Plasmodium salvages cholesterol internalized by LDL and synthesized de novo in the liver
Summary
Our previous morphological studies illustrated the association of sterols with Plasmodium infecting hepatocytes. Because malaria parasites cannot synthesize sterols, they must scavenge these lipids from the host. In this paper, we have examined the source/s of sterols for intrahepatic Plasmodium and evaluated the importance of sterols for liver stage development. We show that Plasmodium continuously diverts cholesterol from hepatocytes until release of merozoites. Removal of plasma lipoproteins from the medium results in a 70% reduction of cholesterol content in hepatic merozoites but these parasites remain infectious in animals. Plasmodium salvages cholesterol that has been internalized by low-density lipoprotein but reduced expression of host low-density lipoprotein receptors by 70% does not influence liver stage burden. Plasmodium is also able to intercept cholesterol synthesized by hepatocytes. Pharmacological blockade of host squalene synthase or downregulation of the expression of this enzyme by 80% decreases by twofold the cholesterol content of merozoites without further impacting parasite development. These data enlighten that, on one hand, malaria parasites have moderate need of sterols for optimal development in hepatocytes and, on the other hand, they can adapt to survive in cholesterol-restrictive conditions by exploitation of accessible sterols derived from alternative sources in hepatocytes to maintain proper infectivity.
Introduction
Malaria parasites that infect mammals usually take up residence in hepatocytes where they actively multiply within a parasitophorous vacuole (PV). The replicative capacity of Plasmodium liver forms is remarkable, achieving one of the fastest growth rates among eukaryotic cells (reviewed in Sinnis and Sim, 1997). In order to meet their anabolic requirements and proficiently multiply within hepatocytes, these parasites must scavenge host cell nutrients that they cannot synthesize. Furthermore, the incompetence of Plasmodium to store host molecules necessitates a constant supply of nutrients to the parasite. To this end, the choice of hepatocytes in the mammalian host may stem from the unique metabolic properties of these cells. Compared with other cells, hepatocytes are particularly proficient at internalizing transport proteins (e.g. transferrin, lipoproteins) via specific surface receptors and at metabolizing many compounds (e.g. lipids, purines, glucose) in large quantities (Morgan and Baker, 1986; Pels Rijcken et al., 1993; Klover and Mooney, 2004; Nguyen et al., 2008).
The nutritional requirements of Plasmodium must be complex, because even rich media do not support the multiplication of parasites maintained in an axenic environment (Jayabalasingham et al., 2010). This observation implies that an intracellular niche is necessary to provide the parasite with a plethora of essential host cell factors during the trophic phase. The important role of the host cell in parasite growth is exemplified by the essentiality of the parasite protein UIS3 located at the PV membrane, which specifically interacts with the host liver-fatty acid binding protein (L-FABP), the main carrier responsible for the delivery of fatty acids to cytoplasmic compartments. The interaction between UIS3 and L-FABP may be necessary to supply the parasite with host fatty acids (Mikolajczak et al., 2007). We showed that the parasite is able to transform its PV into a highly permeable compartment (Bano et al., 2007). Open pores across the PV membrane interconnect the host cytoplasm and the vacuolar spaceand allow the passive diffusion of small molecules < 855 Da from host cytosolic pools. Moreover, the PV membrane displays long tubular extensions that pervade the host cytoplasm, which increases the surface of exchange between the host cell and the PV (Mueller et al., 2005). Of interest, it has been documented that intrahepatic parasites actively upregulate the expression of several host genes involved in metabolite transport and anabolism (Singh et al., 2007; Albuquerque et al., 2009). This brings light to the aptitude of the parasite in modifying the host's basic metabolism to increase the available pool of nutrients. We previously demonstrated that although Plasmodium is unable to synthesize sterols, it does contain cholesterol that must be diverted from host cell compartments and properly delivered to the PV (Bano et al., 2007). Interestingly, the PV membrane forms tight association with the host endoplasmic reticulum (ER) shortly after invasion and during schizogony (Bano et al., 2007; Jayabalasingham et al., 2010). The gathering of host ER to the PV membrane offers an attractive mechanism to situate the host lipid biosynthetic machinery in close proximity to the vacuole.
Hepatocytes play a vital role in cholesterol homeostasis in mammals. Like many cells, hepatocytes are able to acquire cholesterol through two pathways. Cholesterol is synthesized in the ER from acetyl-CoA via the mevalonate pathway (Goldstein and Brown, 1990). Cells can also acquire low-density lipoprotein (LDL)-associated cholesterol from serum through receptor-mediated endocytosis (Goldstein et al., 1985). LDL are degraded in late endosomes and lysosomes to release free cholesterol that then becomes available to other organelles. Besides these two pathways, hepatocytes can acquire a substantial amount of cholesterol through scavenger receptor class B type I (SR-BI)-mediated uptake of cholesterol from high-density lipoproteins (HDL) (Krieger, 1999). Unlike the uptake of cholesterol from LDL, cholesterol ester uptake from HDL does not involve the internalization and degradation of these particles in hepatocytes (Pagler et al., 2006). Hepatocytes are specifically implicated in the export of cholesterol. HDL retro-endocytosis and the secretion of very low- density lipoproteins (VLDL) are two pathways for cholesterol efflux (Olofsson and Boren, 2005). VLDL assemble in the ER lumen, where apolipoprotein B is synthesized and fuses with lipid droplets enriched in triglycerides and cholesteryl esters. The presence of lipid droplets in the ER sets hepatocytes apart from other mammalian cells, which tend to contain cytosolic lipid droplets (Murphy and Vance, 1999). Many intracellular pathogens that require host cholesterol for their growth are equipped to gain access to this lipid from the exogenous and/or endogenous pathway of the host, or from lipid bodies (Coppens et al., 2000; Carabeo et al., 2003; Catron et al., 2004; Kumar et al., 2006; Gilk et al., 2010).
In this paper, we have investigated the potential source/s of host cell cholesterol for Plasmodium developing in liver cells. We have addressed the following questions: Is the PV accessible to host cholesterol until merozoite maturation in the liver? Does the parasite have access to cholesterol synthesized by the host cell and/or to exogenous cholesterol delivered by lipoproteins? If exogenously acquired, is the cholesterol transported by LDL and/or HDL that is preferentially diverted by the parasite? Regardless of the sterol source/s, does the availability of host cholesterol have an impact on parasite development in vitro and in animals? Our results indicate that both host LDL-derived cholesterol from the endocytic network and host cholesterol synthesized in the ER are co-transported to the PV. Infection of cell lines defective in either sterol biosynthetic pathway or LDL supply does not impede Plasmodium growth, suggestive of compensatory activity of the endogenous and exogenous pathways to provide cholesterol to the parasite. Interfering with sterol salvage by pathogenic microorganisms has proven to be immensely powerful in combating the diseases they cause. With respect to preventing malaria infections, evaluation of the different steps in host cholesterol uptake by Plasmodium liver forms as effective points of attack would yield novel approaches to halt parasite replication.
Results
Plasmodium parasites acquire cholesterol from the mammalian host
No metabolic pathway involved in sterol biosynthesis can be identified in Plasmodium genome databases. However, our previous morphological data reported that the PV of malaria liver forms contains sterols (Bano et al., 2007). We wanted to extend these studies by examining the kinetics of cholesterol association with Plasmodium throughout the parasite developmental life cycle both in mosquitoes and mammals. Filipin, a fluorescent compound that interacts with the 3β-OH group of membrane sterols (Volpon and Lancelin, 2000), was used to visualize the distribution of these lipids in Plasmodium yoelii and Plasmodium berghei by light microscopy (Fig. 1). Data showed that, in contrast with mosquito tissues that exhibited a strong filipin-positive staining, P. yoelii oocysts, sporoblasts and sporozoites did not fluoresce (Fig. 1A, a–e). No prominent filipin labelling was observed on membranes of P. yoelii sporozoites maintained 1 h in axenic conditions following salivary gland disruption (Fig. 1A, f). By comparison, P. yoelii sporozoites that have invaded cultured mammalian liver cells showed an intense filipin labelling on the PV at the onset of schizogony (Fig. 1B, a and b). P. berghei parasites infecting hepatoma cells were also filipin-stained (data not shown).
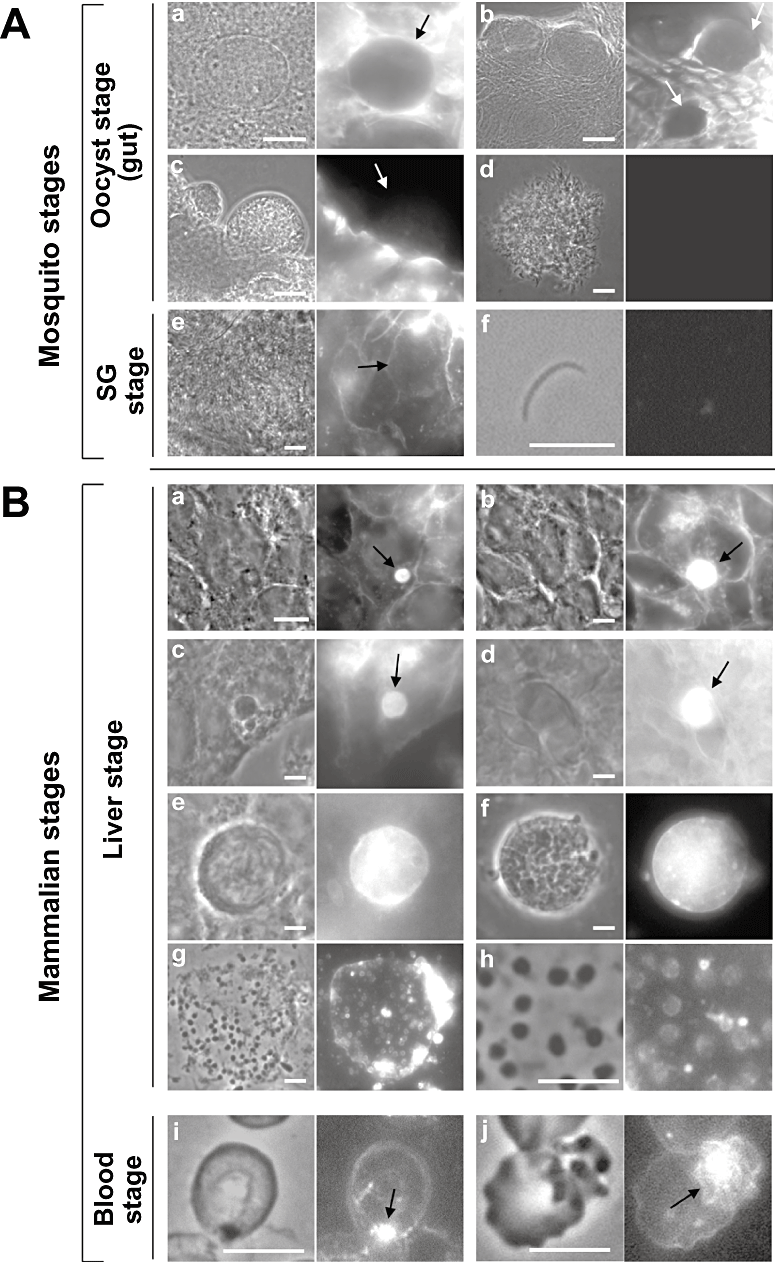
Detection of sterols in both hepatic and blood stages of Plasmodium parasites by fluorescence microscopy. A. Filipin staining of mosquito tissues infected with P. yoelii: static oocyst (a), undifferentiated oocyst (b), differentiated oocyst (c), sporoblast (d), sporozoites either in salivary glands (SG) (e) or isolated from salivary glands and cultivated axenically for 1 h (f). B. Filipin staining of Plasmodium parasites during mammalian cell infection: HepG2-CD81 cells infected with P. yoelii trophozoite during 16 h (a) and schizont 40 h (b), cultured primary rat hepatocytes infected with P. yoelii schizont during 24 h (c), GFP-expressing P. berghei infection C57BL/6 mouse liver 24 h p.i. (d), P. berghei merosomes appending to the surface of HepG2-CD81 cells 55 h p.i, detached from cells 74 h p.i. or lysed, releasing hepatic merozoites (g, h), P. berghei-infected red blood cells at the ring stage (i) and late schizont stage (j). Arrows pinpoint the parasites. Scale bars are 10 µm.
Hepatoma cell lines are a suitable in vitro model system to cultivate Plasmodium. However, these cells are morphologically distinct from hepatocytes, which implies functional differences between the two types of cells (Wilkening et al., 2003). To assess the physiological relevance of our observations, we used primary cultures of hepatocytes isolated from rat as host cells for P. yoelii. Plasmodium yoelii infecting hepatocytes exhibited a similar fluorescence pattern as observed for parasites grown in HepG2 cells (Fig. 1B, c), indicating that the parasite can divert host cholesterol indiscriminately of the nature of the liver cells. Finally, the significance of our in vitro assays was further validated in animals infected with P. berghei. Livers were collected from mice 24 h post-infection (p.i.) before filipin staining, and data showed that the parasites were strongly fluorescent in liver tissues (Fig. 1B, d).
Merozoite-filled vesicles or merosomes can be obtained from in vitro cultures of P. berghei (Sturm et al., 2006). Disruption of merosomes results in the release of hepatic merozoites. A positive filipin staining was observed on merosomes budding from hepatoma cells 55 h p.i. or floating in the culture medium 74 h p.i. (Fig. 1B, e and f). A fluorescent peripheral staining on mature merozoites was clearly visible on the parasites, indicating that newly formed merozoites in the liver contain sterols in their plasma membrane (Fig. 1B, g and h). To further ascertain the presence of sterols in the parasites, we performed biochemical assays to quantify the concentration of these lipids in merozoites. To do so, we developed a protocol to collect pure preparations of merozoites based on density gradient separation using Nycodenz and isopycnic centrifugation (Fig. S1). Assessment of total cholesterol content from merozoite preparations revealed that the parasites contained ∼120 nmol of cholesterol mg−1 protein (Table 1). Compared with a fibroblast or a hepatoma cell, the cholesterol content per merozoite was in the same range of values when the cell volume is taken in consideration. Finally, early and late developmental blood stages of P. berghei in mice also showed bright fluorescence filipin staining (Fig. 1B, i and j), in accordance to previous data on Plasmodium falciparum (Haldar et al., 1991).
| Cell type | Cholesterol content | ||
|---|---|---|---|
| nmol mg−1 proteina | fmol per cellb | fmol cell−1 volume (µm3)c | |
| Merozoites | 117.9 ± 20.4 | 0.1 | 0.034 |
| Fibroblasts | 801.3 ± 46.8 | 32 | 0.026 |
| HepG2 cells | 338.2 ± 40.7 | 67 | 0.011 |
- a. Values are means ± SD of three separate experiments.
- b. Calculated based on 1 mg protein corresponding to 1 × 109 merozoites, 2.5 × 107 fibroblasts and 5 × 106 HepG2 cells.
- c. Calculated based on the mean volume of 3 µm3 for a merozoite, 1200 µm3 for a fibroblast and 6000 µm3 for a HepG2 cell.
- Cells have been cultivated in MEM + 10% FBS. Merosomes of P. berghei have been purified and lysed to release merozoites. After washing, lipids were extracted from cells and total cholesterol concentration was measured by a commercial kit.
Altogether, these data reveal that if accessible to filipin, the membranes of Plasmodium insect forms do not contain sterols. However, once in the mammalian host, the parasite scavenges sterols that may fulfil a significant role in sustaining liver and blood infections.
Plasmodium diverts cholesterol derived from LDL and the mevalonate pathway in hepatocytes
Cholesterol associated with intrahepatic parasites could be originating from sterol-containing lipoproteins present in the culture medium or from the pool of sterols synthesized by hepatocytes, or from both. Experiments were thus designed to provide information about the preferential source/s of sterols for liver forms. To assess a role of lipoproteins such as LDL and HDL in cholesterol supply for the parasite, intracellular P. yoelii were incubated for 24 h in a medium lacking lipoproteins followed by filipin staining (Fig. 2A). Compared with normal conditions [10% fetal bovine serum (FBS)], the PV exhibited a weaker fluorescence staining in 10% lipoprotein-deficient serum (LPDS), which suggests that lipoproteins are in part exploited by the parasite as sources of cholesterol. We next wanted to determine whether delivery of exogenous cholesterol to the parasite involves the host endocytic pathway. We tested the influence of progesterone, which is known to sequester LDL-derived cholesterol in late endosomes and lysosomes (Butler et al., 1992). Incubation of infected cells with 10 µg ml−1 of progesterone for 24 h impaired the transfer of LDL-derived cholesterol to the PV as illustrated by the decreased fluorescence of the schizonts with filipin in progesterone-treated cells compared with untreated cells. To examine whether intrahepatic parasites can take up cholesterol directly from lipoproteins in the extracellular environment, we incubated infected cells with fluorescent NBD-cholesterol incorporated into LDL or HDL freshly isolated from human serum. After 2 h incubation with [NBD-cholesterol]-LDL at 37°C, data showed that the PV was highly fluorescent (Fig. 2B). Exposure of progesterone-treated infected cells to [NBD-cholesterol]-LDL abrogated the staining of the schizonts, confirming the transit of NBD-cholesterol through host endocytic organelles before delivery to the parasites. By comparison, when infected cells were incubated with [NBD-cholesterol]-HDL for 2 h, no staining was associated with the schizonts. These observations were further confirmed by incubating infected cells with LDL or HDL containing [3H]cholesterol to monitor the presence of tritium associated with hepatic merozoites. Lipids from the parasites were separated on thin layer chromatography (TLC) and radioactivity associated with the sterol fraction was counted by scintillation. Only upon incubation with [3H]cholesterol-LDL, radioactive cholesterol were detected in the parasite sterol fraction (Table 2).
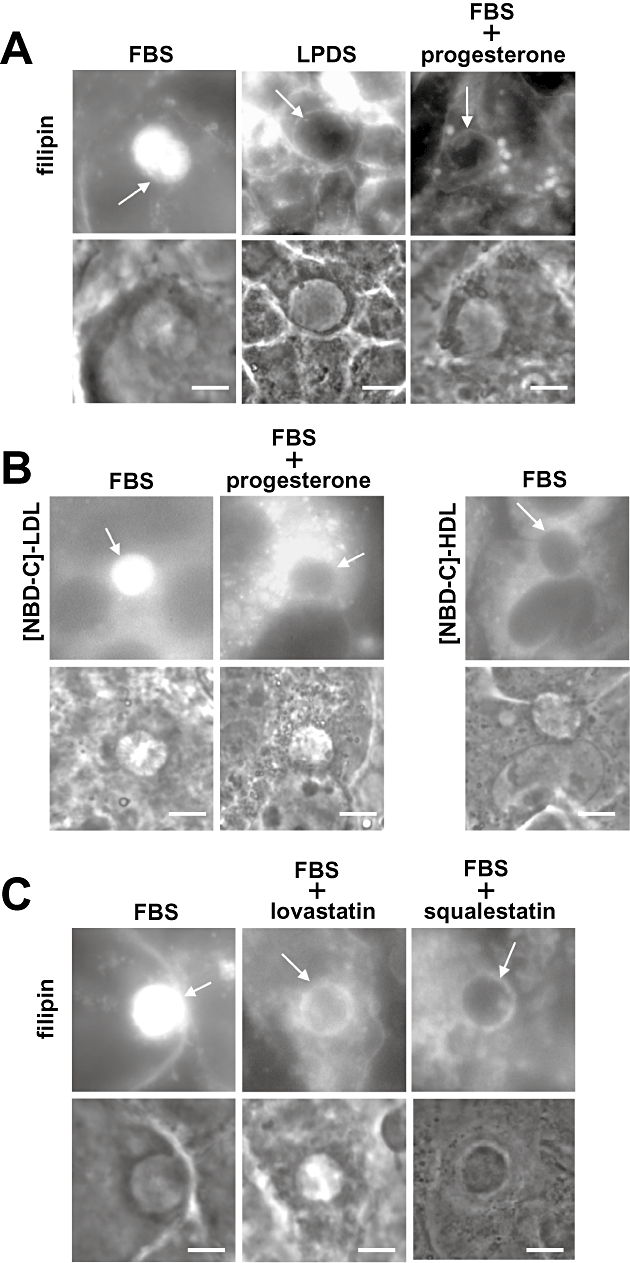
Identification of the source of sterols for Plasmodium in liver cells. A. Filipin staining of PV of P. yoelii in HepG2-CD81 cells under control conditions (10% FBS), in medium depleted in lipoproteins (10% LPDS) for 24 h, or in medium containing 10% FBS in the presence of progesterone (10 µg ml−1). Note the bright filipin staining on large endocytic compartments loaded in cholesterol upon progesterone treatment. B. Fluorescence microscopy of live P. yoelii-infected HepG2-CD81 cells (24 h p.i.) incubated with NBD-cholesterol incorporated into 2 mg ml−1 of LDL ([NBD-C]-LDL) for 2 h under normal conditions (control) or after treatment of infected cells with progesterone (10 µg ml−1 for 24 h), or incubated with NBD-cholesterol incorporated into 2 mg ml−1 of HDL ([NBD-C]-HDL) for 2 h. C. Filipin staining of PV of P. yoelii in HepG2-CD81 cells under control conditions (10% FBS) and in medium containing lovastatin or squalestatin during 24 h after infection. Arrows pinpoint the PV. Scale bars are 10 µm.
| Pulse | Radioactive molecules associated with 109 merozoites |
|---|---|
| [3H]cholesterol-LDL | 2331 ± 299 ng chol |
| [3H]cholesterol-HDL | n.d. |
| [14C]HMG-CoA | 20.9 ± 3.7 pmol HMG-CoA |
- Huh7 cells have been infected with P. berghei for 36 h in normal conditions (MEM + 10% FBS) before addition of radioactive cholesterol in LDL or HDL, or of radioactive HMG-CoA. Floating merosomes were collected and lipids extracted from merozoites for TLC separation to count the radioactivity corresponding to [3H]cholesterol (chol) and [14C]HMG-CoA incorporated into the sterol fraction.
- Data are means ± SD of three assays.
- n.d., not detectable.
To determine whether cholesterol synthesized by hepatocytes can also be scavenged by the parasites, we treated cells with pharmacological inhibitors of the mevalonate pathway, either lovastatin that targets 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the key enzyme of the mevalonate pathway (Krukemyer and Talbert, 1987) or squalestatin that blocks squalene synthase, the first enzymatic step committed to the biosynthesis of sterols (Bergstrom et al., 1995). Data showed that treatment of infected cells with 4 µM lovastatin or 60 µM squalestatin resulted in a less strong filipin staining of the PV in comparison with untreated cells (Fig. 2C). These data were confirmed by incubating infected cells with the 14C radioactive form of the precursor HMG-CoA of cholesterol and collecting merozoites to isolate their lipids. Data showed the presence of [14C]cholesterol associated with the parasites (Table 2).
Jointly, these observations indicate that the parasites are able to retrieve cholesterol derived from metabolized LDL but not from HDL, and from the host endogenous sterol pathway.
Extracellular concentrations of cholesterol does not influence Plasmodium growth in liver cells
We next asked whether the development of Plasmodium in liver cells can be influenced by elevated concentrations of exogenously supplied cholesterol or depletion of cholesterol from the culture medium. The schizont number and size were analysed after cultivation of infected cells in medium devoid of lipoproteins or in medium containing large excesses of cholesterol associated with human LDL, or HDL as negative control. Compared with normal conditions (10% FBS with nominal concentrations of LDL and HDL of 0.11 and 0.21 mg ml−1, respectively), incubation of infected cells in the absence of lipoproteins did not significantly change the level of infectivity (Fig. 3A). This was furthermore confirmed by quantification of the relative burden of liver forms by monitoring the levels of parasite 18s ribosomal transcripts showing no difference among all the culture conditions tested (Fig. 3B). Cholesterol content associated with merozoites grown in LPDS was assessed, and data in Table 3 showed that merozoites did contain cholesterol, although 70% less than control merozoites grown in 10% FBS, in accordance with our morphological observations in Fig. 2A.
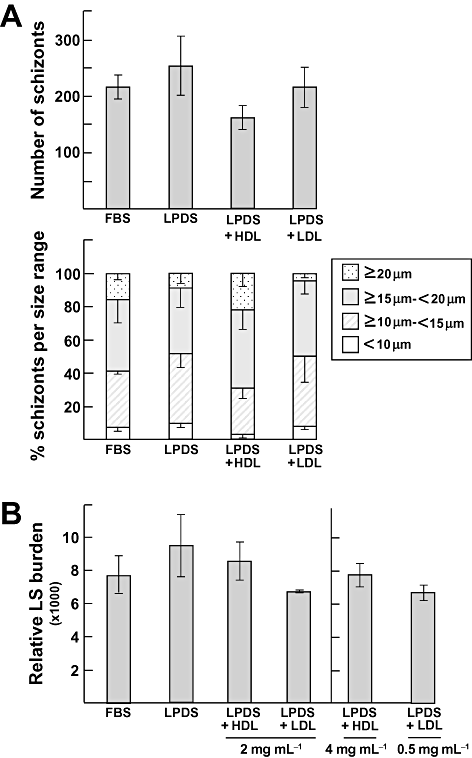
Effect of extracellular sterols on parasite development in liver cells. Quantification of P. yoelii infectivity in HepG2-CD81 43 h p.i. in 10% FBS, 10% LPDS, 10% LPDS plus 2 mg ml−1 HDL or 10% LPDS plus 2 mg ml−1 LDL. A. Schizonts from three replicate cultures were counted and their respective diameter measured for each condition. Data are means ± SD. B. For quantitative PCR, additional experiments were done in three replicates in each condition to monitor Plasmodium 18s ribosomal-RNA in the indicated culture conditions. Data are means ± SD. LS, liver stage.
| Culture conditions | nmol cholesterol/mg protein | |
|---|---|---|
| MEM + 10% FBS | 112.3 ± 15.1 | |
| MEM + 10% LPDS | 34.3 ± 9.0 | P < 0.01 |
| MEM + 10% LPDS + LDL | 121.9 ± 28.2 | |
| MEM + 10% LPDS + HDL | 29.2 ± 28.2 | P < 0.01 |
| MEM + 10% FBS + 60 µM squalestatin | 46.5 ± 11.4 | P < 0.05 |
| MEM + 10% FBS + siRNA SqS | 74.4 ± 19.3 | P < 0.5 |
- After washing, lipids were extracted from merozoites cultivated in the indicated conditions and total cholesterol concentration was measured by a commercial assay. Data are means ± SD of three separate experiments. The differences between control conditions (MEM + 10% FBS) and other conditions that are significant are shown.
Compared with cultures in media with 10% FBS, incubation in media enriched in LDL (up to 0.5 or 2 mg LDL ml−1) did not boost parasite replication (Fig. 3A and B) and cholesterol content in merozoites grown in the presence of 2 mg LDL ml−1 was not significantly different from that of merozoites grown in 10% FBS (Table 3). Excess HDL did not modify the time course of infection (Fig. 3A and B) and these lipoproteins did not contribute to cholesterol supply for the parasite (Table 3). Because the concentration of free cholesterol in LDL is about 7–12 times higher than in HDL, we also monitored parasitegrowth in media containing lipoprotein concentrations adjusted to their cholesterol levels. Intracellular parasites were exposed to 4 mg HDL ml−1 or 0.5 mg LDL ml−1 for direct comparison of parasite development, and data revealed no growth advantage for parasites exposed to 4 mg HDL ml−1 compared with 2 mg HDL ml−1, or to 0.5 mg LDL ml−1 (Fig. 3B).
These data indicate that parasite liver forms divert LDL-derived cholesterol but in moderate amounts. Remarkably, these parasites can achieve normal growth in cholesterol-depleted medium, suggesting that they are likely capable to exploit alternative compensatory source of sterols in case of short supply of LDL.
Reduced expression of LDL receptor in hepatocytes does not affect Plasmodium development
To further verify our hypothesis enounced above, we devised experiments to examine the contribution of the LDL receptor-mediated pathway and the mevalonate pathway of the host cell in supplying cholesterol to the parasite. We downregulated the expression of host LDL receptors by small interfering RNA (siRNA) in Huh7 cells and monitored the development of P. berghei in transfected cells by quantitative PCR. A pool of human LDL receptor siRNA was electroporated into hepatoma cells, and the efficiency of gene downregulation was examined by assaying the expression of LDL receptors relative to that of host β-actin by Western blotting at different time points (Fig. 4A and B). Ninety-six hours post electroporation, we detected the lowest level of LDL receptor expression (∼70–80% less) in cells transfected with LDL receptor siRNA compared with cells transfected with non-targeting control siRNA. Levels of expressed β-actin were similar in both control and LDL siRNA-treated cells whereas a 2.7-fold increase in squalene synthase expression was detected in LDL siRNA-treated cells. In order to determine the impact of silencing of LDL receptor expression on parasite development, Huh7 cells were infected for 2 days with P. berghei sporozoites either 72 h or 96 h after electroporation. Data in Fig. 4C revealed no difference in infectivity between the two siRNA-treated cells, suggesting that under conditions of limited supply of LDL-derived cholesterol through reduced expression of LDL receptors, the parasite can adapt to its new environment and sustain an efficient development likely relaying on the endogenous source of cholesterol.
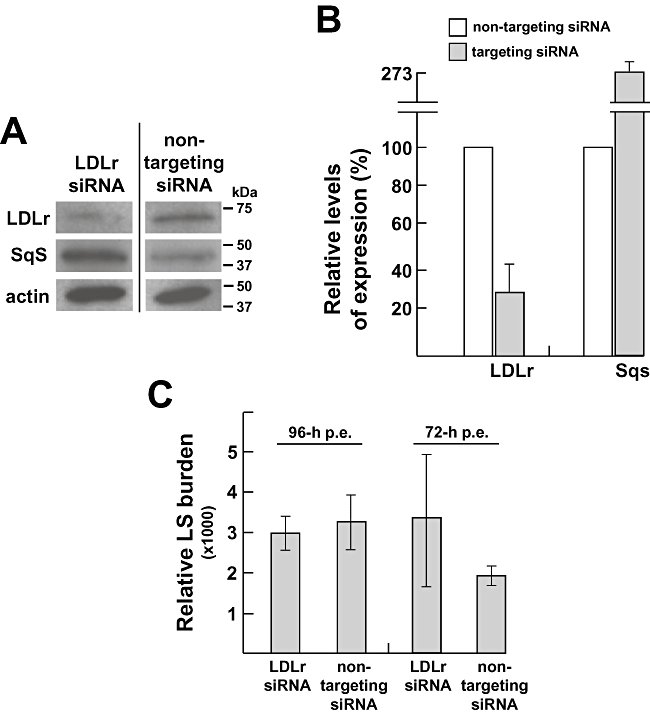
Role of host LDL receptors in cholesterol supply for Plasmodium. A. Immunoblots of lysates from Huh7 cells that have been transfected with LDLr siRNA for 96 h using antibodies against LDL receptor, actin and squalene synthase, compared with immunoblots of lysates from Huh7 cells that have been electroporated with non-targeting siRNA. Molecular weight standards are shown at the right. In Huh7 cells, the expected bands for LDL receptor, squalene synthase and β-actin sizes are approximately 71 kDa, 47 kDa and 42 kDa respectively. B. Quantification of expression levels of LDL receptor and squalene synthase in Huh7 cells electroporated with either LDL receptor siRNA or non-specific targeting siRNA detected by Western blotting were performed by densitometry from three independent assays. C. Quantification of P. berghei development under conditions of silencing of LDL receptor expression or control conditions (non-targeting siRNA) by quantitative PCR 96 h or 72 h post electroporation (p.e.), Huh7 cells were infected with parasites for 48 h before monitoring Plasmodium 18s ribosomal-RNA. These conditions allow the best overlapping between the time course of parasite growth and the time necessary to decrease LDL receptor. Results were done in one biological replicate with three technical replicates. LS, liver stage; LDLr, LDL receptor; Sqs, squalene synthase.
Interference with sterol synthesis in hepatocytes does not impede Plasmodium growth
We then decided to examine the influence of endogenous cholesterol on intrahepatic parasite development using both pharmacological and molecular approaches. First, quantitative experiments were performed to determine the effect of squalestatin on Plasmodium development in hepatocytes. It has been reported that the levels of isoprenoid pyrophosphate intermediates in squalestatin-treated cells are maintained constant by co-ordinate enzyme regulation, thereby ensuring a normal rate of synthesis of nonsterols (Keller, 1996). However, we have carried out experiments to ensure that squalestatin lacks toxic effects in our in vitro system. Exposure of Huh7 cells to the drug from 15–60 µM for 48 h or 72 h led to a decrease in cell proliferation starting at 15 µM at 48 h and 72 h treatment but did not induce any cidal effect (Fig. 5A). The drug activity on cholesterol synthesis inhibition was verified by incubating squalestatin-treated cells with [14C]HMG-CoA. Following treatment at 60 µM, a ∼ 85% reduction in cholesterol production was obtained in Huh7 cells (Fig. 5B). Huh7 cells were then incubated for 24 h with 60 µM squalestatin before infection and treatment was maintained during the entire infection time. After 43 h, infected cells were fixed to monitor parasite development by UIS4 staining. Results revealed a decrease in schizont numbers inversely proportional to squalestatin concentrations, likely because of reduced number of host cells available for the parasites (Fig. 5C). Indeed, after scoring the size of schizonts, no significant difference was noticed between drug-treated cells and untreated cells (Fig. 5C). It is of interest to mention that close examination of treated parasites shows a punctuated localization of UIS4 that contrasts to the continuous staining obtained in the absence of squalestatin (Fig. 5D). Measurement of total cholesterol in merozoites from 60 µM squalestatin-treated cells revealed a ∼ 60% reduction in cholesterol compared with untreated cells (Table 3).
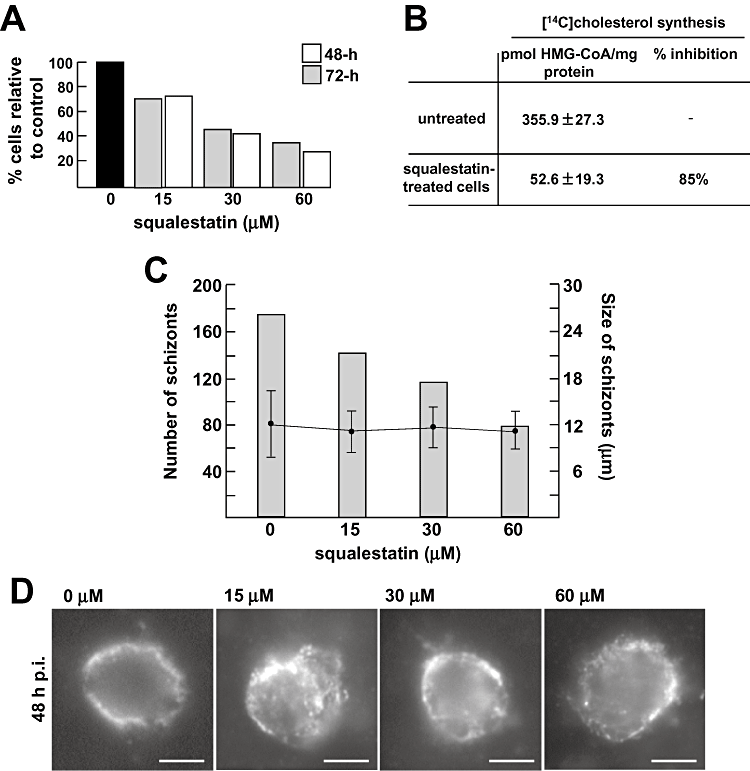
Effect of pharmacological blockade of host sterol synthesis on Plasmodium development. A. Quantification of Huh7 cell proliferation treated with squalestatin. Cells were incubated for 48 h or 72 h with squalestatin at the indicated concentrations before counting in a haemocytometer. Data are expressed in percent of treated cells compared with untreated cells for two representative experiments. The trypan blue exclusion assay was used to determine the number of viable cells present in suspension did not reveal any difference between treated and untreated cells. B. Quantitative measurements of cholesterol synthesis in squalestatin-treated cells and untreated cells. Cells were treated with 0 or 60 µM of squalestatin for 24 h before incubation for an additional 6 h with [14C]HMG-CoA followed by lipid extraction, separation of radioactive cholesterol by TLC and counting by scintillation. Data are means ± SD of three separate experiments. 1 mg protein corresponds to ∼4 × 106 Huh7 cells. Differences between squalestatin-treated cells and untreated cells are significant (P < 0.01). C. Quantification of P. berghei infectivity in squalestatin-treated cells. Huh7 cells were exposed to squalestatin at the indicated concentrations for 24 h before infection with P. berghei. 48 h p.i., infected cells were incubated with anti-PbUIS4 antibodies (PV membrane marker) to measure the relative sizes of the PV in each culture condition, as illustrated in D. The determination of the schizont number was done in two separate assays, and average values are shown. The sizes of these schizonts are expressed as means ± SD. Scale bars are 10 µm.
Second, we performed experiments to evaluate parasite growth under condition of reduced squalene synthase expression by siRNA technology. Twenty-four hours following electroporation of human squalene synthase siRNA into Huh7 cells, squalene synthase expression was decreased by ∼90% compared with cells transfected with control non-targeting siRNA. LDL receptor and actin levels remained constant in both conditions (Fig. 6A and B). Metabolic labelling assays using radioactive HMG-CoA on siRNA-treated cells demonstrated that de novo cholesterol synthesis was inhibited by ∼85% in squalene synthase siRNA-treated cells compared with control (Fig. 6C). Specific effect of downregulation of squalene synthase expression was furthermore confirmed by monitoring the multiplication of Chlamydia trachomatis, an intracellular bacterium that relies on host cholesterol synthesis for growth (Carabeo et al., 2003). Expectedly, bacteria growth in squalene synthase siRNA-treated cells was dramatically impaired and aberrant inclusions were observed in four on five infected cells (Fig. S2). By contrast, P. berghei was not affected by the reduction in squalene synthase levels (Fig. 6D). Additionally, we found that merozoites collected for cells silenced for squalene synthase expression contained ∼35% less cholesterol than control merozoites (Table 3). These data indicate that, similar to parasites exposed to conditions of LDL depletion in the medium, parasites residing in hepatocytes with limited supply of de novo synthesized cholesterol can develop normally.
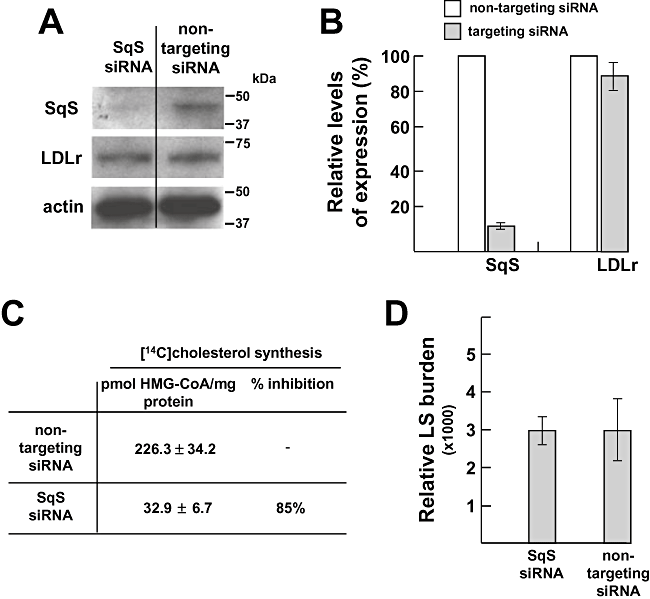
Contribution of host squalene synthase to cholesterol supply for Plasmodium. A. Immunoblots of lysates from Huh7 cells that have been transfected with squalene synthase siRNA expression for 24 h (Sqs siRNA) or non-targeting siRNA using antibodies against LDL receptor (LDLr), actin and squalene synthase (SqS). Molecular weight standards are shown at the right. B. Quantification of expression levels of LDL receptor and squalene synthase in Huh7 cells electroporated with either LDL receptor siRNA or non-specific targeting siRNA by quantitative PCR. Results are means from three independent assays. C. Quantitative measurements of cholesterol synthesis in Huh7 cells silenced for squalene synthase expression or control conditions (non-targeting siRNA). siRNA-treated cells have been incubated for 6 h with [14C]HMG-CoA before lipid extraction, separation of radioactive cholesterol by TLC and counting by scintillation. Data are means ± SD of three separate experiments. Differences between conditions of Sqs siRNA and non-targeting siRNA are significant (P < 0.01). D. Quantification of P. berghei replication under conditions of silencing of LDL receptor expression or control conditions (non-targeting siRNA) 24 h post electroporation by quantitative PCR to monitor Plasmodium 18s ribosomal-RNA 48 h p.i. Results were done in three biological replicates. LS, liver stage.
Merosomes grown under cholesterol-restrictive conditions in vitro are infectious in mice
Our data illustrated that intrahepatic parasites grown either in the absence of lipoproteins in the medium or in cells with strongly limited synthesis of cholesterol can form merozoites at the end of schizogony. However, in both culture conditions, merozoites contained reduced amounts of cholesterol compared with control conditions. We therefore wanted to investigate whether hepatic merozoites produced in these cholesterol-restrictive environments were able to establish a productive infection in mice. P. berghei merosomes were collected from cells cultured in LPDS, FBS + 60 µM squalestatin or FBS alone as control. Mice were then intravenously injected with 250 merosomes for each condition, and blood infection was examined on blood smears 6 days post injection. Data showed that for each culture condition, all the recipient mice were parasitized (Table 4 and Table. S1). At day 13 post injection, dead mice were observed in each group, and the surviving mice exhibited high levels of parasitaemia, indicative of fatal progression of the malaria infection. Thus, these data demonstrate that limited supply of cholesterol either from LDL or the mevalonate pathway does not influence the infectivity of hepatic merozoites in vivo.
| Parasite/Host cell | Culture conditions | No. of merosomesa Injected per mouseb | No. of recipient mice/No. of infected mice Prepatency (days) |
|---|---|---|---|
| P. berghei/Huh7 cells | MEM + 10% FBS | 250 | 5/5 (D6) |
| MEM + 10% LPDS | 250 | 5/5 (D6) | |
| MEM + 10% FBS + 60 µM squalestatin | 250 | 4/4 (D6) |
- a. 81 h p.i.
- b. Swiss-Webster mice.
Exogenous and endogenous sources of cholesterol in hepatocytes are indiscriminately exploited by the parasites and can substitute for each other
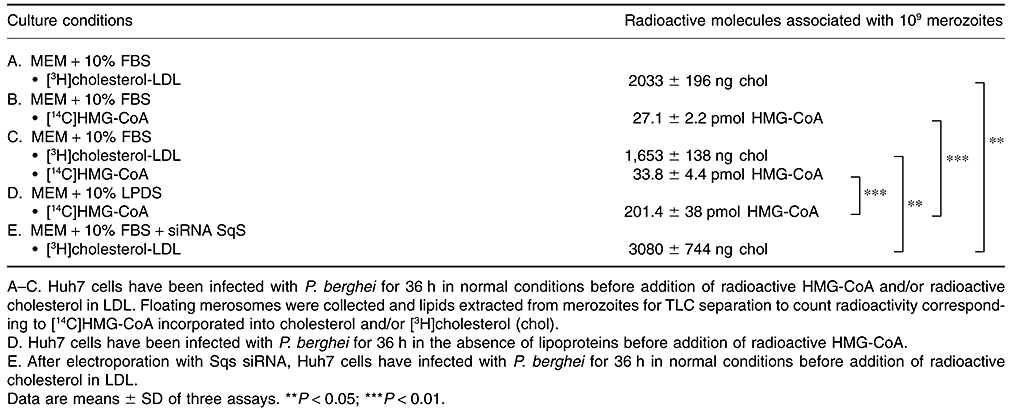
Additional experiments were conducted to substantiate the parasite's ability to intercept cholesterol derived from LDL and synthesized by the cell. P. berghei-infected Huh7 cells were incubated with [3H]cholesterol-LDL or [14C]HMG-CoA to monitor the presence of tritium or 14C associated with the sterol fraction of hepatic merozoites by TLC. As showed in Table 2, parasites were radioactive for each isotope (Table 5A and B). We next designed experiments to analyse the potential co-association of both radiolabelled cholesteryl molecules by co-incubating infected cells with [14C]HMG-CoA and [3H]cholesterol-LDL using the same experimental conditions as described above. Data showed that merozoites contained both [3H]cholesterol and [14C]cholesterol (Table 5C), demonstrating that Plasmodium liver forms simultaneously takes up lysosomal cholesterol provided by LDL and cholesterol synthesized on the ER.
A final set of experiments was devised to determine whether endogenous cholesterol can substitute for LDL-cholesterol in parasites grown in LPDS, and vice versa. Infected cells cultured in LPDS were exposed to radioactive HMG-CoA as described above for direct comparison. After lipid isolation from merosomes and counting of radioactivity associated with the sterol fraction, data revealed that LPDS-treated parasites incorporated ∼7.5 times more endogenous cholesterol than parasite grown in complete medium (Table 5B and D), which is in agreement with the upregulation of the expression of squalene synthase in Huh7 cells treated with LDL receptor siRNA (Fig. 4A and B). Inversely, merozoites isolated from cells with restricted cholesterol synthesis incubated with tritiated cholesterol-LDL contained ∼1.5 times more radioactive cholesterol than parasites incubated in cells producing cholesterol (Table 5A and E).
These data establish that Plasmodium liver forms are able to scavenge both exogenous and endogenous cholesterol, and suggest that, under conditions of blockade of one of these pathways, they can exploit accessible sterols derived from alternative sources in hepatocytes.
Discussion
To date, the cellular and molecular events that take place in the liver infected by the malaria parasite remain largely unexplored. The fast multiplication of intrahepatic Plasmodium necessitates a high demand of lipids for organelle biogenesis. Hepatocytes are professional lipid synthesizers, importers and recyclers (Nguyen et al., 2008), and therefore represent a favourable environment for the replication of the malaria parasite in mammals. Interestingly, a recent microarray-based approach to profile hepatocyte responses to Plasmodium infection revealed that genes coding for sterol syntheses (e.g. squalene epoxidase) and lipid metabolic processes (e.g. SR-BI; membrane bound O-acyltransferase; phosphatidylcholine transfer protein) are specifically upregulated in the host cell (Albuquerque et al., 2009). The cholesterol requirement for Plasmodium residing in the mammalian host may represent a parasite strategy to adapt to an intracellular environment. We previously showed that acute sterol extraction from hepatocytes infected with the malaria parasite using cyclodextrins decreases the flux of solutes across the PV membrane, suggesting that sterols must to some extent contribute to parasite development (Bano et al., 2007). Plasmodium is unable to synthesize the steroid core. We demonstrate here that Plasmodium liver forms are auxotrophic for host cholesterol but require moderate amounts of this lipid to optimally develop in hepatocytes. They are able to intercept cholesterol derived from LDL and the mevalonate pathway. Whether the parasite scavenges endogenous or exogenous sterols separately or indiscriminately from a common reservoir where endogenous and exogenous pathways converge, e.g. from the plasma membrane or ER, remains to be determined. The dispensability of the host LDL receptor pathway for liver form development has been previously reported (Yalaoui et al., 2008). We proposed here that, in the absence of LDL-derived cholesterol, the parasite adapts by diverting cholesterol produced by the mevalonate pathway in order to accommodate its basal needs for sterols. Similarly, when the host cell does not produce sterols, the parasites scavenge LDL-derived cholesterol. This scenario suggests that the liver forms can proficiently acclimatize to cholesterol-restrictive conditions by relaying on available sources of sterols to sustain productive infection.
Changes in plasma lipids and lipoproteins have been reported during acute malaria in humans (Nilsson-Ehle and Nilsson-Ehle, 1990; Shakibaei and Frevert, 1996; Sinnis et al., 1996). The erythrocytic schizogony induces an increase in triglycerides and VLDL, and a decrease in LDL and HDL concentrations, resulting in severe hypocholesterolemia. Although the mechanisms underlying the selective and pronounced decline in LDL and HDL remain obscure, the catabolism of these lipoproteins in the infected liver represents a providential opportunity for Plasmodium to be exposed to even more lipids in hepatocytes. A recent work has proposed a dual stimulatory role of the host SR-BI to parasite development in liver cells (Rodrigues et al., 2008; Yalaoui et al., 2008). First, SR-BI, which is mainly found in cholesterol-enriched membrane microdomains of hepatocytes may serve as the initial docking site to facilitate Plasmodium attachment to the host cell surface. Second, SR-BI seems to be an important factor contributing to parasite replication. The transcription of this receptor is highly upregulated in the infected cell and interference with SR-BI expression has a deleterious impact for infection efficiency. The exact role of SR-BI during parasite maturation is still unknown. It has been proposed that this protein might permit the transport of lipids from intracellular stores to the parasite as host L-FABP expression is activated by SR-BI. Our current data rule out the possibility that the contribution of the SR-BI pathway to parasite growth is linked to cholesterol supply to the PV through HDL intake as we did not observe a trafficking of cholesterol from extracellular HDL to the schizonts. Moreover, Yalaoui and colleagues found that the host LDL receptor pathway could not compensate for the absence of SR-BI, suggesting that SR-BI plays a role in schizont development unrelated to the supply of exogenous cholesterol.
Sterols are essential components of eukaryotic cell membranes as they are required for proper membrane permeability, fluidity, contour, organelle identity and membrane protein function. All the forms of Plasmodium that multiply in mammals contain cholesterol. We previously showed that the PV membrane of hepatic schizonts is particularly enriched in cholesterol (Bano et al., 2007). In mammalian cells, channels through biological membranes are structurally stabilized by sterols. Cholesterol is highly concentrated in bilayers containing gap junctions and plays a significant role in gap junctional assembly and activity (Cascio, 2005) by direct interaction with proteins or other lipids to influence the physical properties of the bilayer (Dart, 2010). By analogy, cholesterol associated with the PV membrane may increase the mechanical strength of this membrane and modulate its porosity to accommodate the parasite's needs. The vacuolar membrane of intraerythrocytic Plasmodium is also permeable and contains small nutrient channels (Desai and Rosenberg, 1997) but this membrane is poor in cholesterol (Jackson et al., 2007). After their isolation from the PV membrane, the nutrient channels of blood forms can be incorporated into liposome bilayers and are functional. Interestingly, liposome-containing channels have the same permeability properties as the channels located within the vacuolar membrane regardless the lipid composition of liposome bilayers (Hsiao et al., 1991). This suggests that the channel function in the parasite does not significantly depend on their lipid environment.
Two potential sources of cholesterol for blood stage parasites have been proposed. One study showed that a major lipid source for Plasmodium is HDL that transfers their lipid cargo to the erythrocyte membrane (Grellier et al., 1991). In accordance to that finding, serum replacement by different lipoprotein fractions revealed that only the HDL fraction is able to assure complete schizogony as well as erythrocyte reinvasion (Grellier et al., 1990). A more recent study demonstrates that the cholesterol-rich detergent-resistant membrane (DRM) components present on the erythrocytic plasma membrane can be incorporated into parasite blood forms (Lauer et al., 2000). Depletion of cholesterol from trophozoite-infected red blood cells induces the expulsion of the parasite, suggesting the importance of cholesterol in maintaining intraerythrocytic infection. During ring to trophozoite development, a tubulo-vesicular membrane network buds from the PV membrane to the periphery of the red blood cell. It has been suggested that the tubulo-vesicular membrane network may be involved in the recruitment of host DRM components to provide vacuolar parasites with cholesterol. The parasite is able to assemble DRM on the plasma membrane. A stomatin protein secreted from the secretory organelle rhoptries and an erythrocyte stomatin-like protein-2 are strong candidates for the formation of these lipid rafts (Hiller et al., 2003). Furthermore, the malaria parasite seems equipped to move cholesterol within its cell as it contains genes coding for sterol translocators (Lipid/Sterol : H symporter; PFA0375C in http://www.PlasmoDB.org), sterol sensors (oxysterol-binding protein; PF11-0327) and sterol transporters (lecithin : cholesterol acyltransferase; PFF1420W).
Provided by diet, sterols are abundant in female Anopheles mosquitoes and necessary for the construction of cellular membranes and the synthesis of ecdysteroids. Lipid transport in mosquitoes is achieved by lipophorins circulating in the haemolymph. Lipophorin-associated lipids include cholesterol, phosphatidylcholine, phosphatidylethanolamine, cholesteryl ester and diacylglyceride (Atella et al., 2006). A recent study demonstrates that lipophorins carry lipids to Plasmodium insect forms. It has been observed that midgut oocysts take up lipophorins to acquire fatty acids and phospholipids from the ingested lipophorins (Atella et al., 2009). Although the transport of cholesterol-derived lipophorins has not yet been investigated, our morphological data reveal that filipin does not bind to Plasmodium in the mosquito vector, which suggests that insect forms do not contain sterols. However, we cannot rule out the possibility that the membrane composition of the insect forms is unique, preventing filipin accessibility to sterols. The molecular processes by which filipin interacts with membrane sterols are still unclear. It has been documented that the effect of filipin on membrane sterols may vary from gentle slowing down of axial rotation to complete immobilization in the membrane, forming solid complexes (Dufourc, 2008). The 3β-OH group of sterols seems required for filipin binding but several studies called attention to the likelihood that the filipin susceptibility of membrane systems also relates to the overall state, e.g. whole shape and hydrophobicity of membrane organization rather than to the binding to a single sterol. To this point, it has been established that the response of a membrane system towards filipin is affected by the apolar aspects of phospholipids and that membranes with high phospholipid : sterol ratio (such as in mitochondria with phospholipid : sterol = ∼40) cannot incorporate filipin (Hsuchen and Feingold, 1973). In addition, a decline in filipin sensitivity could also be due to the abundance of proteins in membranes (such as in clathrin coated pits), which impedes the access of filipin to sterols. Although the lipid and membrane composition of malaria insect forms are not well known, the absence of filipin staining on insect forms may be due to excess phospholipids relative to sterols and/or high protein concentrations in their membranes rather than due to a deficiency in sterols.
Parasite liver forms are equipped to re-route host cholesterol to their PV from the ER and endocytic compartments, and/or a pool where endogenous and exogenous cholesterol converge. Like in the related parasite Toxoplasma (Sinai et al., 1997), the PV membrane of liver forms physically interacts with the host ER, which raises the hypothesis that the lipids manufactured in the ER could be scavenged by these parasites from this organelle. Like intrahepatic Plasmodium, Toxoplasma gondii is auxotrophic for LDL (Coppens et al., 2000). Compared with HDL, LDL are particularly enriched in steryl molecules, which makes these lipoproteins a beneficial source of cholesterol for these parasites. Among the lipoproteins used in this study, human LDL contain ∼350 and ∼1400 molecules of free and esterified cholesterol per particle, respectively, whereas human HDL contain only ∼30 and ∼70 molecules of free and esterified cholesterol per particle respectively (Wilson et al., 1992). In contrast to intrahepatic Plasmodium, Toxoplasma cannot divert cholesterol synthesized de novo by the host cell. Consequently, removal of LDL from the medium impairs parasite growth. Interference with LDL degradation or cholesterol translocation from lysosomes also arrests parasite development. This indicates that unlike Plasmodium, Toxoplasma cannot be rescued by the endogenous pathway in the absence of LDL in the medium. To have access to cholesterol supplied by the endocytic network, Toxoplasma attracts and sequesters host lysosomes in the PV (Coppens et al., 2006). To this point, it could be interesting to analyse how the malaria parasite can divert host endocytic organelles to retrieve their contents.
Downregulation of host cholesterol synthesis in cells infected by microbes has been proposed as a novel innate mechanism of defence against infections. There is increasing evidence that inhibition of the mevalonate pathway by statins may be useful for the prevention and treatment of infections (reviewed in Tleyjeh et al., 2009). For example, lovastatin reduces the intracellular growth of Salmonella sp. in cultured macrophages, as atorvastatin does in Salmonella-infected mice. Lovastatin also reduces the level of infection as a result of Coxiella sp. and Candida albicans in vitro. Various statins are found to interfere severely with the growth of intracellular protozoan parasites of the family Trypanosomatidae, such as Trypanosoma cruzi and Leishmania sp. Both lovastatin and squalestatin inhibit the dengue viral replication (Rothwell et al., 2009). However, the remarkable plasticity of intrahepatic Plasmodium to retrieve cholesterol from LDL in lovastatin- or squalestatin-treated cells precludes the utilization of inhibitors of the mevalonate pathway alone to control malaria infections.
Experimental procedures
Reagents and antibodies
All chemicals were obtained from Sigma Chem. (St. Louis, MO, USA) or Fisher (Waltham, MA, USA) unless indicated otherwise. Analytical grade solvents were used for lipid analysis. The nitrobenzoxadiazole (NBD)-cholesterol was purchased from Molecular Probes (Eugene, OR, USA) and lovastatin was a gift from Merck, Sharp and Dohme. Radiolabelled reagents included [1,2-3H]cholesterol (sp. act: 50 mCi mmol−1) and 3-hydroxy-3-methyl[3-14C]glutaryl coenzyme A (sp. act: 59 mCi mmol−1) from Amersham Corp. (Arlington Heights, IL, USA). Primary antibodies used were rabbit anti-squalene synthase as a generous gift from I. Shechter (Uniformed Service University School of Medecine, Bethesda; Shechter et al., 1992), chicken anti-LDL receptor from Abcam (Cambridge, MA, USA), mouse anti-calnexin from BD Biosciences (San Jose, CA, USA), mouse anti-PfDYN2 (dynamin from P. falciparum) generously provided by I. Florent (Museum National d'Histoire Naturelle, France; Florent et al., 2004; Charneau et al., 2007), mouse PyHsp70 kindly given by F. Zavala (Johns Hopkins University) and rabbit PyUIS4 as generous gift from S. Kappe (Seattle BioMed.).
Cell lines and culture conditions
The cell lines used in this study included the human hepatoma cell line Huh7 (Nakabayashi et al., 1982) generously provided by CM. Rice (The Rockefeller University, New York) and human fibroblasts. Cells were cultured in DMEM supplemented with 10% FBS, 2% penicillin-streptomycin and 0.1 mM non-essential amino acids. The human HepG2 cells expressing the tetraspanin CD81 (HepG2-CD81; Silvie et al., 2006) kindly provided by D. Mazier (Université Pierre et Marie Curie, Paris, France) were cultured in alpha modified Eagle's medium (α-MEM) with 10% FBS and 2% penicillin-streptomycin. All cells were maintained at 37°C in 5% CO2. Primary hepatocytes were isolated from Sprague-Dawley rats and cultured in 50 ml tissue culture flasks as described (Pradel and Frevert, 2001).
Experimental animals
Female C57BL/6 mice and Swiss-Webster mice (6–8 weeks) were purchased from Jackson Lab. (Bar Harbor, ME, USA), and male Sprague-Dawley rats (8–9 weeks) were purchased from Harlan Lab. (Indianapolis, IL, USA). Animal handling was conducted according to institutional animal care and use committee-approved protocols.
Parasite strains and cultivation
Plasmodium yoelii 17XNL, P. berghei ANKA and green fluorescent protein (GFP) expressing P. berghei ANKA (Franke-Fayard et al., 2004) salivary gland sporozoites were collected from infected female in Anopheles stephensi mosquitoes blood-fed on anesthetized Swiss CD-1/ICR mice (performance site for animal use at New York University) as described (Kaiser et al., 2003). Mosquitoes infected with P. yoelii or P. berghei were, respectively, dissected at days 14 to 16, and days 20 to 24 after the first infectious blood meal. Mean number of sporozoites was determined by using a haemocytometer. Purification protocol of P. berghei hepatic merozoites is detailed in Fig. S2.
Bacterial strain and cultivation
The C. trachomatis lab reference serovar E strain (generously provided by JA Carrasco and PM Bavoil, University of Maryland) was cultivated in Huh7 cells at 37°C with 5% CO2 in DMEM supplemented with 10% FBS, 25 µg ml−1 gentamicin and 1.25 µg ml−1 fungizone. Confluent monolayers were pretreated with 30 µg ml−1 DEAE-Dextran before addition of the inoculums suspended in 0.25 M sucrose, 10 mM sodium phosphate and 5 mM L-glutamic acid to yield a 90–100% infection rate.
RNA interference
Huh7 cells were electroporated with 100 nM final concentration of siRNA in Human T-cell solution using the nucleofector device from Amaxa (Walkersville, MD, USA). ON-TARGETplus SMARTpool of human LDL receptor siRNAs and human squalene synthase siRNAs obtained both from Thermo Scientific Dharmacon (Lafayette, CO, USA) were used as targeting siRNA. Non-targeting siRNAs (Thermo Scientific Dharmacon) were used as negative controls. In each replicate, 5 × 105 cells were electroporated and immediately added to 1 ml culture medium in a well of 24-well culture plate. Medium was changed every 24 h. At a predetermined incubation time, cells were infected with 4 × 104P. berghei sporozoites for 48 h and fresh medium was added 24 h p.i. Effect of siRNA was assessed at the gene level by quantitative real-time PCR, at the protein level by Western blotting, or by immunofluorescence assays (IFA).
Quantitative real-time PCR
After 1 min treatment with trypsin at 37°C, cultured cells were collected for total RNA purification using the Qiagen RNeasy Mini Kit (Valencia, CA, USA). cDNA synthesis was performed using the High capacity cDNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). Equivalent amounts of 100 ng RNA was used as template for each assay. The cDNA generated was mixed with SYBR® Green PCR Master Mix (Applied Biosystems) and specific primers for human β-actin, human LDL receptor, human squalene synthase and Plasmodium 18s ribosomal-RNA. Amplification was monitored by the 7000 sequence detection system (Applied Biosystems). Relative expression of genes was normalized the β-actin gene of Huh7 cells for each assay. Primer sequences were for β-actin (sense: 5′-CTATAAGCTTGCCGGGACCTGACTGACT-3′ and antisense: 5′-CATAGAATTCCGCTCGGCCGTGGTGGTG-3′), for LDL receptor (sense: 5′-TGCGAAAGAAACGAGTTCCAGTGC-3′ and antisence: 5′-ATTTGCAGGTGACAGACAAGCACG-3′), for squalene synthase (sense: 5′-CATTGCCACTTTGGCTGCCTGTTA-3′ and antisense: 5′-TTTGACAGCTGGCATATTGGTGGC-3′) and for 18s (sense: 5′-AAGCATTAAATAAAGCGAATACATCCTTAC-3′ and antisense: 5′-GGAGATTGGTTTTGACGTTTATGTG-3′).
Western blotting
After washing in phosphate-buffered saline (PBS) solution, cells were collected and lysed in 50 µl lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 1 mM PMSF and 0.5× 2-mercaptoethanol. Proteins in equal volumes corresponding to 10 µl for each sample were separated on 4–20% Tris-HCl polyacrylamide gel (BIORAD, Hercules, CA, USA). After transfer, PVDF membranes (Millipore, Billerica, MA, USA) were blocked overnight at 4°C in PBS with 5% dry milk and 0.1% Tween-20. Immunoblotting was performed using the primary antibodies against β-actin (as loading control) at 1/100 dilution, LDL receptor at 1/5000 dilution and squalene synthase at 1/1000 dilution, revealed by appropriate secondary antibodies coupled to horseradish peroxidase to stain proteins of interest. Detection of immunoreactive proteins by chemiluminescence was performed using the ECL Plus Western Blotting Kit (Amersham).
Preparation of LDL, HDL, LPDS, lipoproteins containing NBD-cholesterol or [3H]cholesterol
Human LDL (density: 1.019 to 1.063 g ml−1) and human HDL (density: 1.063 to 1.2 g ml−1) were isolated from fresh serum by zonal density gradient ultracentrifugation as described (Poumay and Ronveaux-Dupal, 1985). LPDS was prepared by ultracentrifugation of FBS after adjustment of serum density to 1.215 g ml−1 with KBr (Havel et al., 1955). To incorporate fluorescent cholesterol into LDL or HDL, NBD-cholesterol was dissolved in 25 ml dimethylformamide and added to 210 ml of filtered fresh human plasma for 16 h at 37°C before lipoprotein isolation. Radiolabelling of lipoproteins with 3H-cholesterol was previously described (Coppens et al., 1995).
Metabolic labelling experiments
To study the biosynthesis of sterols in P. berghei, 300 nmol [14C]HMG-CoA was added to infected Huh7 cells pre-permeabilized with digitonin (Leonard and Chen, 1987). After 6 h of pulse, cells were washed and resuspended in chase medium until merosome production. Lipids were then extracted from purified merozoites to quantify the amount [14C]HMG-CoA incorporated into cholesterol associated with the sterol fraction as described (Coppens et al., 1995).
Fluorescence microscopy
Filipin staining. The fluorescent dye filipin (10 mg ml−1 in dimethyl sulfoxide) has been used for cytochemical detection of β-hydroxysterols within membranes of infected mosquito tissues, liver cells, red blood cells and merosomes as described (Coppens et al., 2000). Plasmodium berghei-GFP-infected mouse liver was harvested 24 h post tail injection of 1 million sporozoites. Liver was extensively washed with PBS/heparin solution and fixed overnight in 4% formaldehyde. Several 50 µm sections were generated, and GFP-positive slides were stained with filipin. For drug assays, 24 h cultured cells were infected with sporozoites and simultaneously incubated with progesterone (10 µg ml−1) or squalestatin (60 µM) before filipin labelling. For lovastatin (4 µM), cultured cells were incubated 24 h with the drug before infection, and exposed for additional 24 h to the drug before filipin staining.
NBD-cholesterol staining. To visualize fluorescent cholesterol associated with liver forms, HepG2-CD81 cells were infected with P. yoelii before addition of LDL or HDL containing NBD-cholesterol for 3 h and observed as live by fluorescence microscopy. For drug assay, cultured cells were infected with sporozoites and simultaneously incubated with progesterone (10 µg ml−1) during 24 h before addition NBD-cholesterol-LDL for 3 h.
IFA. For this staining, infected cells were processed as previously described (Karsten et al., 1998) and in this study, incubated with primary antibodies against PyHsp70 at 1/400 dilution or PyUIS4 at 1/250 dilution.
For all fluorescence assays, images were acquired on a Nikon Eclipse E800 microscope equipped with a Spot RT CCD Camera and processed using Image-Pro-Plus software (Media Cybernetics, Silver Spring, MD, USA) before assembly using Adobe Photoshop (Adobe Systems, Mountain View, CA, USA).
In vitro malaria infection assays
All the in vitro assays were conducted in 8-well chambers slide (Permanox, Nalge Nunc International, Rochester, NY, USA). To monitor the effect of lipoproteins on P. yoelii development, 2 × 105 HepG2-CD81 cells per well were cultured in α-MEM + 10% FBS for 24 h before infection with 3 × 104 sporozoites. Three hours p.i., cells were washed to replace the medium by fresh α-MEM + 10% FBS (as positive control); α-MEM + 10% LPDS; α-MEM + 10% LPDS + HDL (2 mg ml−1) or α-MEM + 10% LPDS + LDL (2 mg ml−1). Cultures were maintained 43 h p.i. Parasite development was monitored by quantitative real-time PCR as described (Kumar et al., 2004) and by IFA using antibodies against PyHsp70, a protein selectively expressed by liver forms. To examine the effect of squalestatin on P. berghei development, 2 × 105 Huh7 cells per well were cultured in DMEM + 10% FBS for 48 h or 72 h. Squalestatin was added to the cells for 24 h before infection with 3 × 104P. berghei sporozoites until 48 h of infection. To assess potential squalestatin cytotoxicity, drug-treated Huh7 cells were collected and cell number and viability were determined using a trypan blue dye exclusion assay.
In vivo malaria infection assays
1 × 106 Huh7 cells were cultured in MEM + 10% FBS for 48 h; MEM + 10% LPDS for 48 h; or MEM + 10% FBS + 60 µM squalestatin for 24 h before infection. Cultures were then infected with 1 × 106P. berghei sporozoites and medium were replaced every 24 h. Merosomes were collected 81 h p.i., washed once with MEM + 10% FBS and counted using a haemocytometer. Swiss-Webster mice were infected by intravenous injection of 250 merosomes resuspended in 200 µl MEM + 10% FBS each. Parasitaemia was assessed 6 and 13 days post injection on blood smears stained with Giemsa.
Cholesterol concentration
Total cholesterol content was measured by an enzymatic and colorimetric assay using a reagent mixture from Roche Diagnostics (Indianapolis, IN, USA).
Protein concentration
Protein content was determined by the Bio-Rad Protein Assay using the wavelength scan program of the Beckman DU-640 spectrophotometer (Miami, FL, USA).
Statistical analysis
For comparison of means, P was determined by analysis of variance against control (ANOVA 2).
Acknowledgements
The authors are grateful to the members of the Coppens laboratory for their helpful discussions during the course of this work. We are grateful to the generous providers of strains and antibodies. We wish also to thank Dabeiba Bernal-Rubio, Sandra Gonzalez and Jean Nonan from New York University School of Medicine for their remarkable technical support in the rearing and infection of the mosquitoes. This research was supported by the Johns Hopkins Malaria Research Institute and the Bloomberg Family Foundation. B.J. was a recipient of a predoctoral MRI fellowship.