Francisella infection triggers activation of the AIM2 inflammasome in murine dendritic cells
Summary
The intracellular bacterium Francisella tularensis is the causative agent of tularemia, a potentially fatal disease. In macrophages, Francisella escapes the initial phagosome and replicates in the cytosol, where it is detected by the cytosolic DNA sensor AIM2 leading to activation of the AIM2 inflammasome. However, during aerosol infection, Francisella is also taken up by dendritic cells. In this study, we show that Francisella novicida escapes into the cytosol of bone marrow-derived dendritic cells (BMDC) where it undergoes rapid replication. We show that F. novicida activates the AIM2 inflammasome in BMDC, causing release of large amounts of IL-1β and rapid host cell death. The Francisella Pathogenicity Island is required for bacterial escape and replication and for inflammasome activation in dendritic cells. In addition, we show that bacterial DNA is bound by AIM2, which leads to inflammasome assembly in infected dendritic cells. IFN-β is upregulated in BMDC following Francisella infection, and the IFN-β signalling pathway is partially required for inflammasome activation in this cell type. Taken together, our results demonstrate that F. novicida induces inflammasome activation in dendritic cells. The resulting inflammatory cell death may be beneficial to remove the bacterial replicative niche and protect the host.
Introduction
Intracellular pathogens pose a unique challenge to the mammalian host organism as they invade host cells, thereby hiding from extracellular immune mechanisms, such as circulating antibodies and complement. When infected, migrating cells, like macrophages and dendritic cells (DCs), can serve as propagation vessels for the pathogen to disseminate within the host. Some intracellular organisms like Francisella tularensis, Shigella flexneri and Listeria monocytogenes escape from their phagocytic vacuole and reach the cytosol where they replicate (Ray et al., 2009). Mammalian host cells have evolved mechanisms to detect cytosolic Pathogen-Associated Molecular Patterns from cellular intruders using cytosolic receptors like NOD-like proteins (e.g. NOD1, NOD2, NLRP3 and NLRC4), RIG-I like helicases (e.g. RIG-I, MDA5) or PYHIN proteins (e.g. the DNA receptor AIM2) (Franchi et al., 2009a; Brennan and Bowie, 2010). Upon Pathogen-Associated Molecular Pattern detection, certain cytosolic receptors can initiate the assembly of a protein complex known as the inflammasome (Abdul-Sater et al., 2009). This multiprotein complex recruits and activates the cysteine protease Caspase-1, and serves as a platform for processing cytokine precursors pro-IL-1β and pro-IL-18 to their mature forms (Thornberry et al., 1992). IL-1β and IL-18 are potent pro-inflammatory cytokines and are crucial components for defence against infection through activation of Th1 and Th17 immune responses (Netea et al., 2010). Inflammasome activation also initiates a programmed cell death termed pyroptosis (Bergsbaken et al., 2009). This inflammatory cell death process is increasingly viewed as yet another host defence mechanism that removes the replicative niche of the pathogen and re-exposes it to patrolling immune cells, such as neutrophils (Miao et al., 2010). Inflammasomes are usually named after the receptor that initiates their assembly. For example, the NLRP3 inflammasome responds to a vast number of pathogens and other damages to the cell (Zhou et al., 2010). The AIM2 inflammasome responds to cytosolic dsDNA (Alnemri, 2010). Both NLRP3 and AIM2 receptors require the adaptor protein Asc to recruit pro-Caspase-1 to the inflammasome.
Francisella tularensis is the causative agent of tularemia, of which the pneumonic form can be lethal if untreated (Sjostedt, 2007). F. tularensis ssp. tularensis and F. tularensis ssp. holarctica are the most virulent Francisella subspecies for humans and were reported to be responsible for 100% of tularemia mortality in a recent epidemiological study (Kugeler et al., 2009). Francisella novicida is a close relative of F. tularensis (Sjostedt,2007). This pathogen causes severe disease in immunocompromised humans, but rarely does so in immunocompetent individuals. In addition to its genetic tractability, F. novicida shares some virulence genes with F. tularensis and causes a similar disease in mice, and therefore serves as a good experimental model to study Francisella pathogenesis.
In macrophages, Francisella escapes from its phagocytic vacuole and replicates to high numbers in the cytosol (Clemens et al., 2004; Nano et al., 2004; Checroun et al., 2006). Phagosomal escape and subsequent replication depend on the Francisella Pathogenicity Island (FPI), a 26–30 kb genomic segment that is present, often in duplicate, in all Francisella species identified thus far (Nano et al., 2004; Santic et al., 2005). The FPI is believed to encode a Type VI-like secretion system that secretes effectors important for phagosomal escape and virulence (Barker et al., 2009). Previous research showed that cytosolic F. novicida as well as the attenuated F. holarctica Live Vaccine Strain (LVS) activate Caspase-1-mediated cell death and cytokine release in infected macrophages (Mariathasan et al., 2005; Gavrilin et al., 2006). Lysis of F. novicida and LVS in the cytosol results in DNA leakage, which is detected by the AIM2 cytosolic DNA receptor (Fernandes-Alnemri et al., 2010; Jones et al., 2010; Rathinam et al., 2010). The AIM2 inflammasome assembles in the infected macrophage and elicits AIM2-, Asc- and Caspase-1-dependent cytokine release and cell death. Francisella infection also triggers the release of high amount of interferon-β from macrophages (Henry et al., 2007). This IFN-β pathway is necessary to activate the AIM2 inflammasome (Fernandes-Alnemri et al., 2010; Jones et al., 2010).
Macrophages and DCs are professional antigen presenting cells. They engulf foreign entities, process them and expose antigens at their surface. DCs are key players that link innate and adaptive immunity (Liu, 2001). These sentinel cells engulf pathogens from the periphery and migrate to lymphatic tissues where they present processed antigens to naïve T cells (Liu, 2001; Sundquist et al., 2003). Interactions of bacterial pathogens with DCs do not always mirror their interactions with macrophages. For instance, some virulence factors required for Salmonella typhimurium to infect and survive within macrophages are not required in DCs (Niedergang et al., 2000). Intracellular replication of Legionella pneumophila, while proficient in macrophages, is restricted by induction of rapid apoptosis in L. pneumophila-infected DCs (Neild and Roy, 2003; Nogueira et al., 2009).
Some of the interactions between Francisella with DCs have been studied previously. Li et al. showed that live but not heat-killed LVS induces Caspase-1-dependent IL-1β release in human and mouse DCs, but the mechanisms underlying bacterial detection and the consequences of Caspase-1 activation were not described (Li et al., 2006). In addition, LVS was shown to infect DCs upon airway infection in mice (Bosio and Dow, 2005). The bacterium then uses this niche to traffic to mediastinal lymph nodes where extensive replication occurs (Bar-Haim et al., 2008). It was also shown that F. tularensis ssp. tularensis strain SCHU S4 dramatically alters DC maturation and their ability to prime T cells (Chase et al., 2009; Chase and Bosio, 2010).
The goal of this study is to characterize Caspase-1 activation in DCs upon F. novicida infection. We show that F. novicida escapes into the cytosol of murine DCs where it replicates, and that the AIM2 inflammasome is activated in F. novicida-infected DCs. We demonstrate that AIM2 inflammasome assembly in DCs results in Caspase-1 processing, IL-1β release and rapid pyroptotic cell death. We also show that in DCs, in contrast to macrophages, IFN-β is partially required for inflammasome activation. Because DC infection results in bacterial dissemination in the host, inflammasome activation in DCs is likely a key mechanism to control bacterial infection in vivo.
Results
F. novicida escapes into the cytosol of DCs
Francisella escapes from its phagosome to replicate in the cytosol of macrophages (Clemens et al., 2004; Checroun et al., 2006). To monitor the intracellular life cycle of F. novicida within DCs, we infected bone marrow-derived DCs (BMDCs) with F. novicida strain U112 and monitored the intracellular localization of bacteria over time. At 30 min post infection bacteria strongly colocalized with the lysosomal marker LAMP-1, indicating that they were still within the Francisella-containing phagosome (Fig. 1). However, 4 h post infection, the majority of bacterial cells did not colocalize with LAMP-1, suggesting that the bacteria escaped into the DC cytosol. Importantly, a F. novicida mutant lacking the complete FPI (Weiss et al., 2007a) still localized to LAMP-1-positive vesicles after 4 h (Fig. 1), showing that in BMDCs, like in macrophages, the FPI is needed for phagosomal escape (Santic et al., 2005). Taken together, these observations demonstrate that F. novicida escape the Francisella-containing phagosome and access the cytoplasm of BMDCs by a mechanism that requires the FPI.
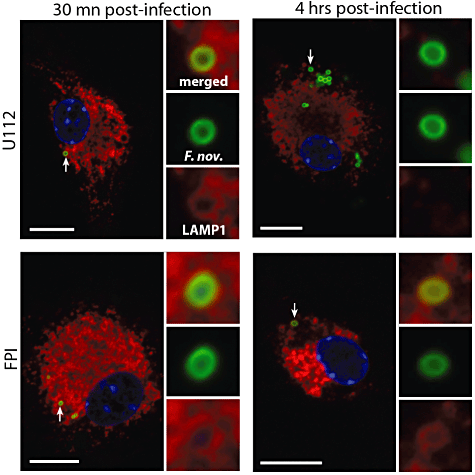
F. novicida escapes into the cytosol of dendritic cells. Immunofluorescence microscopy of BMDCs stained for the lysosomal marker LAMP-1 at 30 min (left panels) and 4 h (right panels) post infection with wild-type F. novicida (U112, top panels) or the FPI deletion mutants (FPI, bottom panel). Scale bars, 10 µm.
F. novicida replicates in murine DCs
It was previously shown that F. tularensis strain SCHU S4 replicates to high numbers in human DCs, growing more than 5 logs over a period of 72 h (Chase et al., 2009). LVS was also shown to replicate in human DC, with greater than 100-fold increase in recoverable colony forming units(cfu) during a 24 h period (Bar-Haim et al., 2008) and a 3 log increase by 72 h (Bosio and Dow, 2005). To characterize the F. novicida life cycle in murine BMDCs, we performed a gentamicin protection assay in which BMDCs were infected with either F. novicida or the isogenic FPI deletion mutant at a multiplicity of infection (moi) of 1 (Fig. 2A). The number of intracellular bacteria was monitored over time by measuring the number of gentamicin-protected CFU from lysed BMDCs. Robust replication was observed in murine DCs with the number of bacteria increasing by more than eightfold over a period of 10 h. Interestingly, the number of intracellular bacteria in WT BMDCs peaked at 10 h, followed by a sharp decrease at 24 h (Fig. 2A, black squares). Replication required the FPI as the number of intracellular FPI deletion mutants did not increase significantly over a period of 24 h (Fig. 2A, open squares).
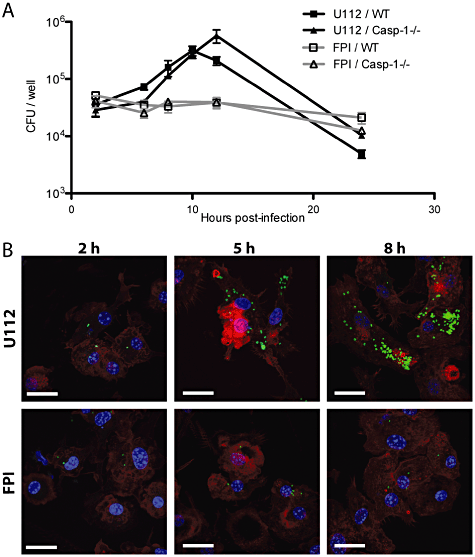
F. novicida replicates in BMDCs. A. BMDCs derived from either wild-type (WT) or Caspase-1-deficient mice (Casp1-/-) were infected with either WT F. novicida (U112) or the FPI deletion mutant (FPI) at an moi of 1. Intracellular bacteria were enumerated by cfu counts. The graph shows mean ± standard deviation (SD) of triplicate wells and is representative of at least four independent experiments. B. Immunofluorescence microscopy of F. novicida-infected BMDCs. BMDCs were infected at an moi of 5. Bacteria were stained with an F. novicida-specific antibody (green), actin was stained with phalloidin (red) and DNA with DAPI (blue). Scale bars, 20 µm.
We confirmed that the increase in gentamicin-protected bacteria was due to an increase in intracellular bacteria by immunostaining infected BMDCs. We observed an increase in the number of intracellular bacteria over time with a peak at around 10 h (Fig. 2B). We also noticed that the cells that had been infected with wild-type F. novicida for 24 h did not stain with phalloidin, which binds filamentous actin, indicating that the integrity of the DCs was compromised (data not shown). In contrast, the amount of intracellular FPI mutants remained the same in BMDCs over the course of the infection (Fig. 2B). In addition, we did not see a difference in the staining of actin filaments, suggesting that DCs infected with the FPI deletion mutant were not compromised during the 24 h infection. Thus, we have shown that F. novicida is capable of replicating in murine BMDCs, and that replication is dependent on the FPI, which is similar to what was previously observed in macrophages (Lauriano et al., 2004; Nano et al., 2004).
F. novicida-infected BMDCs initiate rapid inflammasome-mediated cell death
We next sought to determine whether the decrease in gentamicin-protected F. novicida recovered from infected BMDCs after 10 h was due to host cell death. BMDCs were infected at the indicated moi and cell death was monitored by measuring the amount of lactate dehydrogenase, a host cell cytosolic protein, released into the culture supernatant by cell permeabilization and/or lysis. The wild-type F. novicida strain induced rapid BMDC death in a dose-dependent manner, as more cell death was observed when DCs were infected with an moi of 100 bacteria per host cell compared with an moi of 10 (Fig. 3A and B, black bars, compare U112 10 and U112 100). Moreover, the FPI deletion mutant did not cause cell death in BMDC, similar to what has been described previously in macrophages.
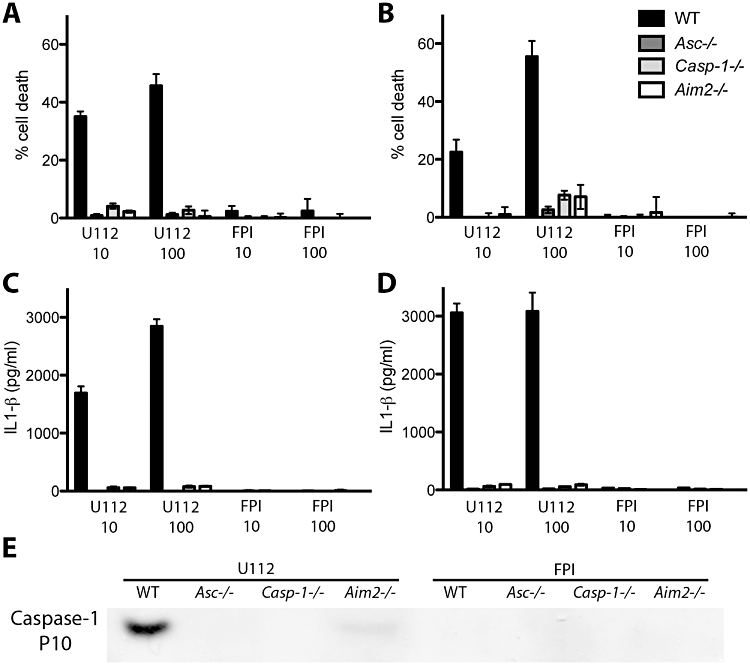
Rapid F. novicida-induced BMDC death and cytokine release depend on the AIM2 inflammasome. Unstimulated (A, C) or Pam3CSK4-stimulated (B, D) BMDCs from WT mice or mice deficient for inflammasome components AIM2, Asc or Caspase-1 were infected with F. novicida at an moi of 10 (U112 10, FPI 10) or 100 (U112 100, FPI 100). Cell death was determined by measuring the release of lactate dehydrogenase in the supernatant at 9 (A) or 7 h (B) post infection. Levels of IL-1β were measured by ELISA at 9 (C) or 7 h (D) post infection. Graphs show the mean ± SD of quadruplicate wells and are representative of at least three independent experiments. (E) Western blot analysis of infected cell supernatants probed with an antibody against the Caspase-1 p10 subunit. Unstimulated BMDCs were infected at an moi of 10 and supernatants were collected 10 h post infection.
We have previously shown in murine bone marrow-derived macrophages that cytosolic F. novicida DNA is recognized by the host molecule AIM2, which recruits Asc and Caspase-1, leading to release of pro-inflammatory cytokines and pyroptosis (Jones et al., 2010). We thus examined whether the F. novicida-induced BMDC death was dependent on the AIM2 inflammasome, similar to what occurs in macrophages. BMDCs were derived from mice that are deficient for either the cysteine protease Caspase-1 (Casp1−/−), the adaptor molecule Asc (Asc−/−) or the cytosolic receptor AIM2 (AIM2−/−). BMDCs were either left untreated or were pre-stimulated with the TLR2 agonist PAM3CSK4 16 h before F. novicida infection. In contrast to WT BMDCs, infection with wild-type F. novicida resulted in very little host cell death in Casp1-/-, Asc-/- and AIM2-/- BMDCs, suggesting that infected BMDCs die through inflammasome-mediated cell death (Fig. 3A and B). A small amount of cell death occurred in pre-stimulated Casp1-/- and AIM2-/- BMDCs infected with wild-type F. novicida, while no cell death was observed in Asc-/- BMDCs (Fig. 3A and B). This is similar to the Asc-dependent, Caspase-1-independent cell death we described previously in bone marrow-derived macrophages (Henry et al., 2007). The FPI deletion mutant did not induce cell death in infected BMDCs. Notably, Western blot analysis showed no increase in AIM2 amounts following infection by F. novicida (data not shown). These results suggest that, similar to macrophages, DCs undergo inflammasome-mediated cell death upon F. novicida infection, and that this cell death is dependent on FPI-mediated vacuolar escape and replication.
We next sought to determine the impact of early inflammasome-mediated cell death on intracellular F. novicida replication. Because death of the host cell results in the removal of the bacterial replicative niche, we postulated that the decrease in gentamicin-protected F. novicida after 10 h of infection was due to inflammasome-mediated cell death. To test this, we monitored F. novicida replication in Casp1-/- BMDCs. F. novicida replicates to higher numbers and for a longer period of time in cells lacking this essential inflammasome component (Fig. 2A, black triangles), consistent with the longer life span of its replicative niche. Eventually the number of gentamicin-protected bacteria recovered from the Casp1-/- BMDCs decreased (Fig. 2A), which is consistent with our observation that some cell death occurred in the Casp1-/- BMDCs (Fig. 3A and B). These observations suggest that inflammasome-mediated cell death limits expansion of Francisella in BMDCs.
Francisella-infected DCs process and release IL-1β
Activation of the inflammasome results in the processing of pro-inflammatory cytokines pro-IL-1β and pro-IL-18 into their bio-active form and release into the extracellular milieu (Franchi et al., 2009b). We monitored the levels of IL-1β released by Francisella-infected DCs. Wild-type F. novicida infection triggered the release of processed IL-1β by BMDCs derived from wild-type mice (Fig. 3C and D). Pre-stimulation of BMDCs with PAM3CSK4 led to the release of higher amounts of processed cytokines (Fig. 3, compare C and D). Importantly, processing and release of IL-1β was dependent on the AIM2 inflammasome, since BMDCs deficient in inflammasome components Asc, Caspase-1 or AIM2 did not release processed IL-1β. Moreover, the FPI deletion mutant failed to induce IL-1β release in WT BMDCs (Fig. 3C and D). Taken together, these results suggest that in addition to pyroptotic cell death, Francisella infection of DCs triggers inflammasome-dependent IL-1β processing and release into the external milieu.
Caspase-1 activation results in its autoproteolytic cleavage into two subunits, p10 and p20 (Thornberry et al., 1992), which are released in the extracellular milieu by a yet unidentified mechanism (Keller et al., 2008). Caspase-1 autocatalytic cleavage is essential for cytokine processing and release (Broz et al., 2010a). To confirm that Caspase-1 is processed in murine BMDCs, we tested supernatants of F. novicida-infected BMDCs for the presence of the caspase-1 p10 subunit by Western blot analysis using a p10-specific antibody. Caspase-1 was processed in WT F. novicida-infected DCs, but not in DCs infected with FPI deletion mutant (Fig. 3E). We did not detect the caspase-1 p10 subunit in supernatants from Asc-/- or AIM2-/- BMDM, indicating that Caspase-1 cleavage was dependent on the presence of AIM2 and Asc (Fig. 3E). Taken together, these results confirm that Caspase-1 is autoproteolytically cleaved in DCs infected with F. novicida, resulting in IL-1β processing and release.
The AIM2 inflammasome assembles in F. novicida-infected DCs
Our laboratory and others have previously shown that AIM2 recognizes and binds to DNA released by lysing intracellular F. novicida in infected macrophages (Fernandes-Alnemri et al., 2010; Jones et al., 2010). These DNA-AIM2 complexes recruit the inflammasome adaptor protein Asc, which then forms a large macromolecular structure termed ASC focus (Jones et al., 2010). Caspase-1 is recruited and activated within this complex (Broz et al., 2010b). To determine whether a similar recognition occurs in BMDCs infected with F. novicida, we visualized the subcellular localization of AIM2 by immunofluorescence microscopy. WT BMDCs infected with wild-type F. novicida pre-labelled with the DNA stain Hoechst contained multiple DNA-AIM2 complexes, likely reflecting AIM2 interaction with DNA leaked from bacterial cells (Fig. 4A). Despite the presence of multiple AIM2-F. novicida DNA complexes per infected DC, only one Asc focus was observed, similar to what was previously described in macrophages (Jones et al., 2010). Asc foci were only observed in BMDCs derived from WT and Casp-1-/- mice, but not from AIM2-/- and Asc-/- mice (Fig. 4B). Moreover, Asc appeared as a diffuse staining throughout the cell and the nucleus and did not aggregate in uninfected BMDCs and in BMDCs infected with the FPI deletion strain (data not shown). These results demonstrate that the ASC foci form in DCs upon detection of cytosolic F. novicida by the AIM2 DNA receptor.
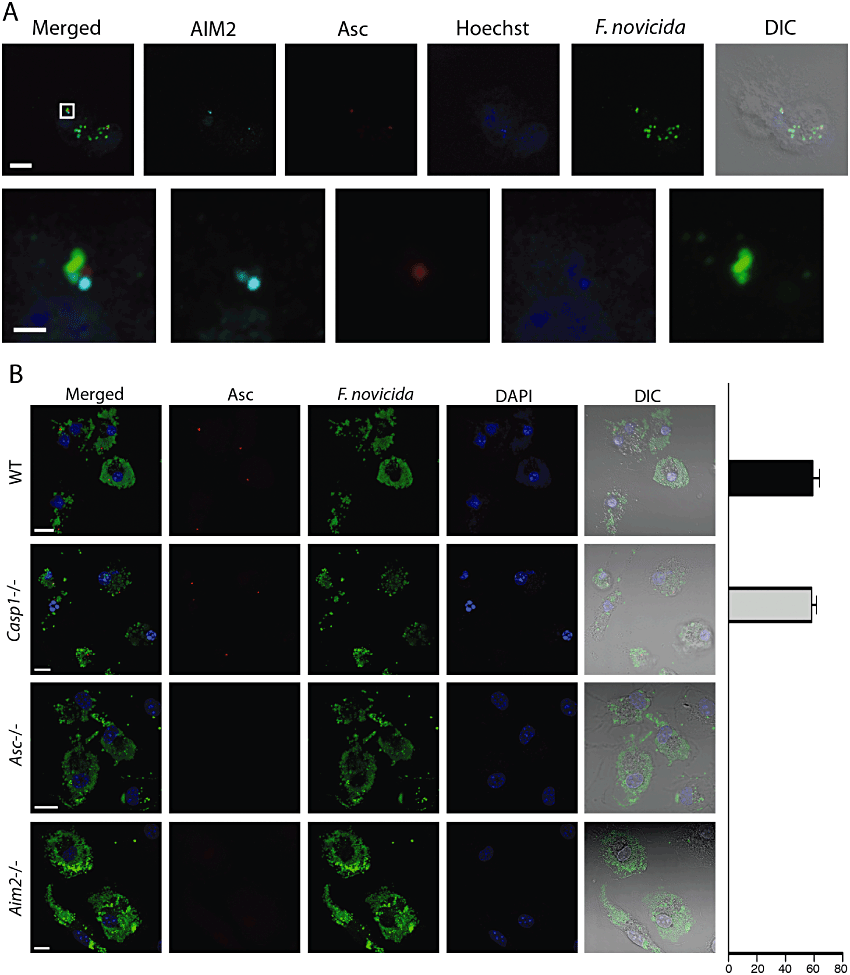
Inflammasome assembly in F. novicida-infected BMDCs. A. Formation of AIM2 aggregates attached to bacterial DNA. Stimulated cells were infected with F. novicida pre-labelled with the Hoechst DNA stain at an moi of 10 and analysed by immunofluorescence microscopy 5 h post infection. Scale bars: 10 µm for upper, 1 µm for lower. B. Formation of Asc aggregates in infected BMDCs derived from either WT or Caspase-1-, Asc- or AIM2-deficient mice. Unstimulated cells were infected with F. novicida at an moi of 10 and analyzed by immunofluorescence microscopy 10 h post infection. The graph bar on the right indicates quantification of cells containing an ASC aggregate. DIC, differential interference contrast. Scale bars, 10 µm.
Type I interferon is partially required for inflammasome activation in BMDC
Francisella infection triggers the release of IFN-β in macrophages. Secretion of IFN-β and signalling through the Type I interferon receptor (IFNR) are required for inflammasome activation in macrophages (Henry et al., 2007; Fernandes-Alnemri et al., 2010; Jones et al., 2010). We sought to verify the requirement of IFN-β signalling for inflammasome activation in DCs. Early after infection with wild-type F. novicida, but not with the FPI deletion mutant, IFN-β was upregulated in BMDC (Fig. 5A).
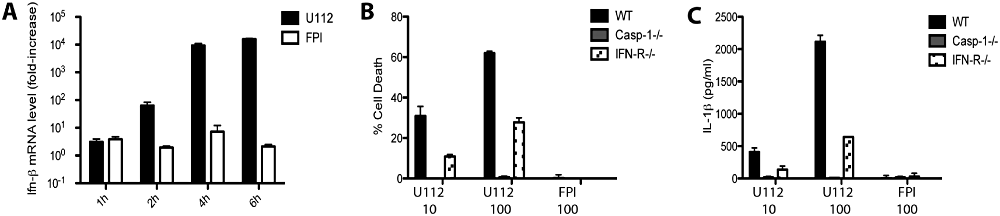
Role of type I interferon signalling in inflammasome activation in BMDCs. A. IFN-β mRNA levels were determined by quantitative RT-PCR following infection of unstimulated WT, casp-1-/- or IFNR-/- BMDCs with F. novicida at an moi of 100. The values represent increase from mRNA levels in uninfected cells. B and C. Cell death and IL-1β release were measured 8 h post infection of unstimulated BMDCs with F. novicida.
To investigate whether IFN-β signalling is required for inflammasome activation, we generated BMDC from mice deficient for the Type I Interferon Receptor. IFNR-/- BMDC were infected with F. novicida and cell death and IL-1β release were monitored. Surprisingly, Caspase-1-mediated cell death (Fig. 5B) and IL-1β release (Fig. 5C) were only partially inhibited in IFNR-/- BMDC. This is in contrast to macrophages where the absence of the interferon receptor abolishes inflammasome activation (Henry et al., 2007). These results suggest that in BMDC, in contrast to BMDM, type I interferon signalling is only partially required for inflammasome activation.
Discussion
Dendritic cells are key players in innate immunity against microbial infections because they engulf potential pathogens. In addition, DCs bridge innate and adaptive immunity by activating T cells through antigen presentation. As potential initial targets for intracellular pathogens, it is not surprising that these cells would have evolved defence mechanisms to prevent intracellular pathogen replication and protect the host from systemic spread of the pathogen. Our study shows that the AIM2 inflammasome is activated by F. novicida in DCs, and that this leads to potent pro-inflammatory cytokine release and pyroptotic cell death.
It was previously demonstrated that LVS-infected monocyte-derived human DCs secrete IL-1β (Ben Nasr et al., 2006). Interestingly, when infected with the highly virulent F. tularensis SCHU S4 strain, human monocyte-derived macrophages, but not DCs, secreted IL-1β (Chase and Bosio, 2010). LVS-infected murine BMDCs and monocyte-derived human DCs were shown to secrete IL-1β in a Caspase-1 dependent way, as secretion was abolished by the use of a Caspase-1 inhibitor (Li et al., 2006). Our work expands this observation and demonstrates that in DCs, IL-1β is processed by the AIM2 inflammasome, as cytokine production is completely abolished in BMDCs derived from mice deficient for any of the three AIM2 inflammasome components examined here.
We show that F. novicida rapidly replicates to high numbers in BMDCs for the first 10 h of infection. However, we observe that the number of intracellular bacteria in our gentamicin protection assay decreases at 24 h. This decrease is due to rapid cell death induced by activation of the AIM2 inflammasome, which exposed intracellular bacteria to gentamicin killing. It was reported that in infected murine BMDCs, F. tularensis SCHU S4 bacterial numbers increase for 72 h before cell death is observed (Chase et al., 2009). LVS was shown to replicate in murine BMDCs within 24 h of infection; however, neither bacterial numbers nor DC death was monitored beyond that point (Bar-Haim et al., 2008). Finally, it was reported that LVS replicates in human monocyte-derived DCs, and that replication results in substantial cell death after 48 h, although the mechanism of cell death was not addressed (Ben Nasr et al., 2006). It is possible that AIM2 sensing dynamics differ between LVS, SCHU S4 and F. novicida leading to differences in the timing of inflammasome-mediated cell death. Future studies investigating inflammasome activation by various Francisella subspecies in macrophages and DCs are needed.
Pyroptosis in response to intracellular pathogens has a beneficial role for the host (Bergsbaken et al., 2009). Lysis of infected cells prevents replication and exposes bacteria to killing by extracellular axes of immunity like neutrophils and circulating antibodies. The importance of pyroptosis in fighting microbial infection was elegantly demonstrated in a recent study by Miao and colleagues. In this study, the authors engineered a S. typhimurium strain to constitutively express flagellin, thereby constantly activating the NLRC4 inflammasome. The authors showed that in contrast to wild-type S. typhimurium, which is lethal to mice, the mutant strain was cleared from infected mice through inflammasome-mediated death of infected cells (Miao et al., 2010). The authors showed that pathogen clearance was mainly due to pyroptotic cell death re-exposing bacteria to neutrophil killing, with little role played by pro-inflammatory cytokines as mice deficient for both IL-1β and IL-18 showed pathogen clearance similar to wild-type mice. The authors also showed that restriction of L. pneumophila and Burkholderia thailandesis in vivo was mainly dependent on pyroptotic cell death (Miao et al., 2010). Therefore, pyroptotic cell death plays a key role in fighting microbial infections.
In addition, pyroptosis may limit the spread of Francisella within the host organism. Bar-Haim and colleagues showed that during intranasal infection, LVS propagates to mediastinal lymph nodes by infecting trafficking DCs (Bar-Haim et al., 2008). Mice pretreated with an inhibitor of airway DC migration exhibited less bacterial burden at mediastinal lymph nodes, which correlated with delayed appearance of disease and animal mortality (Bar-Haim et al., 2008). Therefore, trafficking airway DCs play a role in dissemination of Francisella within the host and pyroptotic cell death of infected DCs could serve to reduce this dissemination. It is tempting to speculate that when Francisella is acquired by the bite of an infected arthropod, rapid inflammasome-mediated death of infected Langerhans cells (DCs in the epidermis) could be important in limiting the ability of bacteria to spread to systemic sites by removing their trafficking vessel.
Francisella-induced pyroptosis in macrophages and DCs are both mediated by the AIM2 cytosolic DNA receptor. Although we observed minor differences in pyroptosis kinetics in the different cell types, with infected BMDCs dying slightly faster compared to BMDM (data not shown), AIM2, ASC and caspase-1 are all required. However, type I interferon signalling, which is indispensable for inflammasome activation in macrophages, is only partially required in DCs (Fig. 5). DC precursors, the plasmacytoid pre-DC (p-preDC) secrete very high amounts of type I interferons, which have also been shown to act as a differentiation/maturation factor for DCs (Tough, 2004). The events regulated by type I interferon in DCs are still poorly understood (Coccia, 2008). It is tempting to speculate that links between the inflammasome platform and type I interferon signalling differ in macrophages and DCs. More studies are necessary to address the precise communication between these two cytosolic innate immunity axes in different cell types.
We have previously shown that mice deficient for inflammasome components Caspase-1, Asc and AIM2 are more susceptible to F. novicida and LVS infection (Mariathasan et al., 2005; Weiss et al., 2007b; Jones et al., 2010). These mice display higher mortality rates compared with WT mice and carry higher bacterial burdens at systemic sites. Therefore, inflammasome activation is key for defence against Francisella infection. The full repertoire of cells invaded by Francisella is still under investigation and whether the bacterium triggers the inflammasome in different cell types is largely unknown. In addition to macrophages and DCs, LVS was shown to invade and replicate within alveolar type II epithelial cells in vitro and in vivo (Hall et al., 2007) and in hepatocytes (Conlan and North, 1992). Inflammasome activation is poorly characterized in nonmyeloid cell lineages (Yazdi et al., 2010). Inflammasome activation has been reported in gut epithelial cells in response to S. typhimurium (Muller et al., 2009) and in skin keratinocytes in response to contact sensitizers (Watanabe et al., 2007). Francisella encounters epithelial cells at its two main entry points: skin (bite from infected arthropod) or lung (aerosolized bacteria). Early inflammasome activation in lung and/or skin DCs may help limit bacterial dissemination from peripheral sites to systemic tissues as well as intracellular replication, while activation in macrophages may limit intracellular replication in systemic sites. In addition, inflammasome activation in other cell types, e.g. epithelial cells, could contribute to mounting an efficient innate and adaptive immune response against this facultative intracellular pathogen.
Experimental procedures
Bacterial strains and cultures
Overnight cultures of F. novicida strains U112 and ΔFPI were started from frozen stocks in Tryptic Soy Broth (TSB) supplemented with 0.2% Cysteine and were grown shaking at 37°C. The FPI deletion strain was generated by gene replacement of the complete FPI by a Kanamycin-resistance gene in the U112 background (Weiss et al., 2007a).
DC culture
Marrow-derived dendritic cells were generated from mouse bone marrow following the standard procedures described in Lutz and colleagues (1999). Briefly, bone marrow from femurs and tibias was flushed with DMEM, washed once with PBS, then plated with DMEM High Glucose (Gibco) supplemented with L-Glutamine, 110 mg l−1 Sodium-Pyruvate, 10% Fetal Bovine Serum (HyClone), 10 mM Hepes, 40 mM β-mercaptoethanol, and 20 µg l−1 recombinant murine Granulocyte Macrophage Colony Stimulating Factor (GM-CSF) (Peprotech, Rocky Hill, NJ, USA). Cells were harvested and used at day 10, at which point FACS analysis showed that greater than 90% were positive for the DC marker CD11c.
Intracellular replication assay
Marrow-derived dendritic cells were seeded into 24 well plates at a density of 2.5 × 105 cells/well and incubated overnight at 37°C. The next day, cells were centrifuged and supernatants were replaced by media containing F. novicida diluted at the appropriate moi. Bacteria were centrifuged onto cells and the plates were incubated for 45 min at 37°C. Infected DCs were washed three times with PBS, with a centrifugation step after each spin to minimize cell loss, and fresh DC media containing 10 µg ml−1 gentamicin was added. At the appropriate time point, cells were washed three times with PBS and lysed with PBS containing 0.1% Saponin for 5 min. The lysates were diluted and plated on modified Mueller–Hinton media for CFU counts.
DC cell death, cytokine release, Western blots and immunofluorescence microscopy
Marrow-derived dendritic cells were seeded onto 96 well plates at a density of 5 × 104 cells per well and were infected as described above at the appropriate moi. Whenever specified, BMDCs were pre-stimulated with 100 ng ml−1 Pam3CSK4 (Invivogen) 16 h before infection. Supernatants were removed and assayed for lactate dehydrogenase release using the CytoTox 96 kit (Promega), and for IL-1β release by ELISA using the Mouse IL-1β/IL-1F2 DuoSet (R&D systems). Western blots for Caspase-1 p10 subunit and immunofluorescence microscopy were performed as previously described (Jones et al., 2010; Broz et al., 2010b).
Acknowledgements
We thank Nobuhiko Kayagaki and Vishva Dixit for providing femurs of Asc-/- and Aim2-/- mice, and Greg Barton for providing femurs of IFN-R-/- mice. We thank Petr Broz for technical advice, useful discussion and comments on the manuscript, Laura McLaughlin for technical advice and Thomas Ruby for technical help and help with FACS experiments. K.B. was supported by a post-doctoral fellowship from the Natural Sciences and Engineering Research Council (NSERC) in Canada. The project described was supported by Award Number U54AI065359 from the National Institute of Allergy and Infectious Diseases to Denise Monack. The content is the sole responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Disease or the National Institutes of Health.