Signalling pathways in the pathogenesis of Cryptococcus
Summary
Efficient communication with the environment is critical for all living organisms. Fungi utilize complex signalling systems to sense their environments and control proliferation, development and in some cases virulence. Well-studied signalling pathways include the protein kinase A/cyclic AMP (cAMP), protein kinase C (PKC)/mitogen-activated protein kinase (MAPK), lipid signalling cascades, and the calcium–calcineurin signalling pathway. The human pathogenic basidiomycetous fungus Cryptococcus neoformans deploys sensitive signalling systems to survive in the human host, leading to life-threatening meningoencephalitis. Known virulence traits of this fungus, including the antioxidant melanin production, the antiphagocytic polysaccharide capsule and the ability to grow at 37°C, are orchestrated by complex signalling networks, whose understanding is crucial to better treat, diagnose and prevent cryptococcosis.
Introduction
Cryptococcus neoformans causes life-threatening fungal meningoencephalitis in immunocompromised and in some cases also immunocompetent hosts (Casadevall and Perfect, 1998). C. neoformans is a dimorphic basidiomycete that proliferates as a budding yeast (α or a mating type) during vegetative growth and switches to hyphal filamentation during mating. Natural niches for C. neoformans are tree hollows and pigeon guano, both of which have been shown to stimulate mating and spore production (reviewed in Lin and Heitman, 2006). Spores and small desiccated yeasts are thought to constitute infectious propagules that are inhaled by humans and animals (Ruiz and Bulmer, 1981; Casadevall and Perfect, 1998; Sukroongreung et al., 1998). There are four pathogenic Cryptococcus serotypes/species: A (C. neoformans var. grubii), B and C (Cryptococcus gattii) and D (C. neoformans var. neoformans). Mating type α is more prevalent in nature and infection and, in some genetic backgrounds, can be more virulent than the a mating type (reviewed in Lin and Heitman, 2006).
What makes C. neoformans virulent? C. neoformans is encapsulated with a polysaccharide coat and acapsular mutants are avirulent; thus, capsule is a key virulence factor (Chang and Kwon-Chung, 1994). C. neoformans produces melanin on media containing diphenolic compounds (Kwon-Chung, 1992) and mutants unable to produce melanin are less virulent in mice (Salas et al., 1996). Other virulence traits of the fungus are the ability to grow at 37°C and the production of urease. Elucidating the mechanisms that govern virulence is crucial for the development of better treatments against cryptococcosis. Here, we review findings on four major signalling pathways in C. neoformans, including the cyclic AMP (cAMP), mitogen-activated protein kinase (MAPK), lipid and calcium/calcineurin signalling pathways with an emphasis on how these signalling routes control virulence of C. neoformans.
Protein kinase A/cAMP signalling pathway
Many pathogenic fungi utilize the cAMP signalling pathway to trigger diverse cellular responses, including virulence. Classic studies were conducted to understand this pathway in the model yeast Saccharomyces cerevisiae. Membrane-bound adenylyl cyclase (Cdc35) converts ATP into cAMP, which serves as a second messenger. cAMP then activates protein kinase A (PKA), which in the resting state is a heteromeric tetramer with coupled regulatory (Bcy1) and catalytic subunits (Tpk1, Tpk2 and Tpk3) (Toda et al., 1987a). In the absence of cAMP, the regulatory subunit is bound to the catalytic subunit and inhibits kinase activity, and binding of cAMP to the regulatory subunits dissociates the catalytic and regulatory subunits activating PKA (Toda et al., 1987b). Subsequent phosphorylation of downstream targets by PKA induces cellular responses, including nutrient sensing, growth regulation, stress responses and pseudohyphal growth (reviewed in Lengeler et al., 2000). Adenylyl cyclase is a membrane-bound protein activated by nutrient sensing via the Ras-GTPase (Ras1 and Ras2), a G-protein-coupled receptor system (Gpr1–Gpa2) (Toda et al., 1985), and Cap/Srv2 (Shima et al., 2000).
In C. neoformans, the cAMP-mediated signalling pathway also contributes to cellular responses, including virulence traits such as melanin production, capsule formation and invasive hyphal growth (Fig. 1). Disruption of CAC1 encoding adenylyl cyclase impairs melanin production, capsule formation and mating (Alspaugh et al., 2002). Aca1, an adenylyl cyclase-associated protein, is also required for mating and hyphal differentiation (Bahn et al., 2004). Two laccase genes, LAC1 and LAC2, are known to be regulated by the cAMP pathway (Pukkila-Worley et al., 2005). Thus, the cAMP pathway is a central regulatory conduit governing virulence of C. neoformans.
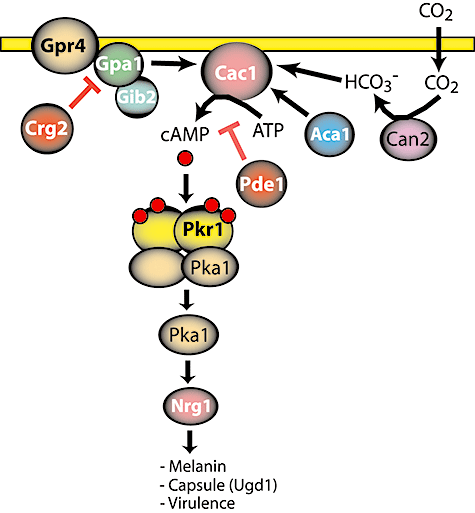
Protein kinase A (PKA)/cyclic AMP (cAMP) signalling pathway in pathogenesis of C. neoformans. Adenylyl cyclase (Cac1) is activated by the Gα subunit (Gpa1) or HCO3- resulting in production of cAMP. cAMP then binds to regulatory subunits (Pkr1) of the PKA complex to release an active form of the catalytic subunit (Pka1), which phosphorylates downstream proteins including Nrg1. A GTPase-activating protein (Crg2) and phosphodiesterase (Pde1) negatively regulate Gpa1 and production of cAMP respectively. Gpr4, G-protein-coupled receptor; Can2, carbonic anhydrase; Aca1, adenylyl cyclase-associated protein; Gib2, Gβ subunit.
An upstream component of the adenylyl cyclase–cAMP–PKA pathway includes a G-protein-coupled receptor in C. neoformans, as in S. cerevisiae. Disruption of GPA1, which encodes a Gα subunit, leads to loss of virulence resulting from lack of melanin and capsule production and a concomitant reduction in mating ability (Alspaugh et al., 1997). However, Gpb1, a Gβ subunit, is exclusively required for mating and monokaryotic fruiting rather than virulence (Wang et al., 2000). On the other hand, overexpression of Gib2, a Gβ-like/RACK1 homologue, which functions as a Gβ subunit in the cAMP signalling cascade, suppresses the virulence factor defects of a gpa1 mutant (Palmer et al., 2006). Gpr4, a G-protein-coupled receptor, was identified and shown to be involved in activating the cAMP–PKA pathway. gpr4 mutants display decreased levels of endogenous cAMP resulting in impaired capsule production (Xue et al., 2006). Therefore Gpr4–Gpa1 is an upstream G-protein-coupled receptor system in C. neoformans. C. neoformans encodes three RGS (regulators of G-protein signalling) proteins including Crg1, Crg2 and Crg3, which act as GTPase-activating proteins (GAP) that are negative regulators of Gα subunits. Crg2 regulates the cAMP-mediated signalling pathway for virulence as well as pheromone-mediated mating (Shen et al., 2008; Xue et al., 2008). Crg1 is solely involved in pheromone-mediated mating (Wang et al., 2004). The functions of Crg3 are as yet not known.
In S. cerevisiae, two cAMP phosphodiesterases with low affinity and high affinity, Pde1 and Pde2, respectively, are known to function as negative regulators of cAMP production (Thevelein and de Winde, 1999). In C. neoformans, the Pde1 phosphodiesterase also modulates cAMP production (Hicks et al., 2005).
Cryptococcus neoformans also encodes a PKA complex, the downstream target of adenylyl cyclase. A pkr1 disruption mutant, which lacks the regulatory subunit of PKA, produces enlarged capsules and is hypervirulent (D'Souza et al., 2001). Interestingly, the requirement of the catalytic subunits of PKA is divergent in the serotype A and D lineages. In serotype A, a pka1 mutant is avirulent with defects in melanin production and capsule formation as well as mating, whereas Pka2 is not required for virulence (D'Souza et al., 2001). In contrast, in serotype D, mutations in pka1 and/or pka2 do not lead to a loss of virulence although the pka2 mutant does display decreased melanin and capsule production (Hicks et al., 2004). Transcriptional profiling analysis in pka1, pkr1 and wild type of serotype A identified that the cAMP–PKA pathway induces protein sets for synthesis and secretion of virulence factors (Hu et al., 2007). In this study, components in the secretory machinery for virulence factors were suggested as possible drug targets (Hu et al., 2007). Nrg1, a transcription factor, was identified as a downstream target of the cAMP pathway, and a transcriptome analysis between wild type and the nrg1 mutant revealed that the transcription factor regulates Ugd1, a UDP-glucose dehydrogenase involved in capsule production (Cramer et al., 2006).
The Cryptococcus capsule is one of the pre-eminent virulence factors and is considered to protect the pathogen from the host defence (Casadevall and Perfect, 1998). Capsule production is induced in response to carbon dioxide (CO2) levels present in the host (Granger et al., 1985). Therefore, C. neoformans senses CO2 to produce this virulence factor, in which the balance between CO2 and HCO3- is mediated by Can2, a carbonic anhydrase. CO2 diffuses across the plasma membrane followed by hydration of CO2 into HCO3- by Can2, and then HCO3- directly binds to and activates adenylyl cyclase, encoded by the CAC1 gene (Fig. 1) (Bahn et al., 2005a; Mogensen et al., 2006). Adenylyl cyclase plays central roles in CO2 sensing and signalling in both C. neoformans and Candida albicans (Klengel et al., 2005; Mogensen et al., 2006). Thus, the cAMP–PKA pathway is involved in a signalling relay through both a CO2 sensing system and a G-protein-coupled receptor system to produce and properly locate virulence factors. Taken together, the cAMP–PKA signalling pathway is a potential target for developing therapeutic drugs against fungal infections.
MAPK pathways
One of the ways eukaryotic cells sense and respond to changes in the outside environment involves MAPK pathways, cascades of phosphorylation reactions that convey signals from the cell surface to the nucleus. The core of MAPK pathways consists of three kinases. The downstream MAPK is activated by a MAP kinase kinase (MAPKK), which in turn is activated by a MAP kinase kinase kinase (MAPKKK). The mechanisms of sensing and response to external signals vary and determine the specificity of each pathway. MAPK pathways often involve specialized scaffold proteins, which further contribute to signalling fidelity. Sensors are heterotrimeric G-protein-coupled receptors or other membrane-bound receptors. The response typically involves transcriptional activation/repression of appropriate genes.
In bakers' yeast there are at least five MAPK pathways, which mediate the response to pheromone during mating, trigger filamentous growth during nitrogen starvation, respond to osmotic shock and other stresses, cope with cell wall stress or initiate sporulation (reviewed in Chen and Thorner, 2007). Among human pathogenic fungi, MAPK pathways have been characterized in greatest detail in C. albicans (Monge et al., 2006). This is partly due to the fact that the MAPK pathway mediates the dimorphic yeast to hypha transition, a morphological change essential for infection with Candida. Similar to other pathogenic fungi, MAPK pathways in C. neoformans are crucial for its survival in the host (reviewed in Roman et al., 2007). Some aspects of this MAPK system are similar to the S. cerevisiae paradigm. However, there is increasing evidence for the unique character of MAPK pathways in C. neoformans contributing to its virulence. In the following section we describe MAPK pathways that play important roles during cryptococcal infection (Fig. 2).
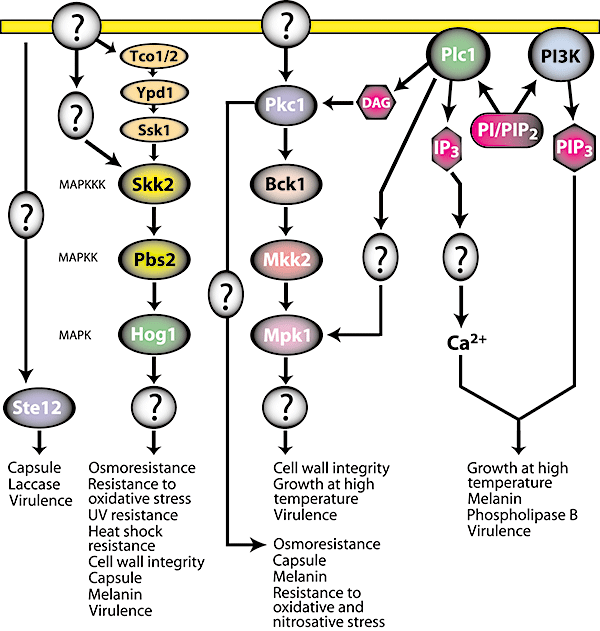
MAPK signalling pathway and PI3K/IP3 signalling pathway in C. neoformans. Protein kinase C (Pkc1) phosphorylates MAPKKK (Bck1) and subsequent phosphorylations of MAPKK (Mkk2) by Bck1 and MAPK (Mpk1) by Mkk2 induce downstream components for virulence traits. Pkc1 also signals through a MAPK-independent pathway. The MAPK Hog1, which is activated by Pbs2, triggers virulence factor production. Although the exact architecture of the upstream part of the HOG pathway is unknown, the likely players are hybrid sensor kinases Tco1/2, Ypd1 and the response regulator Ssk1. Ste12 is a transcription factor involved in the mating MAPK pathway, but how it contributes to virulence is unclear. Phospholipase (Plc1) activates the PKC/MAPK pathway through two different routes, one of which involves diacylglycerol (DAG). IP3 is involved in the release of cytosolic Ca2+. PI3K signalling induces virulence traits through PIP3. PI, phosphatidylinositol; PIP2, phosphatidylinositol bisphosphate.
In S. cerevisiae, response to high temperature is mediated by the cell integrity pathway that involves a MAPK cascade. A similar pathway was found to coordinate responses to high temperature in C. neoformans (Kraus et al., 2003). The core component of this pathway, the MAP kinase Mpk1, was found to be essential for growth at 37°C. Growth of the mpk1Δ mutant at 37°C can be restored by osmotic stabilization, and perturbation of the cell wall induces Mpk1 phosphorylation, indicating Mpk1 is involved in maintaining cell wall integrity.
The response to osmotic stress in S. cerevisiae is mediated by the MAPK Hog1 through the conserved high-osmolarity glycerol (HOG) pathway (reviewed in Hohmann et al., 2007). The signal reaches Hog1 via two routes. One involves a three-component phosphorelay system composed of the transmembrane osmosensor Sln1, Ypd1 and Ssk1. Under normal conditions, Sln1 in complex with Ypd1 phosphorylates Ssk1, which prevents Ssk1 from interacting with and activating the MAPKKK Ssk2. The other route involves the transmembrane protein Sho1 as a sensor, which upon stress activates a p21-activated kinase (PAK) Ste20 through Cdc42, and Ste20 then cooperates with Cdc50 to activate the MAPKKK Ste11. Downstream elements of the Sho1 branch are shared by two other MAPK pathways: the mating and pseudohyphal growth pathways. Both routes converge to activate the MAPKK Pbs2, which in turn activates Hog1. Activated Hog1 enters the nucleus and triggers transcriptional responses, although a critical role for Hog1 nuclear import has been challenged recently (Westfall et al., 2008).
Identical genes encoding Hog1 are found in C. neoformans genomes of both serotypes A and D. Interestingly, the relatively more virulent serotype A strain H99 is more resistant to osmotic shock than the serotype D strain JEC21 (Cruz et al., 2000), which suggests that co-opting the HOG pathway was one way C. neoformans acquired increased virulence. Indeed, whereas in some strains of serotype D the HOG pathway operates in a similar fashion to the canonical HOG pathway of S. cerevisiae, the pathway in serotype A has been adapted to regulate virulence (Bahn et al., 2005b). Specifically, in serotype A Hog1 is constitutively phosphorylated under normal conditions and dephosphorylated in response to osmotic stress, which is the exact opposite to the budding yeast paradigm. Serotype A Hog1 also reveals a distinct localization pattern after exposure to osmotic stress. Strikingly, this peculiar Hog1 behaviour is observed in several clinical isolates of serotype A obtained from diverse geographical regions, whereas serotype D shows more variability in Hog1 responses among different isolates. The precise molecular basis of the variability in Hog1 phosphorylation remains unclear, but the key component responsible for this virulence-associated pattern is the MAPKKK Skk2 (Bahn et al., 2007).
Bahn et al. found that Hog1 of serotype A represses the cAMP–PKA pathway resulting in decreased production of melanin and capsule. The hog1Δ and pbs2Δ mutants are less virulent as compared with the wild-type serotype A strain. Given that both mutants show significantly increased melanization and production of capsule, it provides evidence that the ability to cope with stress and the production of capsule and melanization are both important for virulence.
There are several unanswered questions regarding the HOG pathway in C. neoformans. First, it is unclear how Hog1 is regulated at a mechanistic level, especially since there are no direct orthologues of the osmosensor proteins Sln1 and Sho1 in Cryptococcus. Second, the detailed mechanics of the unusual regulation of Hog1 in serotype A remain elusive. Third, the exact mechanism of cross-talk between the HOG pathway and the cAMP pathway requires further investigation.
A recent study of C. albicans showed that regulation of genes encoding chitin synthases depends on three signalling pathways: the calcineurin, protein kinase C (PKC) and HOG pathways (Munro et al., 2007). This study illustrates that the signalling pathways do not operate in isolation but rather form a fine-tuned network consisting of cross-talk, feedback loops and compensatory mechanisms. Cell wall biogenesis and cell integrity are crucial for survival of fungal cells and there is increasing evidence for multiple pathways employed in these processes in C. neoformans. Gerik et al. showed recently that the localizations of chitin and chitosan are impaired in the pkc1Δ strain. Interestingly, Pkc1 acts through Mpk1 to maintain cell integrity but signals independently of Mpk1 in response to oxidative and nitrosative stresses (Gerik et al., 2008). It will be of interest to probe whether Hog1 is involved in Pkc1-dependent response to these stresses. Furthermore, whereas a pkc1Δ mutant has defects in capsule and decreased melanin production, these virulence traits are not affected in strains deleted for BCK1, which encodes the MAPKKK acting downstream of Pkc1 (Gerik et al., 2005). It is possible that melanin and capsule defects are indirect effects of impaired cell wall organization, especially since pkc1Δ has a more severe phenotype than the bck1Δ mutant. More studies are needed to elucidate how Pkc1 coordinates these multiple pathways.
Recently a two-component signal transduction module has attracted considerable attention in medical mycology due to the fact that it is not present in mammalian cells and thus is attractive as a potential drug target (reviewed in Kruppa and Calderone, 2006; Bahn, 2008). In S. cerevisiae, this module constitutes the upstream pathway of the HOG signalling cascade. Although the exact architecture of the upstream part of the HOG pathway in C. neoformans is unknown, the likely players are hybrid sensor kinases Tco1/2, Ypd1 and the response regulator Ssk1 (Bahn et al., 2006). Skn7 homologue, which in S. cerevisiae is involved in oxidative and cell wall damage stress responses was shown to be involved in serotype A responses to oxidative stress and to contribute to virulence (Wormley et al., 2005). It has also been proposed that in serotype D, Skn7 allows intraphagocytic survival by contributing to resistance to phagolysosomal killing (Coenjaerts et al., 2006). It will be important to identify other components of the pathway mediated by Skn7, perhaps at least in part shared with the HOG pathway.
The HOG pathway in Cryptococcus illustrates how components of MAPK signalling underwent specialization leading to increased virulence. Another example is the role of the transcription factor Ste12 in C. neoformans. Ste12 is the homologue of the S. cerevisiae protein that acts during mating in response to pheromone stimulation. Davidson et al. and others found that the upstream components of the C. neoformans MAPK mating pathway do not contribute to virulence, whereas Ste12 is crucial in a serotype D strain (Chang et al., 2000; Davidson et al., 2003). Importantly, the STE12 gene is part of the MAT locus and the two STE12a and STE12α alleles differ, which may in part explain why α cells are more virulent than a type. Ste12 is sufficient but not essential for mating and filamentation responses (Chang et al., 2001; Davidson et al., 2003) and it stimulates expression of known virulence genes (Wickes et al., 1997; Chang et al., 2000), confirming roles of Ste12 beyond promoting mating and filamentation.
Chang et al. (2001) examined whether Ste12α and Ste12a could complement each other by exchanging the genes between strains of opposite mating type. In this interesting approach, both Ste12α and Ste12a complemented each other functionally. However, while expression of Ste12a efficiently complemented the ste12αΔ strain with respect to hypha formation on filamentation agar, complementation by Ste12α in the ste12aΔ mutant was much less effective. This indicates that the effects of the STE12 genes on haploid fruiting depend on other, yet unidentified mating type-specific genes. In the same study both Ste12α and Ste12a contributed to the capsule size of the yeast cells derived from brain smears of infected mice and both genes were important for the expression of several virulence-associated genes (Chang et al., 2001). It would be of interest to test whether expressing Ste12α in the congenic a type strain would enhance virulence or whether a MATα strain with Ste12α replaced by Ste12a would be impaired in virulence.
In contrast to serotype D, deleting STE12 in a serotype A strain had no effect on virulence (Yue et al., 1999). Interestingly, the opposite relationship was found for another MAPK pathway component, the PAK Ste20 (Wang et al., 2002). C. neoformans contains two PAK kinases, Ste20 and Pak1, which are involved in mating, differentiation and virulence. Ste20 is mating type-specific, whereas Pak1 is not. A serotype A ste20αΔ mutant was attenuated for virulence but the serotype D ste20αΔ mutant was as virulent as the respective wild type. In contrast to STE20, deletion of PAK1 attenuated virulence in both serotypes. It would be of interest to test whether a serotype A α strain with STE20α replaced with STE20a proves less virulent than the wild type.
In summary, MAPK pathways in C. neoformans play an essential role in its ability to proliferate in the host. There is a high degree of specialization within these pathways, which is both mating type- and serotype-specific and thus results in differences in virulence between strains. There is also an apparent cross-talk between MAPK pathways and other signalling routes, which adds to the complexity of the signalling networks in C. neoformans that enable it to respond appropriately, both to the environment and to the host.
Phosphatidylinositol 3-kinase and inositol 1,4,5-triphosphate
Recent reports demonstrate that the phosphatidylinositol 3-kinase (PI3K) signalling pathway contributes to the pathogenesis of C. neoformans via an involvement in melanin production (Fig. 2) (Hu et al., 2008). PI3K is responsible for the synthesis of phosphatidylinositol 3-phosphate (PIP3) and in S. cerevisiae, the class III Vps34 PI3K forms a membrane-associated signalling complex with Vps15 to regulate protein sorting and autophagy. Vps34 forms a complex with Vps15, Vps30 and Vps38 to regulate vesicular transport of hydrolases to the vacuole lumen and also forms another complex with Vps15, Atg14 and Vps30 to trigger autophagy in nutrient-limited conditions (Herman and Emr, 1990; Stack et al., 1993; Kametaka et al., 1998; Burda et al., 2002). Vps34 is activated by the pheromone-activated GTP-bound Gα subunit (Gpa1) at the endosomal membrane (Slessareva et al., 2006).
In C. neoformans, a PI3K deletion mutant displays significantly decreased virulence in a murine infection model and no obvious autophagic vesicles form after phagocytosis by macrophages (Hu et al., 2008). This suggests that this pathogenic fungus utilizes PI3K signalling as a regulatory mechanism for the expression of virulence factors and for survival in stress conditions.
The C. neoformans phospholipase C (PLC) has been shown to play a role in pathogenesis. plc1, plc2 double deletion mutations exhibit defects in induction of virulence traits including melanin production, growth at 37°C and release of phospholipase B (Plb1) (Fig. 2) (Siafakas et al., 2007). The regulation by PLCs is accomplished through PKC/MAPK (Chayakulkeeree et al., 2008). PLC mediates production of inositol 1,4,5-triphosphate (IP3), which serves as a secondary messenger in Ca2+ signalling pathways. IP3 receptors (IP3R) are a family of Ca2+ channels localized on the endoplasmic reticulum (ER) in eukaryotic cells, including a choanoflagellate (Cai, 2008). In response to IP3, the IP3 receptor releases Ca2+ to the cytoplasm to trigger key cellular responses (reviewed in Foskett et al., 2007). Given the absence of ryanodine and IP3 receptor orthologues in the genomes of ascomycetes and basidiomycetes, internal Ca2+ release from vacuoles to the cytosol might occur through transient receptor potential (TRP) channels, as in S. cerevisiae (Denis and Cyert, 2002). Most interestingly, IP3 induces the internal release of Ca2+ from vacuoles in yeast (Belde et al., 1993). In Neurospora crassa, a Ca2+ gradient at hyphal apices is modulated by IP3 and adenophostin A, an IP3 receptor agonist (Silverman-Gavrila and Lew 2001). A question arises as to how cellular IP3 is sensed. One explanation is that there might be a Ca2+ channel that responds to IP3. Alternatively, IP3 might be further phosphorylated by an IP3 kinase, producing higher phosphorylated forms of inositol as signalling molecules. In S. cerevisiae, Ipk2 is known to convert IP3 to IP5, which controls transcription of a subset of genes, suggesting inositol phosphate (IP) signals are not restricted to Ca2+ release (Odom et al., 2000). The roles of IP3 in C. neoformans are as yet unknown although two PLCs are known to be expressed (Siafakas et al., 2007). However, a recent finding that myo-inositol induces mating and pathogenicity of C. neoformans on plants (Xue et al., 2007) indicates that Cryptococcus might sense inositol or utilize inositol and its derivatives for signal transduction. Investigation of roles for IP3 in pathogenesis will further strengthen the link between phospholipid signalling and virulence in this human pathogenic fungus.
Ca2+–calcineurin pathway
Ca2+ is a second messenger that mediates cellular responses to changes in the external environment. An increased level of calcium is sensed by calmodulin, which activates the Ca2+/calmodulin-dependent protein kinases (CaMK) and the conserved serine/threonine phosphatase calcineurin. Calcineurin consists of two subunits: a 60 kDa catalytic A subunit and a 19 kDa regulatory B subunit, both of which are essential for enzyme activity (Stie and Fox, 2008). When intracellular calcium levels are low, calcineurin is inactive as a C-terminal inhibitory domain blocks the active site. Elevated cytosolic calcium is sensed by calmodulin, which binds the C-terminal region of calcineurin A and induces conformational changes which relieve this autoinhibition. Subsequently, activated calcineurin stimulates transcription of a number of genes whose products enable the cell to cope with environmental stress and maintain calcium homeostasis. In multicellular eukaryotes, well-characterized substrates of calcineurin are members of the nuclear factor of activated T cell (NFAT) family of transcription factors (Crabtree and Olson, 2002) that activate calcium-responsive genes, including interleukin 2. This pathway is the target of the immunosuppressive drugs cyclosporin A (CsA) and FK506, which inhibit T-cell activation.
A comparative genome analysis indicated that Ca2+ signalling elements are well conserved among fungi (Zelter et al., 2004). In S. cerevisiae calcineurin responds to high cation concentrations, cell wall stress and prolonged exposure to mating pheromone (Cyert, 2003). A stress response in S. cerevisiae leads to calcineurin-mediated dephosphorylation and activation of the transcription factor Crz1, which elevates transcription of more than 160 genes. In the fission yeast Schizosaccharomyces pombe, the calcineurin pathway is triggered by a variety of stresses, including nitrogen starvation and low temperature (Yoshida et al., 1994; Plochocka-Zulinska et al., 1995). The calcineurin pathway has been also implicated in the regulation of cell cycle progression (Rasmussen et al., 1994; Miyakawa and Mizunuma, 2007; Zhang and Rao, 2008).
The immunosuppressive drugs CsA and FK506 also possess antifungal activity. CsA and FK506 diffuse into the cell and bind to the cyclophilin A and FKBP12 proteins respectively. The resulting protein–drug complexes are potent inhibitors of calcineurin. The finding that the calcineurin A gene CNA1 is essential for the virulence of C. neoformans opened a potential avenue for the development of novel antifungals (Odom et al., 1997; Steinbach et al., 2007). Although the detailed architecture of calcium signalling in Cryptococcus is far from being solved, considerable advances have been made in this area recently. Here we describe key findings on calcineurin signalling in C. neoformans (Fig. 3).
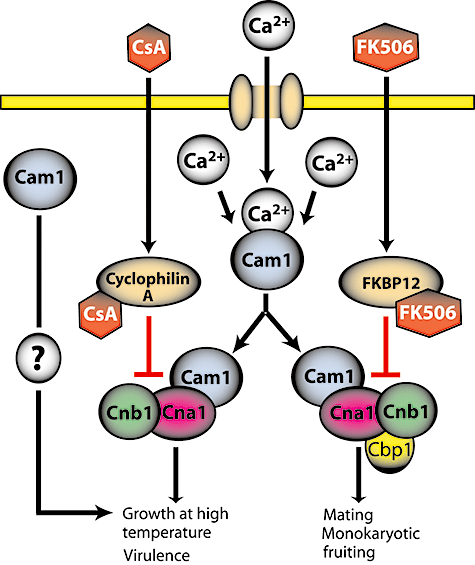
Ca2+/calcineurin signalling in C. neoformans. Cytosolic Ca2+ is sensed by calmodulin (Cam1), which activates CaMKs (not shown) and the calcineurin complex (Cna1 and Cnb1) to support growth at 37°C and virulence. Calmodulin may also act independently of the calcineurin complex. Cyclosporine A (CsA) and FK506, bound to cyclophilin A and FKBP12, respectively, inhibit calcineurin. Cbp1: calcipressin, a calcineurin effector.
The levels of cytosolic calcium in fungi are coordinated by calcium pumps and channels located in the plasma, vacuolar and ER membranes. A plasma membrane calcium channel Cch1 (Liu et al., 2006) and the ER calcium pump Eca1 (Fan et al., 2007) are implicated in virulence of C. neoformans, underscoring the importance of calcium signalling in this pathogen. Unlike S. cerevisiae, calcineurin is essential for growth of C. neoformans at temperatures above 35°C. In addition to temperature sensitivity, cna1Δ mutants are sensitive to increased levels of CO2, alkaline pH and high concentrations of cations, indicating that calcineurin is essential for survival of Cryptococcus in conditions found in the host (Odom et al., 1997). Similar to CNA1, the gene encoding the calcineurin regulatory B subunit, CNB1, is also essential for growth at 37°C, survival of Cryptococcus in the host and CsA/FK506 action (Fox et al., 2001). Interestingly, loss of calcineurin function leads to elevation of transcript levels of FKS1, a gene encoding a component of β-1,3-glucan synthase complex, an essential cell wall-synthesizing enzyme, and this effect is dependent on Mpk1 (Kraus et al., 2003). Thus, the calcineurin and Mpk1 cell integrity pathway coordinately regulate C. neoformans responses to high temperature stress.
Calmodulin, encoded in C. neoformans by an essential gene, CAM1, acts in response to high temperature by two distinct mechanisms, only one of which involves Ca2+ and calcineurin (Kraus et al., 2005). The effectors of the other response that cooperate with calmodulin remain elusive. Ca2+/calmodulin-dependent protein kinases (CaMKs) require calcium to respond to calmodulin and thus are not likely candidates. Calmodulin participates in the internalization step of endocytosis in budding yeast in a Ca2+-independent manner (Kübler et al., 1994) and a similar role of calmodulin in Cryptococcus is possible.
The substrates involved in calcineurin-mediated stress responses in Cryptococcus are largely unknown. No clear homologue of the CRZ1 gene, encoding the S. cerevisiae calcineurin-activated transcription effector, has been identified in C. neoformans serotype A or D. It is possible that C. neoformans contains different or more than one transcription factor responsive to calcineurin. This could explain the apparent difficulty in obtaining multicopy suppressors that function downstream of the phosphatase (see below). Alternatively, the effects of calcineurin in C. neoformans could be post-transcriptional. By analogy to yeast, the pH response may involve regulation of the plasma membrane or vacuolar H+-ATPase (Mendoza et al., 1994; Garrett-Engele et al., 1995; Hemenway et al., 1995), whereas the response to Li+ likely involves homologues of the Li+/Na+ pump Pmr2A. To identify the substrates of calcineurin that mediate high temperature responses, a multicopy suppressor screen was performed (Fox et al., 2003). The screen revealed a novel gene, CTS1, which encodes a leucine zipper-containing phospholipid-binding protein. Strains lacking CTS1 are hypersensitive to FK506 and temperature sensitive. However, epistasis analysis indicated that calcineurin and Cts1 function in parallel pathways. Because Cts1 is essential for proper septation, overexpression of Cts1 may ameliorate defects in the cna1Δ mutant associated with cell wall perturbations (Kraus et al., 2003).
In addition to a role in responses to stress, calcineurin is essential for hyphal elongation during mating and monokaryotic fruiting of C. neoformans (Cruz et al., 2001). Although not directly necessary for virulence, these pathways are relevant because spores produced as a result of either mating or fruiting are thought to be the infectious propagules (possibly in addition to small yeast cells). A conserved member of the calcipressin family, Cbp1/Rcn1, has been shown to play a role in mating in C. neoformans (Görlach et al., 2000; Fox and Heitman, 2005). Members of this family interact with calcineurin and act as calcineurin effectors or feedback regulators. Interestingly, Cbp1 is not necessary for Cryptococcus survival at high temperature (Fox and Heitman, 2005), indicating that the two roles of calcineurin, responses to stress and filamentation, are distinct and operate with different components.
In summary, C. neoformans has developed elaborate signalling mechanisms to cope with the high temperature stress in the host. These mechanisms involve at least two cooperating pathways, the calcineurin pathway and the cell wall integrity MAPK pathway. The challenge in future studies will be to identify the effectors and transcription factors that respond to calcineurin and to define the cross-talk between these two major pathways and other pathways mediating responses to stress.
Conclusion
Similar to other eukaryotes, there is an extensive cross-talk between individual signalling pathways in C. neoformans. This is generally due to two factors. First, multiple pathways often control a common process. Second, many signalling outcomes impact other processes through feedback loops and compensatory responses. An interesting feature of signalling in Cryptococcus is a high degree of specialization in individual serotypes and between the two mating types, which adds to the complexity of these signalling networks. A combination of global expression studies, like the recent genome-wide analysis of virulence factors (Liu et al., 2008), coupled with genetics and detailed mechanistic analysis at a molecular level should lead to a better understanding of the architecture of the signalling routes in Cryptococcus that can be targeted for therapy.
Acknowledgements
We thank all contributors to studies on signalling in C. neoformans and we apologize to colleagues whose studies were not cited due to space limitations. Our research is funded by the NIH/NIAID.