The Entamoeba histolytica methylated LINE-binding protein EhMLBP provides protection against heat shock
Summary
Adaptation to environmental stress is a key process that allows the unicellular parasite Entamoeba histolytica to survive in its human host. We previously characterized EhMLBP as an essential protein for the growth and the virulence of the parasite. EhMLBP binds to methylated repetitive DNA, and is one of the core proteins of the parasite's epigenetic machinery. Here, we show that EhMLBP and heat shock proteins have common properties. EhMLBP is induced by heat shock and its expression is regulated by a heat shock element binding site that is located in its 5′ non-coding region. Following heat shock, the perinuclear localization of EhMLBP in control trophozoites is replaced by an even distribution within the nucleus alongside with an enhanced recruitment of EhMLBP to the reverse transcriptase of a long interspersed nucleotide element (LINE) DNA. Constitutive overexpression of EhMLBP protects trophozoites against heat shock and reduces protein aggregation. This protective function is lost in trophozoites that overexpress a mutated form of EhMLBP which is devoid of its heat shock domain. To the best of our knowledge, this is the first report of a methyl DNA-binding protein that plays a protective role against heat shock.
Introduction
Amoebiasis is an acute or chronic gastrointestinal disease that is caused by the protozoan parasite, Entamoeba histolytica, and has two major clinical manifestations, haemorrhagic colitis and liver abscesses (Blessmann et al., 2002). It is estimated that approximately 50 million people worldwide are infected with the parasite, thereby making the disease a serious global health problem (WHO, 1997). Infection by the parasite occurs after ingesting water or food that is contaminated with faeces that contains amoebic cysts (Andersen, 2000). The host's response against E. histolytica infection is complex, and it often includes fever in cases of liver amoebiasis (Salles et al., 2003).
The heat shock (HS) response, in which a sudden temperature rise triggers the synthesis of heat shock proteins (HSPs), is the most evolutionary conserved defensive mechanisms that organisms have developed in order to protect themselves against an acute exposure to elevated temperatures (Shamovsky and Nudler, 2008). Most HSPs are molecular chaperons that bind to non-native proteins and prevent the accumulation of toxic protein aggregates that are formed following HS (Hendrick and Hartl, 1993). In E. histolytica, two HSPs, HSP 100 and HSP 70, have been extensively studied due to their association with some epigenetic components of the parasite. For example, methylated cytosines are present in the 5′ untranslated region of the HSP100 gene (Bernes et al., 2005), and HSP 100 expression is induced by 5-azacytidine, an inhibitor of DNA methyltransferase. The second one is HSP 70 whose expression is upregulated consequently to the overexpression of the E. histolytica DNA methyltransferase 2 (Ehmeth) (Fisher et al., 2006).
The E. histolytica methyl-binding protein (EhMLBP) is a protein that specifically binds to methylated long interspersed nucleotide element (LINE) retrotransposons and ribosomal DNA (rDNA) in E. histolytica (Lavi et al., 2006; 2008). We have recently shown that EhMLBP is essential for the growth and virulence of E. histolytica (Lavi et al., 2008). In this report, we show that EhMLBP plays an unexpected role in the protection of the parasite against HS. Our results suggest that the underlying mechanism of the protective action of EhMLBP is to reduce the amount of toxic protein aggregates. Finally, we observed that the binding properties of EhMLBP to DNA are regulated by HS and that EhMLBP is involved in the control of LINE expression. This work represents the first example of amethylated DNA-binding protein that shares properties with HSPs.
Results and discussion
EhMLBP expression is regulated by HS
EhMLBP was originally characterized as a protein that binds to specific methylated DNA targets through its lysine-rich C-terminal domain (Lavi et al., 2006; 2008). In order to obtain functional information about EhMLBP in E. histolytica, we did a hidden Markov Models (HMM-HMM) comparison for homology detection and structure prediction using the HMM-HMM comparison tool (HHpred). Typically, psi-blast searches with the sequence of interest as input against the non-redundant sequence database from NCBI is automatically performed by the software to build a multiple alignment from it. This alignment will then be used to construct a concise statistical representation (a hidden Markov model or HMM) and to search the selected HMM databases for homologues (in our case, the PDB70 database was searched). Therefore, there is no need to generate manually a HMM model as this is automatically done by the software. The results of the HMM-HMM comparison revealed that the N-terminal of EhMLBP is homologous to the fibrinogen alpha chain, and its middle part contains a domain of HSP (HSD) (Fig. 1).

Schematic representation of EhMLBP and its 5′ non-coding region. Analysis of the 5′ non-coding region of EhMLBP from nucleotide −313 to +1 using the transcription factor search (TFSEARCH) tool shows that the region from −33 to +1 contain heat shock elements that are characterized by the classical inverted repeat, GAA GAA TTC (top box, underlined nucleotides). Analysis of the coding region of EhMLBP using the HMM-HMM comparison tool shows that this region contains two different domains: a 30–450 bp fibrinogen alpha chain domain and a 450–645 bp heat shock domain. A third domain localized between 645 and 912 bp is the DNA-binding domain (Lavi et al., 2006).
Heat shock proteins are classified according to their molecular size. Small HSPs have molecular masses that range from 15 to 40 kDa, and the molecular mass of EhMLBP is 32 kDa (Klemenz et al., 1991). Small HSPs are found in the three domains of life, Archea, Bacteria and Eukarya, and share a common α-crystallin domain. Phylogenetic analysis on these small HSPs revealed that this α-crystallin domain was already present even before the prokaryotes and eukaryotes diverged (Kriehuber et al., 2010). A remarkable feature is that EhMLBP lacks this conserved α-crystallin domain.
In order to gain insights into the regulation of EhMLBP expression, we explored the 5′ non-coding region of EhMLBP for transcription factor binding sites using the transcription factor search (TFSEARCH) tool. Interestingly, binding sites for heat shock elements (HSEs) (Pelham, 1982) were predicted by the TFSEARCH search tool. HSEs are short inverted repeats of the sequence nGAAn that are found in multiple copies around the transcriptional start sites of HSP genes (Pelham, 1982). Four putative HSE binding sites were identified in the −33 to +1 region of the start codon by the search tool. This 33 bp DNA fragment contains the only inverse repeat (TTC) of HSE that was found in the 5′ non-coding region of EhMLBP in addition to the repeats of the sequence GAA thus making this region to contain a perfect triplicate of HSE. The presence of HSEs has been reported in the promoter region of proteins that are not HSPs, such as interleukin-6 (A.A. Parikh, 1988), p35 (Chang et al., 2006) and E. histolytica Rab GTPase (Romero-Diaz et al., 2007). The results of a recent analysis on the whole-genome sequence of Drosophila melanogaster showed that only 10% of the genes that contain HSE are indeed upregulated by HS (Gonsalves et al., 2011). Therefore, the presence of an HSE in the promoter region of genes does not necessarily cause upregulation of transcription following HS. In E. histolytica, the response to HS results in an overall downregulation of gene expression, and only a few genes are upregulated (Weber et al., 2006). Hence, these observations raise a question of whether HS can regulate EhMLBP expression.
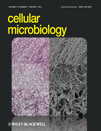
In order to test whether HS can regulate EhMLBP expression, time-course of EhMLBP expression was determined in control and in E. histolytica trophozoites exposed to HS by Northern blotting. After 20 min exposure to heat (42°C), EhMLBP mRNA levels in the heat-shocked trophozoites were significantly higher than those in the control trophozoites (Fig. 2A). High level of EhMLBP mRNA was also observed in trophozoites exposed to HS for 40 and 60 min. An identical induction profile was observed for HSP 70 (Fig. 2A). These data were corroborated on a protein level by Western blot analysis using a polyclonal antibody against EhMLBP (Lavi et al., 2006) and a monoclonal antibody against HSP 70 as a positive control (Fig. 2B). These results indicate that EhMLBP expression is upregulated by HS. Trophozoites maintained 2 h at 37°C after removal of the heat stress had their HSP 70 level returned to the level before heat stress. In contrast, the upregulation of EhMLBP expression was still observed in trophozoites brought back to 37°C for 12 h (Fig. 2C). One example of non-heat shock protein that is upregulated during recovery from HS is the A-rich-binding protein PABP1. This protein is an important regulator of mRNA synthesis and is involved in translation initiation rate during recovery (Ma et al., 2009). The ongoing expression of EhMLBP after HS suggests that this protein plays also an important role in the recovery process.
Time-course of EhMLBP expression in response to heat shock and recovery at 37°C. A. Northern blot analysis of RNA that was prepared from control trophozoites (37°C) and heat-shocked trophozoites (20, 40 and 60 min at 42°C). The EhMLBP, HSP 70 and actin probes were prepared by PCR using the primers described in Table 1 and E. histolytica genomic DNA as template. B. Nuclear protein fractions from control trophozoites (37°C) and heat-shocked trophozoites (20, 40 and 60 min at 42°C) were probed with EhMLBP, HSP 70 and actin antibodies, and analysed by Western blotting. C. Nuclear protein fractions from control trophozoites (37°C) and trophozoites exposed to 42°C for 60 min followed by a recovery period (2, 4 or 12 h at 37°C) were probed with EhMLBP, HSP 70 and actin antibodies, and analysed by Western blotting. Data presented in this figure are representative of at least three independent experiments.
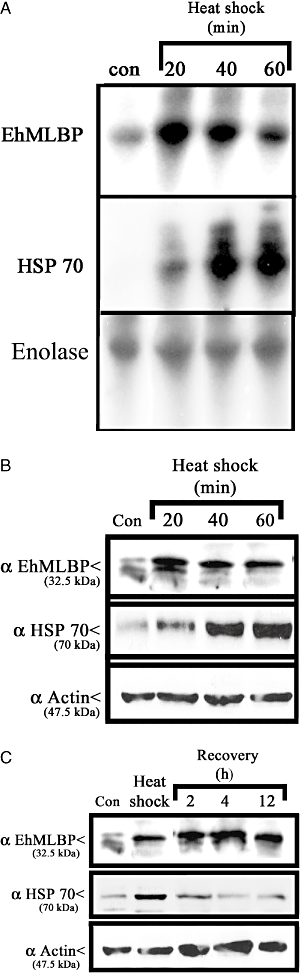
The expression of E. histolytica HSP 100 (EhHSP100) was reported to be regulated by a 25 kDa HSE transcription factor which binds to its HSE binding site in the EhHSP100 promoter (Bernes et al., 2005) (Fig. 3A). In order to determine whether HS causes upregulation of EhMLBP expression by the same mechanism, we assessed the extent of binding of HSE proteins to the EhMLBP promoter by Southwestern blotting. The nuclear protein fraction of heat-shocked trophozoites contained a 25 kDa protein which could bind to both the EhHSP100 promoter (used as the positive control) and the 5′ non-coding region of EhMLBP (Fig. 3A). Since the size of this protein that binds EhHSP100 promoter is identical to that that binds to the 5′ non-coding region of EhMLBP, this finding suggests that the same HSE-binding protein binds to both sites.
Role of heat shock elements in the transcription of EhMLBP. A. Southwestern blot analysis: lanes 1, 3, 5, 7: nuclear protein fractions from control trophozoites; lanes 2, 4, 6, 8: nuclear protein fractions from heat-shocked trophozoites. Lanes 1 and 2: nuclear protein fractions were probed with a radiolabelled probe for the 5′ non-coding region of E. histolytica HSP 100. Lanes 3 and 4: nuclear protein fractions were probed with a radiolabelled probe for the EhMLBP 5′ non-coding region. Lanes 5 and 6: nuclear protein fractions were probed with a radiolabelled probe for the mutated EhMLBP 5′ non-coding region (devoid of its HSE). Lanes 7 and 8: nuclear protein fractions were probed with a radiolabelled probe for the EhMLBP 5′ non-coding region and an unlabelled probe for HSP 100 5′ non-coding region as a competitor. B. Chloramphenicol acetyl transferase (CAT) gene was expressed under the control of the 5′ non-coding region of EhMLBP (from −313 to the start codon) or under the control of a mutated EhMLBP 5′ non-coding region (devoid of its HSE). CAT expression among the different transfectants was determined under control (37°C) or heat shock condition (60 min at 42°C). Upper panel shows Ponceau S staining of total protein fraction from the different transfectants. Lower panel shows Western blot analysis on CAT expression under control and heat shock conditions in the different transfectants. Data presented in this figure are representative of at least three independent experiments.
In order to determine whether these two 25 kDa HSPs are identical, we compared their binding abilities using a radiolabelled DNA probe of the 5′ non-coding region of EhMLBP in the presence of 5 µg ml−1 of an unlabelled DNA probe of the EhHSP100 promoter (Fig. 3A). Interestingly, the 25 kDa HSE-binding protein cannot bind to a mutated form of the 5′ non-coding region of EhMLBP devoid of its HSE binding sites (33 to +1 region). This finding suggests that the HSE binding sites is essential for the binding of the HSE-binding protein to the 5′ non-coding region of EhMLBP. In presence of unlabelled DNA probe of the EhHSP100 promoter, the binding of the radiolabelled probe of the 5′ non-coding region of EhMLBP to the 25 kDa protein was completely abolished (Fig. 3A). This result strongly suggests that the identical 25 kDa HSE-binding protein binds to both EhHSP100 and the 5′ non-coding region of EhMLBP in heat-shocked E. histolytica trophozoites.
To further demonstrate the role of the HSE-binding domain in the expression of EhMLBP in heat-shocked E. histolytica trophozoites, we cloned a DNA fragment of the 5′ non-coding region of EhMLBP (−313 to +1) and of the 5′ non-coding region of EhMLBP deleted of the HSE-binding domain in front of the CAT reporter gene (p-full and p-truncated constructions). The expression of CAT was measured in p-full and p-truncated transformed trophozoites. The p-full construct was sufficient to drive the expression of CAT in control trophozoites and to upregulate its expression in heat-shocked trophozoites (Fig. 3B). In contrast, the p-truncated construct was unable to upregulate CAT expression in heat-shocked E. histolytica trophozoites.
Taken together, these data confirm that the HSE that is present in the 5′ non-coding region of EhMLBP is involved in the regulation of EhMLBP expression in heat-shocked E. histolytica trophozoites.
Effect of HS on the nuclear localization of EhMLBP
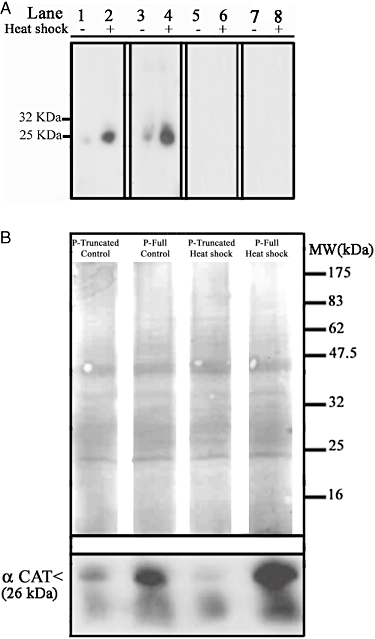
The upregulation of EhMLBP expression in heat-shocked trophozoites and the presence of an HSD in the protein suggest that EhMLBP is involved in the parasite's response to HS. In order to test this hypothesis, EhMLBP or a mutated form that does not have the HSD was constitutively overexpressed in E. histolytica trophozoites that were transfected with the plasmids, p-EhMLBP over and p-EhMLBPm respectively. The overexpression of EhMLBP and its mutated form was confirmed by Western blot analysis. We observed that the expression of EhMLBP in the p-EhMLBP over transfectants was 2.5-fold higher than that of the p-control transfectants (densitometry analysis) (Fig. 4A). Indeed, the level of EhMLBP expression in the p-over transfectants cultivated at 37°C was similar to that of the heat-shocked p-control transfectants (Fig. 4A). EhMLBPm is detected by the EhMLBP antibody in the Western blots as a 27 kDa protein which corresponds to the theoretical mass of the mutated protein (Fig. 4A).
Expression of EhMLBP in heat-shocked p-control E. histolytica transfectants, p-EhMLBP over E. histolytica transfectants and p-EhMLBPm E. histolytica transfectants. A. Western blot analysis of EhMLBP expression in control and heat-shocked p-control, p-EhMLBP over and p-EhMLBPm E. histolytica transfectants. Nuclear and cytoplasmic protein fractions from all transfectants were probed with an antibody against EhMLBP (upper panel), and an antibody against actin (lower panel). B. Upper panel: laser scan immunofluorescence analysis of HM1:MSS untransfected E. histolytica trophozoites, p-control, p-EhMLBP over and p-EhMLBPm E. histolytica transfectants. EhMLBP was detected using a primary antibody against EhMLBP and a secondary Cy3-conjugated antibody (red). The nuclei (blue) were stained by TO-PRO®-3. Data presented in this figure are representative of at least three independent experiments.
EhMLBP has a perinuclear localization in E. histolytica trophozoites when they are grown under standard culture conditions at 37°C (Lavi et al., 2006). The behaviour of EhMLBP in heat-shocked E. histolytica trophozoites was examined by confocal immunofluorescence microscopy using an EhMLBP antibody (Fig. 4B). Computer-assisted image overlay of the EhMLBP and the TO-PRO®-3 signals confirmed the perinuclear localization of EhMLBP in control trophozoites. The perinuclear localization of EhMLBP was also observed in p-control and p-EhMLBPm transfectants grown at 37°C. Interestingly, we observed that EhMLBP was evenly distributed within the nucleus of heat-shocked trophozoites and in p-EhMLBP over transfectants that were grown at 37°C. This distributed nuclear localization was also observed in the heat-shocked p-EhMLBP over and p-control transfectants, but was lost in the heat-shocked p-EhMLBPm transfectants where a perinuclear localization of the protein was observed (Fig. 4B). In addition to its perinuclear localization, EhMLBPm was also detected in what looks like some secretory vesicles (Ghosh et al., 1999) (Fig. 4B). The meaning of this additional localization is still unknown.
The fact that EhMLBP in the p-EhMLBPm transfectants is unable to spread within the nucleus suggests that the HSD is essential for this phenomenon and that EhMLBPm acts as a dominant-negative protein.
Binding of EhMLBP to reverse transcriptase (RT) LINE DNA following HS
The redistribution of nuclear proteins under stress was reported to be highly dynamic and regulated process that involves transcription regulation, translation and chromatin processing (Messaoudi et al., 2007). The specificity and stability of protein–DNA complexes can be influenced by changes in the environment. For example, oxidative stress lead the bacterial DNA-binding proteins from starved cells (Dps) protein to spread across the DNA in order to prevent damage by oxidative radicals (Martinez and Kolter, 1997). The transcription factor Sp1 is another example of a protein that provides protection in acidic pH conditions and increases its DNA binding properties when the intracellular pH is low (Torigoe et al., 2003). In line with these evidences the spreading of EhMLBP into the nucleus of heat-shocked trophozoites suggests that EhMLBP binds differentially to its DNA targets following HS. The reverse transcriptase (RT LINE Accession No. AB097129.1) is one of the genes whose expression is upregulated by HS (Weber et al., 2006), and is also a known EhMLBP DNA target (Lavi et al., 2006). Therefore, the binding of EhMLBP to RT LINE was examined by ChIP in control and heat-shocked trophozoites (Fig. 5). We observed that the recruitment of EhMLBP to RT LINE DNA was significantly enhanced in heat-shocked trophozoites. In contrast, no enhanced recruitment of EhMLBP to rDNA was observed in control and heat-shocked trophozoites (Fig. 5A and B). HS has been reported to induce the transcription of long terminal repeats (LTR) and non-LTR retroelements in E. histolytica (Weber et al., 2006) and other organisms (Bradshaw and McEntee, 1989; Kimura et al., 1999; Li et al., 1999; Rudin and Thompson, 2001). Our results suggest that the enhanced recruitment of EhMLBP to RT LINE DNA in heat-shocked trophozoites is part of the mechanism that leads to the upregulation of RT LINE transcription. In order to test this hypothesis, the transcription of RT LINE was examined in control and heat-shocked p-control, p-EhMLBP over and p-EhMLBPm transfectants using reverse transcriptase (RT)-polymerase chain reaction (PCR) (Fig. 5C) and Northern blot analysis (Fig. 5D). As previously reported, HS enhances the transcription of RT LINE (Weber et al., 2006) and EhMLBP (Fig. 2A). Indeed, the overexpression of EhMLBP led to increased expression of RT LINE under control condition (Fig. 5C and D). Due to the strong conserved sequence homology between the various RT LINEs (Sharma et al., 2001), the signal obtained from the Northern blot assay probably reflect the steady-state level of RT LINEs expression in the parasite. These finding suggest that EhMLBP is an activator of RT LINE expression. To determine if this function applies to the regulation of short interspaced interspersed nuclear elements (SINEs) expression (Cruz-Reyes et al., 1995; Van Dellen et al., 2002), the transcription of SINE was examined in the non-transfected HM1:MSS strain and in the p-control, p-EhMLBP over and p-EhMLBPm transfectants using Northern blot analysis (Fig. 5D). No significant difference in the amount of low molecular weight SINE mRNA species was observed between the non-transfected HM1:MSS strain and the transfectants. However, the presence of high-molecular-weight SINE mRNA species was consistently observed in p-EhMLBP over and p-EhMLBPm transfectants. Theses high-molecular-weight SINE mRNA species may correspond to new transcripts or are the result of post-transcriptional modifications such as polyadenylation which is known to increase the life time of SINEs (Borodulina and Kramerov, 2008). SINEs RNA are upregulated during HS and HS recovery (Li et al., 1999; Kimura et al., 2001). It is tempting to speculate that the upregulation of EhMLBP expression by HS and during recovery (Fig. 2C) is linked to SINEs kinetics; a hypothesis that will require further investigation to be answered.
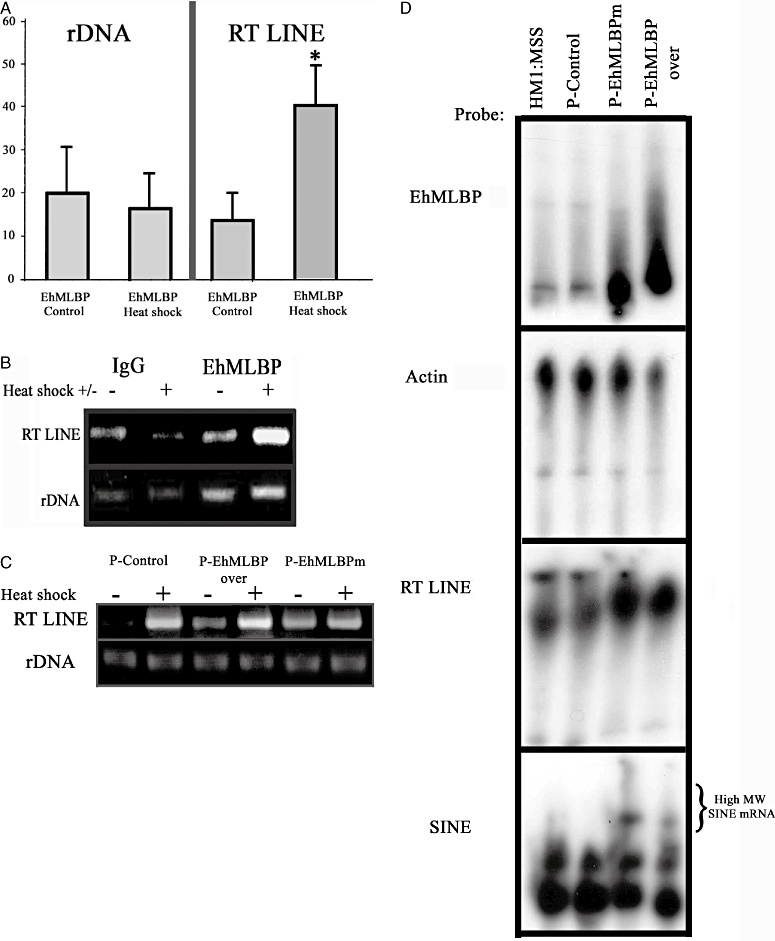
DNA binding pattern of EhMLBP following heat shock. A. Chromatin immunoprecipitation assays were performed on control and heat-shocked trophozoites. Formaldehyde cross-linked protein–DNA complexes were isolated. Sheared protein–DNA complexes were immunoprecipitated with a non-specific antibody (IgG), or an antibody against EhMLBP. Isolated fragments were amplified by PCR to detect the enrichment of the precipitated DNA. Genes that were amplified by PCR were rDNA and RT LINE. The graph displays the results of the densitometry quantification of four independent chromatin isolations and immunoprecipitations [mean arbitrary units ± standard error (SE)] normalized to the value for the IgG control (taken as 0). *P < 0.05 and is the significance of the difference between the control and heat-shocked values. B. Agarose gel analysis of one representative immunoprecipitation experiment following PCR amplification of rDNA and RT LINE. C. RT-PCR analysis on mRNA extracted from control (−) and heat-shocked (+) EhMLBP transfectants. The primers that were used to amplify rDNA and RT LINE are described in Table 1. D. Northern blot analysis of the steady-state RT LINE and SINE mRNA levels in non-transfected HM1:MSS trophozoites, p-control, p-EhMLBP over and p-EhMLBPm E. histolytica transfectants. The primers that were used to amplify the probes for EhMLBP, actin, RT LINE and SINE are described in Table 1. Data presented in (D) are representative of three independent experiments.
This putative role of EhMLBP as activator of RT LINE transcription conflicts with the established function of methyl DNA-binding domain (MBD) proteins, namely, they are repressors of retrotransposons transcription (Muotri et al., 2010). However, it has been reported that MBD proteins under certain cellular conditions can act as a cofactor for transcriptional activation (Fujita et al., 2003). The role of EhMLBP as a positive regulator of RT LINE transcription may be beneficial to the parasite as retroelements were reported to regulate the level of the host gene expression following HS (Mariner et al., 2008).
It has been reported that the binding of EhMLBP to RT LINE is mediated through the C-terminal domain of EhMLBP (Lavi et al., 2006). However since EhMLBP also has a separate domain which is homologous to chaperone protein, we investigated whether EhMLBP can act as a chaperone protein and protect the cell from HS (Kitagawa et al., 2000).
Overexpression of EhMLBP provides protection against HS
The resistance to HS of the p-EhMLBP over and p-EhMLBPm transfectants were compared with that of the p-control transfectants. The p-EhMLBP over transfectants were substantially more resistant to HS than the p-control transfectants (Fig. 6). Interestingly, this protective function was lost in EhMLBPm trophozoites. This result indicates that EhMLBP is involved in the resistance of the parasite to HS. Furthermore, the HSD is essential for this unexpected protective function because those trophozoites that expressed a mutated form of EhMLBP without an HSD are as sensitive to HS as the p-control trophozoites.
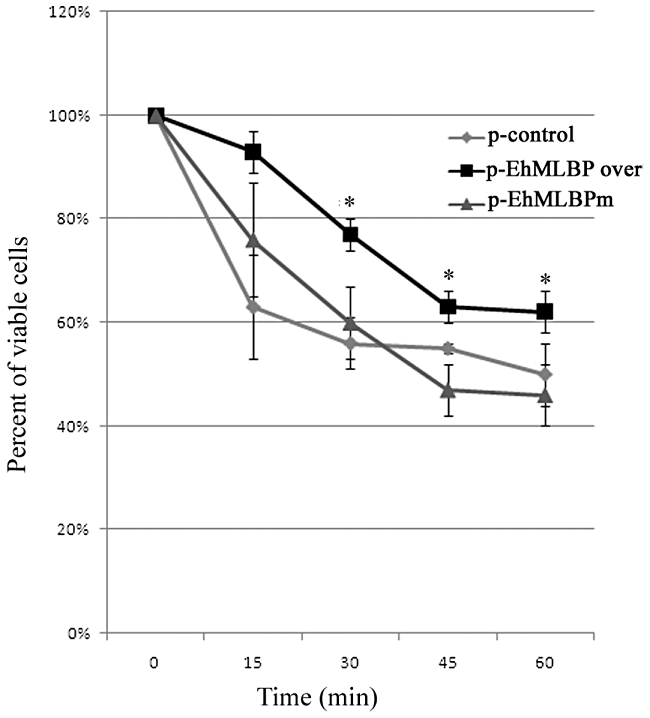
Viability of heat-shocked EhMLBP transfectants. P-control, p-EhMLBP over and p-EhMLBPm E. histolytica transfectants were exposed to 42°C for 1 h. Their viability was determined every 15 min using the ability of viable trophozoites to exclude eosin. The graph shows the survival curve from five independent experiments T-test was used in order to determine the difference of p-EhMLBP over from p-EhMLBPm and p-control (*Pvalue < 0.05).
Overexpression of EhMLBP reduces protein aggregates
Misfolded and denatured proteins by HS often aggregate and interact inappropriately with other cellular constituents, and the subsequent impairment of many biological processes (Stefani and Dobson, 2003). Therefore, the accumulation of aggregated proteins has detrimental effects on the cell viability, and their presence is the trigger for the HS response. One of the main function of the HSPs is to prevent the accumulation of aggregated proteins (Friant et al., 2003), and the overexpression of HSP 70 was reported to diminish aggregation (Nollen et al., 2001). In line with this background, we hypothesized that the overexpression of EhMLBP prevents the accumulation of aggregated proteins. In order to test this hypothesis, the amount of aggregated proteins was measured in the untransfected E. histolytica trophozoites strain HM1:MSS, p-control transfectants, p-EhMLBP over transfectants and p-EhMLBPm transfectants (Fig. 7, upper panel). Protein aggregates were detected in all the trophozoites and HS substantially increased their amount. However, the amount of aggregated proteins in the pEhMLBP over transfectants was the lowest of all the trophozoites, irrespective of whether they were or were not heat-shocked. No significant decrease of the amount of aggregated proteins was observed in the p-EhMLBPm transfectants when compared with that of HM1:MSS and p-control transfectants. Aggregated protein fractions were reported to contain ubiquitinated proteins and HSPs (Kopito, 2000; Gilks et al., 2004; Goldbaum and Richter-Landsberg, 2004; Ryhanen et al., 2009). The presence of ubiquitinated proteins, HSP 70 and EhMLBP in the aggregated protein fractions was therefore tested by Western blot analysis (Fig. 7, lower panels). Ubiquitinated proteins, HSP 70 and EhMLBP were detected in the aggregated protein fractions of all the cell lines examined. The higher amount of EhMLBP was observed following HS in the aggregated proteins prepared from p-EhMLBP over transfectants. The presence of EhMLBP in this fraction suggests that this protein interacts with denatured proteins in order to suppress their aggregation. This hypothesis is supported by the fact that the presence of a 27 kDa EhMLBP protein devoid of its HSD in the aggregated protein fractions of p-EhMLBPm transfectants (Fig. 7, lower panel) impedes the activity of the endogenous EhMLBP.
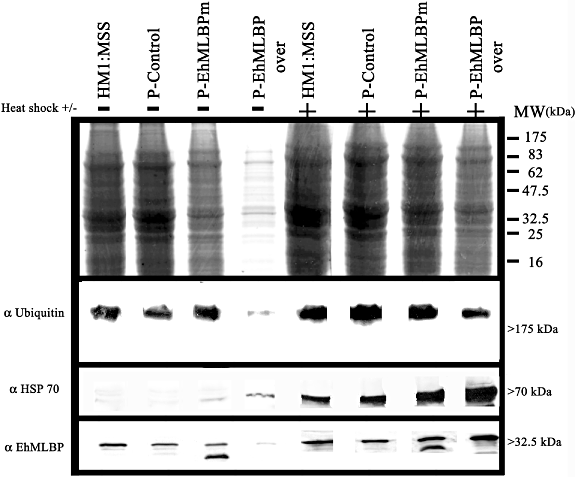
Overexpression of EhMLBP reduces the amount of aggregated proteins. Aggregated proteins from E. histolytica HM-1: IMSS trophozoites, p-control, p-EhMLBP over and p-EhMLBPm transfectants were isolated, as described in Experimental procedures. Upper panel: Coomassie blue staining of the aggregated proteins. Lower panel: Western blot of the aggregated proteins which were probed for ubiquitin, HSP 70 and EhMLBP, as indicated. Data presented in this figure are representative of three independent experiments.
In summary, EhMLBP is a MBD protein whose expression is upregulated by HS. Our results suggest that EhMLBP has a dual function: regulation of RT LINE and possibly SINE expression by is DNA-binding domain and the protection of the parasite against HS that requires its HSD. Therefore, we further propose that EhMLBP serves as a bridge between environmental stress response and epigenetic regulation in E. histolytica.
Experimental procedures
Parasite and cell culture conditions
Entamoeba histolytica trophozoites strain HM-1: IMSS were grown under axenic conditions in Diamond's TYI-S-33 medium at 37°C. Trophozoites in the exponential phase of growth were used in all experiments, and trophozoites were exposed to 42°C for 20, 40, 60 min in the kinetic assay and for 60 min in the other HS assays.
HHpred search of the EhMLBP protein against the PDB70 database
HMM-HMM comparison was performed using the HHpred server (http://toolkit.tuebingen.mpg.de/hhpred#) with the EhMLBP sequence as query sequence against the PDB70 database and a Max. HHblits iterations of 3.
Computational analysis of EhMLBP 5′ non-coding region
The presence of transcription factor binding sites in the 5′ non-coding region of EhMLBP was predicted using the transcription factor search (TFSEARCH) tool (http://www.cbrc.jp/research/db/TFSEARCH.html) and the yeast binding site profile matrix.
Northern blotting
Total RNA was prepared using the RNA isolation kit TRI Reagent (Sigma). RNA (10 µg) was size fractionated on a 4% polyacrylamide denaturing gel that contained 8 M urea and subsequently blotted to a GeneScreen membrane (NEN Bioproducts, Boston, MA). Under stringent conditions, hybridization was done overnight at 65°C in hybridization buffer [0.5 M Na-phosphate (pH 7.2), 7% sodium dodecyl sulfate (SDS), 1 mM EDTA and 50 µg ml−1 hybridization buffer of salmon sperm] with the following DNA probes (0.004 µg ml−1): actin, HSP 70, EhMLBP, RT LINE and SINE. The probes were prepared by PCR using the primers described in Table 1 and E. histolytica genomic DNA as template.
| Primers | Primer sequence 5′–3′ | Direction | Underlined restriction sites |
|---|---|---|---|
| rDNA 5′ | ATGGTGAACAATCATACCTT | Sense | |
| rDNA 3′ | TTATCGGATGTGTGAGCCC | Antisense | |
| HSP 70 cDNA 5′ | TAAGAAGAGTGGCAAAAAGAGTATTG | Sense | |
| HSP 70 cDNA 3′ | TGTATTCTACTTCAATTAATGGTTTAT | Antisense | |
| EhMLBP cDNA 5′ | ATGAACGCATTTATTCTCCTC | Sense | |
| EhMLBP cDNA 3′ | GCATATTTGTTTGCAAGTTGTG | Antisense | |
| Actin 5′ | ATGGGAGACGAAGAAGTTCAAGCAC | Sense | |
| Actin 3′ | ATCTTCATTGTTGGTGGTGCTAA | Antisense | |
| HSP 100 promoter 5′ | ATGAATAAGAAAGTGTGAATAATAG | Sense | |
| HSP 100 promoter 3′ | TGAGTATTTAAAGGAACTTGAAG | Antisense | |
| EhMLBP promoter 5′ | GGTTTTAATTAAAAATCAACA | Sense | |
| EhMLBP promoter full 3′ | CTTCAGCAGTAGCAAGTGCA | Antisense | |
| EhMLBP promoter truncated 3′ | GTTATTAGTTCTTTCTTAAATACCTA | Antisense | |
| Kpn1EhMLBP promoter 5′ | GGTACC GGTTTTAATTAAAAATCAACA | Sense | GGTACC |
| Kpn1EhMLBP promoter full 3′ | GGTACCCTTCAGCAGTAGCAAGTGCA | Antisense | GGTACC |
| EhMLBP Kpn 5′ | GGTACCATGAACGCATTTATTCTCC | Sense | GGTACC |
| EhMLBP Bamh1 3′ | AGGATCCTTATTTCTTAGCGATCTTTTTGG | Antisense | GGATCC |
| EhMLBP 645 Xba1 5′ | TCTAGAGTTGCTAGAAATAATGTTGAG | Sense | TCTAGA |
| EhMLBP 447 Xba1 3′ | TCTAGATTTCTTAATAGTAGCTTGTGT | Antisense | TCTAGA |
| RT LINE 5′ | TGATGTTAAATCCAATTGAAAAACAA | Sense | |
| RT LINE 3′ | AACATTGTTTAATCCATTGATGATT | Antisense | |
| SINE 5′ | AGTTATTATCTGGTTTGACG | Sense | |
| SINE 3′ | TCATCTATTCTTCCTGCTC | Antisense | |
| RT LINE (probe) 5′ | ATGATGAAATATGGAAAAATGC | Sense | |
| RT LINE (probe) 3′ | TGTTTAGATAATACATCAATAGATA | Antisense |
The membrane was then washed at 65°C for 20 min with washing buffer 1 [5% SDS, 40 mM sodium phosphate (pH 7.2) and 1 mM EDTA], followed by three 30 min washes at 65°C with washing buffer 2 [1% SDS, 40 mM sodium phosphate (pH 7.2) and 1 mM EDTA]. The DNA probes were randomly labelled with p32 dCTP (Amersham) using a DNA labelling kit (Biological Industries).
Fractionation of trophozoites and Western blot analysis
Nuclear and cytoplasmic protein fractions of E. histolytica trophozoites were prepared, using a previously published protocol (Lavi et al., 2006). The proteins were resolved by 12% SDS-polyacrylamide gel electrophoresis, and then transferred to nitrocellulose membranes. The membranes were first blocked with 3% skim milk in phosphate-buffered saline (PBS)-Tween, and then probed overnight at 4°C with primary antibodies against (i) 1:1000 EhMLBP (Lavi et al., 2006), (ii) 1:1000 actin (MP Biomedicals), (iii) 1:1000 HSP 70 (StressMarq), and (iv) 1:1000 chloramphenicol acetyl transferase (CAT) (Sigma-Aldrich) and 1:1000 ubiquitin (zymed, Invitrogen). At the end of the incubation, the membranes were incubated with an appropriated conjugated secondary antibody (1:10 000) (Jackson ImmunoResearch), and the blots were then developed by enhanced chemiluminescence (EZ-ECL, Biological Industries).
Plasmid construction and transfection in E. histolytica
A 313 bp sequence upstream to the EhMLBP start codon was amplified from E. histolytica genomic DNA using the primers, EhMLBP promoter 5′ and EhMLBP promoter full 3′ (Table 1). The truncated 5′ EhMLBP non-coding region was amplified using the primers, EhMLBP promoter 5′ and EhMLBP promoter truncated 3′ (Table 1), and the amplified products were then cloned into a pGEM®-T easy vector (Promega, Biological Industries).
For construction of the plasmids p-full or p-truncated that were used in the CAT reporter gene assay, the 5′ non-coding region (−313 to +1 or −313 to −33) of EhMLBP was first amplified using primers that contain a KpnI restriction site, and then cloned into the pEhNeo/CAT vector (Hamann et al., 1995; Isakov et al., 2008) that was previously linearized by digestion with the KpnI restriction endonuclease.
For construction of the plasmid, p-EhMLBP over, in which EhMLBP is overexpressed in E. histolytica trophozoites, EhMLBP was first amplified from E. histolytica cDNA using the primers, 5′ EhMLBP KpnI and 3′ EhMLBP BamHI. The resulting PCR product was then cloned into the expression vector pEhNEO/CAT, which was previously linearized by digestion with the KpnI and BamHI restriction endonucleases.
For construction of the plasmid, p-EhMLBPm, in which EhMLBP devoid of its HSP domain is expressed in E. histolytica trophozoites, the 5′ region of EhMLBP was amplified using the primers, 5′ EhMLBP and 3′ EhMLBP 450 XbaI. The 3′ region of EhMLBP was then amplified using the primers, 5′ EhMLBP 645 XbaI and 3′ EhMLBP. These two EhMLBP fragments were then digested with XbaI, ligated and cloned into the pGEM®-T Easy vector. The deletion of the HSP domain was confirmed by sequencing. Mutated EhMLBP was amplified using the primers, 5′ EhMLBP KpnI and 3′ EhMLBP BamHI, and the amplified product was subcloned into pEhNeo/CAT vector that has been previously linearized with KpnI and BamHI. The vector (p-scrambled pept-3) (Lavi et al., 2008) was used as the control vector (p-control).
The transfection of E. histolytica trophozoites with these different vectors was done using a previously described protocol (Fisher et al., 2006).
Southwestern analysis
The protein lysates of control and heat-shocked E. histolytica trophozoites were first separated on 12% SDS-PAGE, and then transferred to nitrocellulose membranes. The membranes were then hybridized overnight at 4°C in a standard binding buffer (20 mM Tris–HCl, pH 8.0; 50 mM NaCl; 1 mM EDTA) with the following radiolabelled DNA probes (0.01 µg ml−1): EHSP100 putative promoter (Bernes et al., 2005), the 5′ non-coding region (−313 to +1) of EhMLBP or the 5′ non-coding region of EhMLBP that lacks the HSE (−313 to −33). At the end of the incubation, the membranes were then washed three times in standard binding buffer, and exposed to X-ray film.
Detection of EhMLBP in trophozoites by immunofluorescence microscopy
Control and heat-shocked E. histolytica trophozoites were transferred to 8 mm round wells on glass slides, and then incubated for 15 min at 37°C so that they could become attached to the glass surface. The following indirect immunofluorescence assay was then performed using the attached trophozoites. Briefly, the amoebae were washed with PBS, fixed with cold methanol for 10 min at −20°C, and then permeabilized with 0.1% Tween. The slides were then blocked with 5% goat serum. The samples were then probed with 1:1000 EhMLBP antibody overnight at 4°C. After four rounds of washing with PBS buffer containing 2% BSA and 0.1% Tween, the samples were then incubated with goat Cy3-conjugated anti-mouse (Jackson ImmunoResearch) 1:300. The nuclei were stained with 1:1000 TO-PRO®-3. Confocal immunofluorescence microscopy was performed using a Radiance 2000 Bio-Rad laser scan microscope with a 60× oil immersion objective.
ChIP assay
The ChIP assay was performed using a previously described protocol (Anbar et al., 2005; Lavi et al., 2006) with minor modifications. E. histolytica trophozoites strain HM-1: IMSS (4 × 107 for each experiment) were grown for 48 h, and were then either heat shocked or used as control experiment. Each ChIP assay was performed in triplicate and immunoprecipitation was performed either using an antibody against EhMLBP or with pre-immune serum. The primers that were used to amplify RT LINE and rDNA are described in Table 1. The PCR products were separated by electrophoresis on 1.5% agarose gels, stained with ethidium bromide, and the intensity of the bands were quantified by densitometry.
RT-PCR
Entamoeba histolytica total RNA was treated with DNase-free RNase (Promega, Biological Industries) and then reverse transcribed using the EZ-First Strand cDNA Synthesis Kit (Biological Industries), according to the manufacturer's instructions.
Viability assay
Trophozoites (1 × 106) were exposed to 42°C for 1 h in TYI-S-33 medium without serum. Every 15 min, an aliquot of the culture was stained with eosin (0.1% final concentration), and the number of living trophozoites were counted in a counting chamber under a light microscope (Bausch and Lomb).
Isolation of aggregated proteins
Entamoeba histolytica trophozoites (3 × 106) from control or EhMLBP transfectants were incubated at 37°C or exposed to 42°C for 1 h. At the end of the incubation, the trophozoites were washed twice in ice-cold PBS, and then lysed in ice-cold extraction buffer (Akakura et al., 2001; Gilks et al., 2004) that contained 10 mM Tris, pH 7.4, 1 mM MgCl2, 0.2% Tween 20, 10 mM sodium molybdate for stabilizing the unstable chaperone complex (Akakura et al., 2001), and protease inhibitors. The lysates were then sonicated twice for 3 min in a Bioruptor UCD-200 sonicator (Diagenode) using 20 s bursts and 20 s between the bursts at the low setting in ice water. The samples were then immediately centrifuged at 14 000 g for 20 min at 4°C, and the supernatants were collected. The supernatant were then boiled in 2% SDS, and then precipitated using 60% acetone. The precipitates were then resuspended in equal volumes of reducing SDS-PAGE sample buffer before SDS-PAGE gel electrophoresis and immunoblotting as described.
Acknowledgements
This study was supported by grants from the Israel Science Foundation, the Israel Ministry of Health, the Rappaport Family Institute for Research in the Medical Sciences, the Niedersachsen-Technion programme and the Deutsche Forschungsgemeinschaft (DFG).