2 at 37°C for 30 min. Immunohistochemistry was then performed using the Vectastain ABC system (Vector Labs, USA) according to the manufacturer's instructions. To stain the sections for plasminogen or M. hyopneumoniae, slides were treated with a 1:500 dilution of anti-porcine plasminogen, or a 1:1000 dilution of M. hyopneumoniae-specific antiserum (Jenkins et al., 2006) respectively. Negative control slides were treated with PBS instead of primary antibody. Bound antibodies were detected with a chromagen solution containing 3-amino-9-ethylcarbazole and 0.015% hydrogen peroxide in dimethylformamide. Slides were then counterstained with haematoxylin and mounted with Faramount aqueous mounting medium (Dako, Denmark).
PK15 adherence assay
Protein-coated microspheres were used to investigate the ability of P102 proteins to promote adherence to PK15 cells. The assay was performed as described by Seymour et al. (2010) and Burnett et al. (2006). Briefly, fluorescent microspheres were coated with recombinant P102 proteins and incubated with PK15 monolayers for 1, 4 or 7 h. Monolayers were washed to remove unbound cells before fixing. Fixed PK15 cells were stained with a 1:200 dilution of Vybrant® Dil cell-labelling solution (Invitrogen) and imaged using a Nikon A1 Confocal Microscope. BSA coated microspheres were employed as a negative control and F1P116 coated microspheres as a positive control. F1P116 is a M. hyopneumoniae adhesin (Seymour et al., 2010).
Acknowledgements
Lisa M. Seymour is a recipient of an Australian Postgraduate Award. This work was partially funded by an Australian Research Council Linkage grant LP776711. We acknowledge Jake Matic (University of Wollongong, NSW, Australia), Shayne Fell and Paul Young (Elizabeth Macarthur Agricultural Institute, NSW, Australia) for their technical assistance. We also acknowledge Alison Seymour (Elizabeth Macarthur Agricultural Institute) for the provision of antisera.