Staphylococcus epidermidis polysaccharide intercellular adhesin induces IL-8 expression in human astrocytes via a mechanism involving TLR2
Summary
Staphylococcus epidermidis is an opportunistic biofilm-forming pathogen associated with neurosurgical device-related meningitis. Expression of the polysaccharide intercellular adhesin (PIA) on its surface promotes S. epidermidis biofilm formation. Here we investigated the pro-inflammatory properties of PIA against primary and transformed human astrocytes. PIA induced IL-8 expression in a dose- and/or time-dependent manner from U373 MG cells and primary normal human astrocytes. This effect was inhibited by depletion of N-acetyl-β-d-glucosamine polymer from the PIA preparation with Lycopersicon esculentum lectin or sodium meta-periodate. Expression of dominant-negative versions of the TLR2 and TLR4 adaptor proteins MyD88 and Mal in U373 MG cells inhibited PIA-induced IL-8 production. Blocking IL-1 had no effect. PIA failed to induce IL-8 production from HEK293 cells stably expressing TLR4. However, in U373 MG cells which express TLR2, neutralization of TLR2 impaired PIA-induced IL-8 production. In addition to IL-8, PIA also induced expression of other cytokines from U373 MG cells including IL-6 and MCP-1. These data implicate PIA as an important immunogenic component of the S. epidermidis biofilm that can regulate pro-inflammatory cytokine production from human astrocytes, in part, via TLR2.
Introduction
Many neurosurgical procedures require the insertion of medical devices such as external ventricular drains/devices (EVDs) or cerebrospinal fluid (CSF) shunts to relieve intracranial pressure (Key et al., 1995; Yogev and Bisno, 2000). The use of such devices can be associated with infective complications most often as a result of microbial colonization and the development of biofilm on the surface of the shunt or EVD. Coagulase-negative staphylococci (CoNS), particularly Staphylococcus epidermidis, are frequently associated with indwelling medical device infections including neurosurgical shunts and EVDs (Diaz-Mitoma et al., 1987), and there is evidence demonstrating the involvement of biofilm (Diaz-Mitoma et al., 1987; Younger et al., 1987; Kockro et al., 2000).
The formation of S. epidermidis biofilm on a device surface involves host and bacterial factors (Costerton et al., 1987; Mack et al., 1992). One of the primary components is an extracellular mucoid polysaccharide, known as polysaccharide intercellular adhesin (PIA), which facilitates cell–cell adhesion and accumulation into a mature biofilm (Mack et al., 1992). PIA is a homoglycan, composed of poly β-(1→6)-N-acetylglucosamine, partially O-succinylated and partially de-N-acetylated (Mack et al., 1996; Sadovskaya et al., 2005). Its production is associated with carriage of the intercellular adhesion operon (ica) consisting of four biosynthetic protein-encoding genes (icaADBC) (Heilmann et al., 1996; Gerke et al., 1998) and icaR, a negative regulator (Conlon et al., 2002). The poly N-acetylglucosamine backbone of the polymer is neutral; some negative charges are present due to partial O-succinylation and the introduction of positive charges along the molecule is dependent on the deacetylating activity of IcaB. Deacetylation of PIA has been reported to be important for both biofilm formation and immune evasion (Vuong et al., 2004).
A major component of the innate immune response is the Toll-like receptor (TLR) family, which are a group of transmembrane receptor proteins expressed on the surface of sentinel cells of the immune system and other cell types (Tosi, 2005). In the central nervous system (CNS) TLRs are expressed on the surface of astrocytes, microglia and cerebrovascular endothelial cells (Bailey et al., 2006; Lehnardt et al., 2006). Microbial recognition by TLRs is based on their ability to recognize conserved microbial structures and their function is to coordinate an efficient pro-inflammatory immune response. Both TLR2 and TLR4 can recognize a variety of conserved microbial agonists from both Gram-positive and -negative bacteria. The current dogma is that TLR2 and TLR4 recognize lipopeptides and lipopolysaccharide (LPS) respectively. The signalling cascade leading to NFκB activation, and the production of pro-inflammatory cytokines, from these receptors occurs via the adaptor proteins Myd88 and Mal/TIRAP.
TLR2 has an important functional role in the host response to staphylococcal infection. While it remains a matter for debate TLR2 has been reported to recognize staphylococcal peptidoglycan and lipoteichoic acids (LTA) (Fournier and Philpott, 2005). In the CNS astrocytes are the most abundant cell type that assume a pro-inflammatory functional role. They express TLRs (Bailey et al., 2006; Lehnardt et al., 2006) and TLR-associated signalling proteins (Bsibsi et al., 2002; Kielian et al., 2002; Bowman et al., 2003; Carpentier et al., 2005). It has previously been demonstrated that astrocytes are capable of recognizing peptidoglycan via TLR2, and that their stimulation with Staphylococcus aureus cell-wall material induced IL-1β, TNF-α, MIP-1 and MCP-1 production (Esen et al., 2004). More recently, TLR2 has also been found to be important in the regulation of acute stage brain abscess formation caused by S. aureus and in neurodegeneration induced by group B streptococci in microglia (Kielian et al., 2005; Lehnardt et al., 2006). In addition to this, other stimulants such as LPS, a known TLR4 agonist and the TLR9 agonist uCpG DNA, have been shown to induce cytokine production from astrocytes (Lee et al., 2004; Yoo et al., 2008).
To date, little is known regarding the host response to S. epidermidis biofilm-related infections, and whether TLR signalling is involved. In the context of device-related infections, it has been previously demonstrated that the glycocalyx produced by CoNS can induce cytokine production from murine peritoneal macrophages (Stout et al., 1994). There is mounting evidence supporting the recognition of carbohydrates by TLRs and that these represent a unique group of TLR agonists with immunogenic properties that are charge-dependent (Kalka-Moll et al., 2002; Wang et al., 2006). Considering this, we investigated the immunogenic properties of PIA in human astrocytes and the role of TLRs in these events.
Results
PIA induces IL-8 production from human astrocytoma U373 MG cells
Polysaccharide intercellular adhesin is a major component of the S. epidermidis biofilm. We investigated PIA's immunogenicity against U373 MG astrocytoma cells. The PIA was prepared from the deproteinated biofilm extract of a PIA-overproducing S. epidermidis 5 (CIP 109562) strain; the polymer displayed ∼20% deacetylation (Sadovskaya et al., 2006). Dose–response experiments (0.01–100 μg ml−1 PIA) performed at 24 h determined 10 μg ml−1 as the optimum dose of PIA for induction of IL-8 (Fig. 1A). IL-1β also induced IL-8 and was used as a positive control. Time-course studies confirmed 24 h to be the optimal period with no further increase evident in IL-8 levels at 48 h (data not shown). THP-1 monocytic cells were also tested for PIA responsiveness. Compared with control cells (61 ± 20 pg ml−1 IL-8) PIA at 10 or 100 μg induced 1147 ± 126 and 1814 ± 171 pg ml−1 IL-8 respectively (P ≤ 0.05 for both).
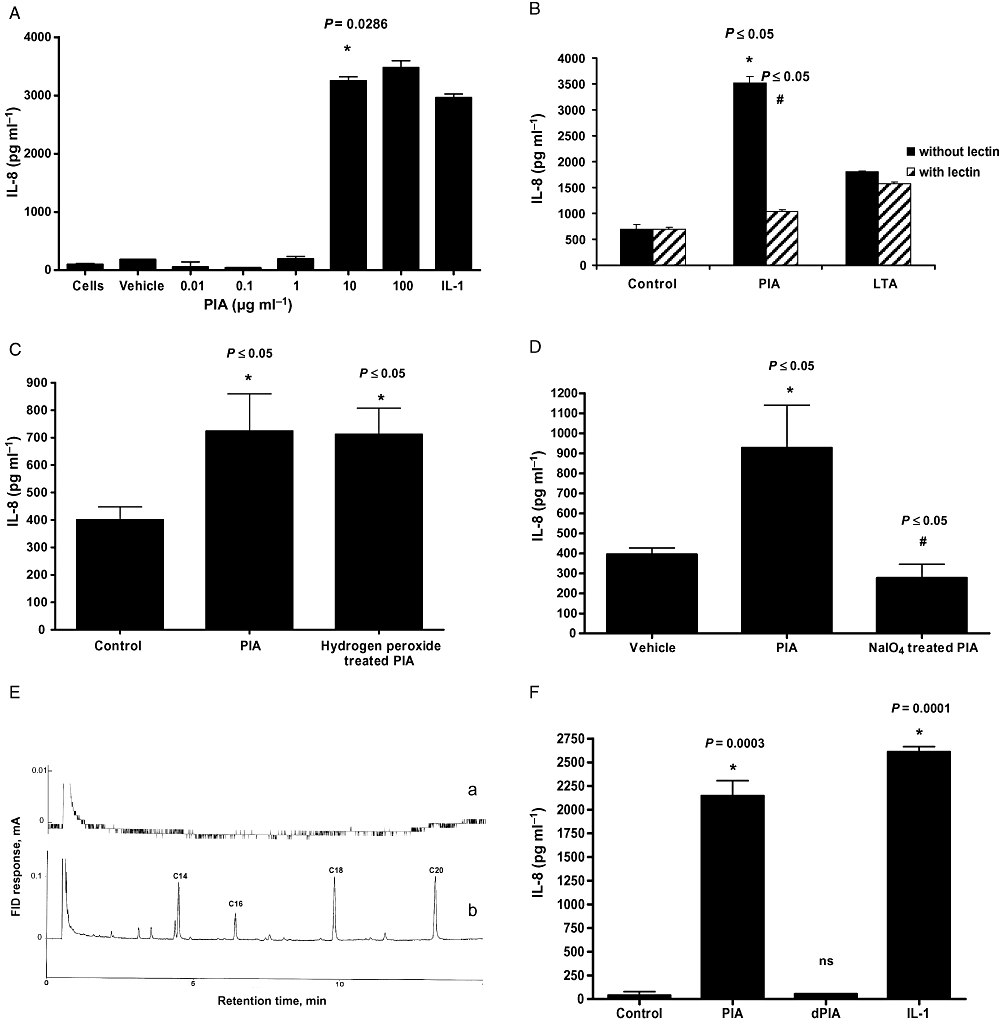
PIA induces IL-8 production from U373 MG astrocytoma cells.A–D. U373 MG astrocytoma cells (1 × 105 ml−1) were left unstimulated or stimulated with (A) vehicle, IL-1 (10 ng ml−1) or PIA as indicated for 24 h, (B) PIA or LTA (10 μg ml1, 24 h) with or without prior treatment with lectin, (C) PIA or H2O2-treated PIA (10 μg ml−1, 24 h), (D) PIA or NaIO4-treated PIA (10 μg ml−1, 24 h) and production of IL-8 was quantified in cell supernatants by ELISA.E. GLC chromatographic profiles of FAME derived from PIA preparation (a, 50 μg μl−1) and S. epidermidis LTA (b, 2 μg μl−1). Assignments of FAME was made tentatively using different standards (SupelcoTM 37 component FAME mix; olive oil FAME). FID, flame ionization detector.F. IL-8 ELISA of supernatants from PIA (10 μg ml−1), deacetylated PIA (dPIA) (10 μg ml−1) or IL-1 (10 ng ml−1) treated cells (24 h).*Versus untreated cells, or #versus agonist.
Lipopeptides, teichoic acids and nucleic acids are not involved in PIA induction of IL-8 in U373 MG cells
Purity of the PIA preparation was verified by 1H-NMR, monosaccharide analysis, fatty acid analysis and UV absorbance. The PIA preparation contained no contaminating nucleic acids as measured by UV spectra or sugar analysis. A number of additional experiments were performed in order to prove that the induction of IL-8 production from U373 MG cells was indeed due to PIA and not to the impurities of highly active lipopeptides or LTA, which could be present in the preparation in trace amounts below the detection limits of our analytical methods. However, great care initially was taken to only use high-molecular-weight fractions during the PIA preparation process to ensure the low-molecular-weight extracellular teichoic acids were well separated.
Polysaccharide intercellular adhesin is composed of repeating N-acetylglucosamine units. Lectin from Lycopersicon esculentum specifically binds oligomers of N-acetylglucosamine and, as such, is a useful tool for determining the purity of PIA preparations. In order to ensure that the PIA-induced IL-8 response from U373 MG cells was as a result of stimulation with PIA and not contaminating cell-wall material, e.g. LTA, PIA was left untreated or treated with L. esculentum lectin (which should bind to and remove PIA) and then added to the cells. Figure 1B shows that the removal of N-acetylglucosamine polymer from the PIA preparations using lectin caused a significant decrease in IL-8 production (P ≤ 0.05) in comparison with cells that were stimulated with lectin-untreated PIA. Stimulation of U373 MG cells with LTA led to induction of IL-8; however, this response was unaffected by pre-treatment with lectin, which does not bind LTA.
Hydrogen peroxide can destroy the IL-8-inducing capacity of lipid-containing molecules without affecting PIA. Figure 1C shows that following treatment with H2O2[1%, 37°C for 4 h (Zähringer et al., 2008)], PIA retains its ability to induce IL-8. The ability of sodium meta-periodate-treated PIA to induce IL-8 activity was also tested. Degradation of PIA with sodium meta-periodate should destroy its IL-8-inducing activity. Figure 1D shows that treatment of PIA with sodium meta-periodate abolishes its IL-8-inducing capacity.
Although we were unable to exclude the possibility of trace amounts of lipopeptides in our PIA preparations, from our GLC fatty acid analysis (Fig. 1E) we calculated that no more than 0.005% (w/w) fatty acids could be present. This equates to 0.05 ng of lipoprotein per 10 μg of PIA. Stimulation of U373 MG cells with a dose of lipopeptide even 10 times higher (0.5 ng) failed to induce IL-8 expression (data not shown).
Treatment of U373 MG cells with cell-wall teichoic acid (CWTA) (0–500 μg ml−1) isolated from S. epidermidis RP62A led to no detectable increase in IL-8 production (data not shown) indicating that CWTA is unlikely to be a contaminating immunogenic factor in the PIA preparations used here.
Acetylation of PIA is important for its ability to induce IL-8 expression from U373 MG cells
We next assessed the role of acetylation in the ability of PIA to induce IL-8 production from U373 MG cells. As before stimulation of cells with PIA displaying ∼20% deacetylation resulted in a significant induction of IL-8 production (P = 0.0003), whereas PIA that was fully deacetylated failed to induce a response (Fig. 1F).
Induction of IL-8 from U373 MG cells by an adhered biofilm produced by ica− and ica+S. epidermidis isolates
Next we investigated whether carriage of the ica operon led to increased IL-8 production from U373 MG astocytoma cells by intact, adhered S. epidermidis biofilm (Fig. 2). Compared with control cells, BM13, a PIA-producing ica+ isolate, but not BM3, an ica- isolate, significantly increased IL-8 production (Fig. 2A) from U373 MG cells. The IL-8 induction was similar to that stimulated with an adhered biofilm, formed by the strong biofilm-producing S. epidermidis strain RP62A. Figure 2B shows the degree of biofilm produced on the glass surface following staining with crystal violet.
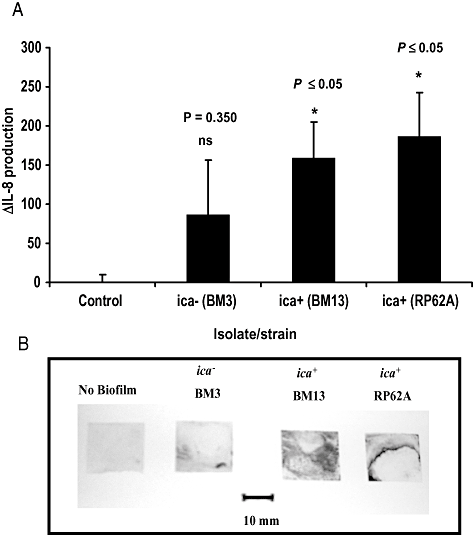
IL-8 induction from U373 MG cells by S. epidermidis biofilm.A. ΔIL-8 expression (pg ml−1) from U373 MG astrocytoma cells (1 × 106 cells ml−1) following stimulation for 24 h with an adhered biofilm on a glass surface, formed by ica- (BM3) or ica+ (BM13) S. epidermidis isolates. The known ica+ biofilm-positive RP62A was included as a positive control.B. Adherent biofilm on the glass surface was stained with crystal violet (0.4% w/v). A glass slip with no adhered biofilm acted as a control.
ΔMyd88 and ΔMal, but not IL-1 receptor antagonist, inhibit PIA-induced IL-8 expression in U373 MG cells
We investigated the mechanism by which PIA induces IL-8 from U373 MG cells by focusing on the IL-1R/TLR family. Having ruled out the involvement of interleukin-1 type I receptor (IL-1RI), by demonstrating that IL-1 receptor antagonist (IL-1ra) could not significantly inhibit PIA-induced IL-8 production while effectively blocking IL-1-induced IL-8 expression (Fig. 3A), we then determined the effect of ΔMyD88 on the PIA effect. Figure 3B shows that expression of a ΔMyD88 transgene significantly inhibits PIA-induced IL-8 expression. IL-1 was used as a positive control as ΔMyD88 is known to inhibit IL-1RI signalling (Muzio et al., 1997).
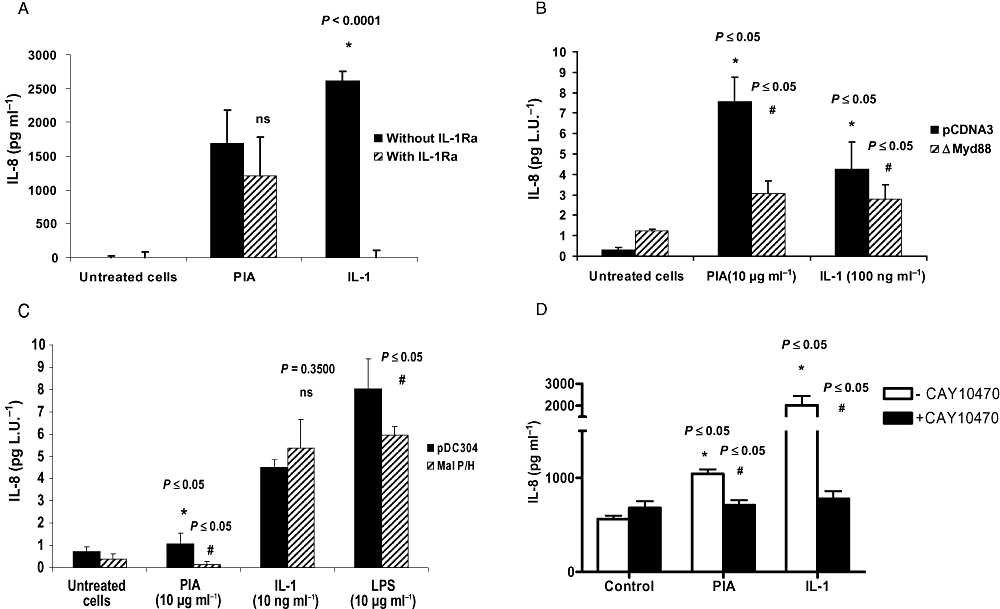
MyD88, Mal and NFκB, but not IL-1, are involved in PIA-induced IL-8 expression. U373 MG astrocytoma cells (1 × 105 cells ml−1) (A) were left unstimulated or stimulated with PIA (10 μg ml−1) or IL-1 (10 ng ml−1) for 24 h with or without prior treatment with IL-1ra (0.1 µg ml−1 for 1 h). IL-8 production was quantified in cell supernatants by ELISA (*versus untreated cells, or #versus agonist), or were co-transfected for 24 h with pRLSV40 (a constitutive luciferase reporter gene) plus (B) an empty vector (pCDNA3) or ΔMyd88 expression plasmid or (C) an empty vector (pDC304) or Mal P/H expression plasmid then left untreated or stimulated with PIA (10 μg ml−1), IL-1 (10 ng ml−1) or LPS (10 μg ml−1) as indicated for 24 h. IL-8 levels were corrected for transfection efficiency and expressed in pg per light unit (L.U.), or (D) PIA (10 μg ml−1) or IL-1 (10 ng ml−1) with or without prior treatment with CAY10470 (20 nM, 1 h) (*versus untreated cells, or #versus agonist).
MyD88 is an adaptor protein for the IL-1R/TLR family. Mal is a homologue of MyD88 involved in TLR2 and TLR4 signalling. Figure 3C demonstrates that expression of a functionally inactive version of Mal (Mal P/H) significantly inhibited PIA-induced IL-8 production from U373MG cells. LPS- but not IL-1-induced IL-8 expression was also inhibited. The PIA response in Fig. 3C is lower than in Fig. 3B due to differences in transfection efficiencies between the two experiments.
We also evaluated the effect of CAY10470, an inhibitor of NFκB, on PIA-induced IL-8 expression. Figure 3D shows that PIA-induced IL-8 is inhibited by CAY10470, further implicating an NFκB signalling cascade in this response. IL-1 was used as a control.
PIA signals via TLR2 to induce IL-8 production in U373 MG cells
To determine if PIA-induced IL-8 production was TLR4-dependent, we stimulated a stably transfected TLR4-only-expressing HEKTLR4 cell line with PIA. In comparison with LPS treatment, which led to a significant increases in IL-8 protein production at doses of 1 or 10 μg, PIA treatment of HEKTLR4 cells had no measurable effect on IL-8 (Fig. 4A), suggesting that TLR2 rather than TLR4 may be the receptor mediating PIA's effect in U373 MG cells. LPS at 1 and 10 μg maximally induced IL-8 expression from HEKTLR4.
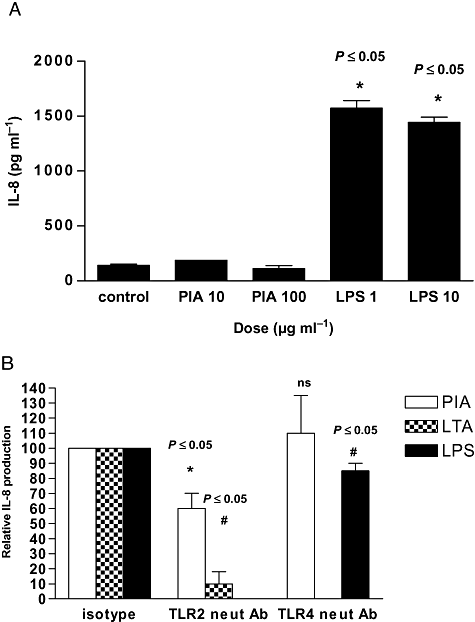
TLR2, not TLR4, mediates the PIA response in U373 MG cells.A. HEKTLR4 cells (1 × 105 cells ml−1) were stimulated with PIA (10 or 100 μg ml−1) or LPS (1 or 10 μg ml−1) for 24 h and production of IL-8 was quantified in cell supernatants by ELISA (*versus untreated cells).B. U373 MG cells (1 × 105 cells ml−1) were left untreated or stimulated with PIA (10 μg ml−1), LTA (10 μg ml−1) or LPS (10 μg ml−1) ± IgG2a control isotype antibody, TLR2.1- or TLR4-neutralizing antibodies (25 μg ml−1) for 1 h as indicated. Production of IL-8 was quantified in cell supernatants by ELISA (*versus untreated cells, or #versus agonist).
To confirm the role of TLR2, we quantified the effect of TLR2- and TLR4-neutralizing antibodies on inhibition of PIA-induced IL-8 production in U373 MG cells. Figure 4B shows that inhibition of TLR2 decreased PIA-induced IL-8 production by ∼40% (P ≤ 0.05). An isotype control antibody was used as a control. The TLR2-neutralizing antibody also blocked IL-8 production induced by LTA, a known TLR2 agonist. The TLR4-neutralizing antibody had no effect on the PIA response but did significantly decrease LPS-induced IL-8.
U373 MG cells express TLR2 on the cell surface
Quantitative fluorescence microscopy was used to detect expression of TLR2 and TLR4 on U373 MG cells. Figure 5A illustrates that these cells express detectable TLR2 on their surface. The median channel fluorescence emitted by the FITC-linked anti-TLR2 antibody is significantly greater than that of the isotype antibody (P < 0.0011). Expression of TLR4 by these cells was considerably higher. This may explain in part the relatively small inhibition of the LPS response by the TLR4-neutralizing antibody seen in Fig. 4B.
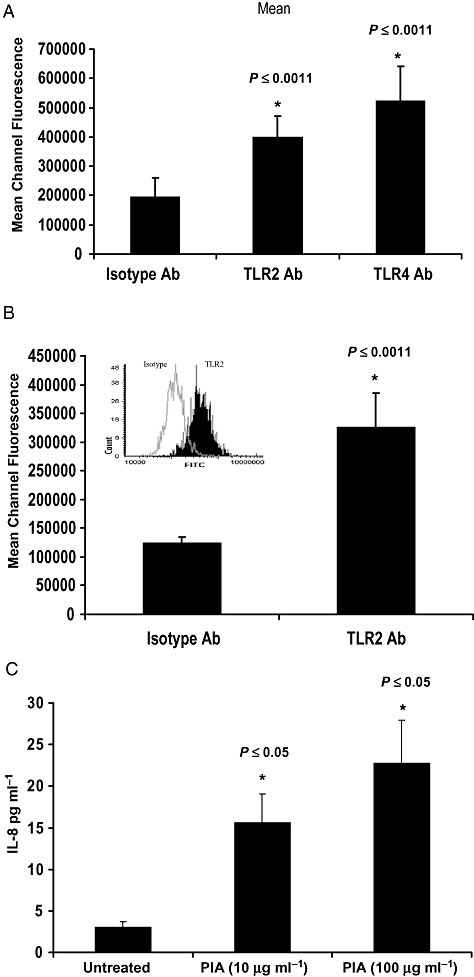
Primary and transformed human astrocytes express TLR2 and respond to PIA.A and B. (A) U373 MG or (B) primary human NHA astrocytes (1 × 105 ml−1) were grown in chamber slides, Fc blocked, and labelled with FITC-conjugated anti-TLR2, anti-TLR4 or an isotype control antibody (Ab). Cells were counterstained with PI and TLR surface expression was quantified by laser-scanning microscopy. Graphs show median channel fluorescence ± SEM (*versus isotype, n = 6). Inset in (B) shows representative histogram of FITC fluorescence comparing isotype- (clear) and anti-TLR2 Ab- (solid) labelled samples.C. NHA cells (1 × 105 cells ml−1) were left untreated or stimulated with PIA (10 or 100 μg ml−1) for 48 h. ELISA was used to quantify levels of IL-8 in cell supernatants (*versus untreated cells).
Primary human astrocytes express TLR2 and respond to PIA
Figure 5B shows that, similar to the transformed astrocytoma cells, primary normal human astrocytes (NHA) also express cell surface-exposed TLR2. Furthermore these cells produce detectable levels of IL-8 and respond to PIA stimulation in a dose-dependent manner (Fig. 5C).
PIA regulates expression of multiple cytokines
The effect of stimulation of U373 MG cells with PIA on a range of cytokines was assessed by cytokine array analysis. PIA induced production of a number of cytokines (Fig. 6A) and representative examples of those displaying the most obvious increases in production compared with unstimulated cells were selected for further study, namely IL-8, IL-6 and MCP-1. The array findings were validated by performing cytokine-specific ELISAs on the same cell supernatants (Fig. 6B). Finally levels of IL-8, IL-6 and MCP-1 were quantified in different CSF samples. Figure 6C shows that CSF isolated from an individual infected with S. epidermidis contained significantly increased levels of IL-8, IL-6 and MCP-1 compared with normal CSF or inflamed, sterile CSF.
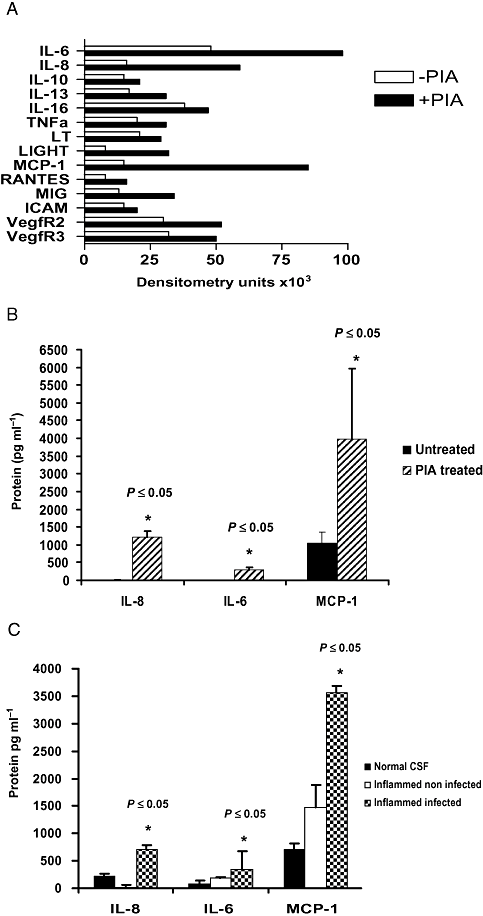
PIA induces the expression of cytokines associated with S. epidermidis meningitis.A. U373 MG astrocytoma cells (1 × 105 cells ml−1) were left untreated or stimulated with PIA (10 μg ml−1) for 24 h. Cytokine array analysis was performed on pooled supernatants from triplicate samples and increases in the production of selected cytokines are depicted in densitometry units.B and C. IL-8, IL-6 and MCP-1 concentrations in (B) the same samples (*versus untreated cells) or (C) different categories of CSF were measured by ELISA (*versus normal CSF).
Discussion
Here we link PIA, a major component of the S. epidermidis biofilm, to activation of TLR2 and expression of IL-8 in human astrocytes.
Biofilm formation is a primary virulence factor of S. epidermidis. The production of PIA and the subsequent formation of biofilm have an important role in protecting the bacteria from phagocytosis (Johnson et al., 1986; Falcieri et al., 1987). Conversely staphylococcal glycocalyces, including that produced by S. epidermidis, have been reported to activate the innate immune response at doses of 10–100 μg ml−1 leading to production of prostaglandin E2 (PGE2), IL-1 and TNF-α in murine peritoneal macrophages (Stout et al., 1994). This is in keeping with our finding that S. epidermidis PIA induces a potent IL-8 response from human astrocytes at similar doses and another recent report describing how a capsular polysaccharide from the Gram-negative Bacteroides fragilis can activate NFκB in a TLR2-dependent manner (Wang et al., 2006).
Myd88 belongs to a family of TLR adaptor proteins. While MyD88 signals for all TLRs with the exception of TLR3, a second adaptor protein termed Mal/TIRAP (Fitzgerald et al., 2001) is involved only in TLR2 and TLR4 signalling (Horng et al., 2001; Yamamoto et al., 2002). Human astrocytes express mRNA for both Myd88 and Mal/TIRAP (Farina et al., 2007). By blocking signalling through these adaptor proteins, neutralizing TLR2 and 4 and studying the effect of PIA on TLR4-only-expressing HEKTLR4 cells we confirmed the likely involvement of TLR2 in the PIA response.
TLR2 has a role in the recognition of structural components of a variety of Gram-positive bacteria, such as S. epidermidis and S. aureus, as well as fungal and protozoan structures (Kopp and Medzhitov, 2003). Kielian et al. (2005) have shown that TLR2 recognizes S. aureus peptidoglycan in a brain abscess model. Furthermore, it was reported that TLR2 expression on astrocytes is necessary for the recognition of Gram-positive peptidoglycan, and stimulation of these cells by cell-wall material of S. aureus has been reported to result in the production of pro-inflammatory mediators (Esen et al., 2004). Recently Zähringer et al. (2008) proposed that TLR2 is activated only by lipoproteins or lipopeptides, and that the activity of all other putative compounds so far reported as TLR2 agonists is due to contaminating lipopeptides/proteins. Having thoroughly determined the purity of our PIA preparations via NMR, fatty acid analysis and lectin, NaOH, H2O2 and sodium meta-periodate inhibition studies we are confident that our observed effects represent a true phenomenon.
TLR2 can signal as a heterodimer. In conjunction with TLR1 it confers responsiveness to LTA and triacylated lipopeptides while with TLR6 it can respond to diacylated MALP-2. In airway epithelial cells TLR2 and TLR5 are involved in the flagellin response (Soong et al., 2004), thus it is possible that TLR2 may signal cooperatively with another TLR in response to PIA. TLR2's role may not however be via a direct interaction with PIA. In other cells types CD36 and RP105 act as co-receptors for TLR2 (Nagai et al., 2005; Triantafilou et al., 2006).
We found that native PIA from S. epidermidis CIP 109562, displaying ∼20% deacetylation (Sadovskaya et al., 2006), was capable of inducing a significant increase in IL-8 production. However, PIA that was 100% deacetylated failed to induce the same response. The deacetylation of N-acetylglucosamine residues, through the activity of IcaB, introduces positive charges along the length of the molecule, and this appears to be important in PIA-mediated biofilm formation and immune evasion (Heilmann et al., 1996; Vuong et al., 2004). Using a mouse model of device-related infection, it was shown that a S. epidermidisΔicaB mutant was significantly impaired in its ability to establish infection compared with a wild-type strain (Vuong et al., 2004). Kalka-Moll et al. (2002) reported that bacterial polysaccharides from B. fragilis, S. aureus and Streptococcus pneumoniae with zwitterionic charge motifs represent an unusual group of carbohydrates with possibly unique immunogenic properties. Since PIA can be considered as a zwitterionic polymer the immunogenic properties of this polysaccharide may also be charge-dependent. Our observation that deacetylation of PIA with NaOH abolishes its IL-8-activating activity supports this.
Until recently, there has been much circumstantial evidence supporting TLR expression by astrocytes. This evidence has been based on the ability of known ligands, such as LPS, Gram-positive cell-wall antigens and unmethylated CpG DNA to induce cytokine production from the astrocytes (Chung and Benveniste, 1990; Schumann et al., 1998; Takeshita et al., 2001). Here we show that both LTA and LPS can induce IL-8 production in U373 MG astrocytoma cells, and provide direct evidence for the presence of TLR2 on these cells. Moreover, we demonstrate the previously unreported expression of TLR2 in primary NHA cells. Our observations support the work of Bowman et al. (2003), who demonstrated constitutive, low-level expression of mRNA-encoding TLR2, as well as TLR4, TLR5 and TLR9 in cultured astrocytes and Lehnardt et al. (2006) who reported TLR2 expression by murine astrocytes. Others have reported that cultured human astrocytes display low basal level expression of TLR2, along with TLR3 and TLR4 (Bsibsi et al., 2002). Although the IL-8 response induced by PIA in the primary NHA cells was less potent than in the transformed cell line it is possible that other S. epidermidis factors are likely to enhance cytokine expression by astrocytes in vivo. Although S. epidermidis CWTA failed to induce IL-8 production from U373 MG cells, other biofilm mediators such as the cell-wall proteins Aap (Rohde et al., 2005; Stevens et al., 2008), Bap/Bhp (Lasa and Penadés, 2006) or AtlE (Heilmann et al., 1997) that were not present in our PIA preparations could potentially activate TLR2 or other TLRs in vivo.
Stimulation of U373 MG cells with an intact S. epidermidis biofilm, adhering to a glass surface and produced by known meningitis-causing isolates with variant ica genotypes caused different degrees of IL-8 production. The only significant change however was with a known meningitis-causing S. epidermidis isolate with an ica+ genotype. While it is unlikely that the IL-8 production was solely the result of stimulation by PIA present in the biofilm, other cell-wall antigens, such as LTA but not CWTA, and peptidoglycan may possibly have been involved. It is therefore possible that other components of S. epidermidis biofilm may also contribute to biofilm-related meningitis in vivo.
Our cytokine array analysis revealed that in addition to IL-8 production, PIA stimulation of astrocytes also led to increased production of other NFκB-activated genes including IL-6 and MCP-1. In vivo astrocytes produce IL-8, IL-6 and MCP-1 as well as other important mediators of astrocyte function, such as RANTES and IL-1β (Farina et al., 2007). IL-6 has an important immunomodulatory role in the CNS in enhancing the initial local innate immune response. Such activation can have profound effects in the blood–brain barrier, increasing permeability and allowing for the translocation of peripheral circulating immune cells into the CNS (Farina et al., 2007). Production of IL-8 and MCP-1 by astrocytes is important in the attraction of immune cells into the CNS and MCP-1 in particular has been reported to have an important role in the recruitment of phagocytes in response to staphylococcal infections (Fournier and Philpott, 2005).
Interestingly, IL-6, IL-8 and MCP-1 were also detected in CSF taken from a patient clinically diagnosed with active meningitis, resulting from S. epidermidis infection. The S. epidermidis isolate responsible for this infection possessed the genetic determinants (icaADBC) necessary for PIA production and was capable of biofilm formation in vitro (data not shown). All three cytokines were present at higher concentrations in the CSF from this patient, compared with patient CSF that displayed signs of inflammation due to non-infective causes, and a patient CSF that displayed no signs of inflammation or infection. Additional studies using more patient samples may reveal a real association between the factors.
It is now widely accepted that the primary strategy of S. epidermidis immune evasion involves production of a biofilm. Although expression of PIA is necessary for protection against phagocytosis and killing by antibacterial peptides (Schroder, 1999; Vuong et al., 2004), we have demonstrated here that PIA also has pro-inflammatory immunogenic properties.
Experimental procedures
Cell culture and treatments
U373 MG human astrocytoma cells were obtained from the European Collection of Cell Cultures (ECACC), primary NHA from Lonza, UK and HEKTLR4 cells from Invivogen. U373 MG cells were cultured in Eagle's minimal essential medium (MEM) (Gibco®) supplemented with 10% FCS, 1% l-glutamine, 1% sodium-pyruvate, 1% non-essential amino acid (Gibco®) and 1% penicillin/streptomycin (Invitrogen Life Sciences). NHA cells were cultured in the supplied astrocyte basal medium (Clonetics®, Lonza) supplemented with 3% FCS, 0.3% ascorbic acid, 0.1% recombinant human EGF, 0.1% GA-1000, 0.25% Insulin, 1% l-glutamine (Clonetics®, Lonza) and 1% penicillin/streptomycin (Invitrogen Life Sciences). HEKTLR4 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (Gibco®) supplemented with 10% FCS, 1% penicillin/streptomycin (Invitrogen Life Sciences) and 1% blastocidin (10 mg ml−1) (Invivogen). Twenty-four hours prior to agonist treatment, cells were placed under serum-free conditions or in media containing 1% FCS for LPS stimulations. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2.
Agonist treatments were performed in serum-free conditions or 1% FCS conditions as appropriate. Unless otherwise stated, treatments were performed on 1 × 105 cells per millilitre.
Preparation of PIA and CWTA
Polysaccharide intercellular adhesin was prepared from a crude extract of S. epidermidis 5 (CIP 109562), a strain from our collection as described previously by Sadovskaya et al. (2005). Briefly, the bacteria were grown statically in 750 ml of TSB in 3-1 Erlemeyer flasks at 37°C for 24 h. Bacterial biofilms on the bottom of the flasks were washed twice with NaCl (0.9% w/v) and collected in 0.9% NaCl using 6-mm-diameter glass beads. The suspension was then sonicated twice, 30 s each, on ice using an Ikasonic sonicator (IKA Labortechnik) set to 50% amplitude and 50% duty cycle. The sonicate was clarified twice by centrifugation (2000 g, 4°C, 15 min, then 8700 g, 4°C, 10 min). Following this, the supernatant was concentrated using an Amicon 10 kDa cut-off ultrafiltration cell. TCA was added to a concentration of 5% and the precipitate was removed by centrifugation (8700 g, 4°C, 10 min). The now protein-free extract was fractionated on a Sephacryl S-300 column (1 × 80 cm), eluted with water. Amino sugar-rich fractions corresponding to HMW PIA were pooled and lyophilized.
CWTA was obtained from the SDS-treated S. epidermidis RP62A as described previously (Sadovskaya et al., 2004).
Purity of PIA assays
The purity of the PIA was checked by 1H-NMR (Varian UNITY INOVA 500 instrument) and monosaccharide analysis, as described previously (Sadovskaya et al., 2005). Glucosamine was the only monosaccharide present in the PIA preparation. In order to exclude the possible contamination with LTA, S. epidermidis LTA and the PIA preparation were analysed for the presence of fatty acids as fatty acids methyl ester (FAME) by gas liquid chromatography (GLC). LTA of S. epidermidis RP62A was prepared from defatted cells by hot phenol extraction as described in Iwasaki et al. (1986). The purified S. epidermidis LTA (0.5 mg) was heated in 2 M HCl in absolute methanol at 85°C for 6 h. The reaction mixture was evaporated to dryness under a stream of nitrogen, the residue dissolved in 250 μl of chloroform and analysed by GLC on Shimadzu GC-14 gas chromatograph equipped with a flame ionization detector (FID) and a Zebron ZB-5 capillary column (30 m × 0.25 mm), with hydrogen as carrier gas using a temperature gradient 170°C (3 min), 260°C at 5°C min−1. PIA preparation (0.5 mg) was treated similarly, and the residue was taken in 10 μl of chloroform. No peaks corresponding to fatty acids were detected in the PIA preparation (Fig. 1E).
UV spectra of the PIA preparation and GlcNAc standard (50 μg ml−1) were detected using a Helios β spectrophotometer in the range of 200–300 nm.
Lycopersicon esculentum Lectin (1 mg ml−1) (Sigma) binds N-acetyl-β-d-glucosamine oligomers and was used to determine the purity of the PIA preparations. Fifty micrograms of lectin was incubated in a 1:1 ratio with PIA (1 mg ml−1) or LTA (1 mg ml−1) at 25°C for 1 h and centrifuged at 14 000 r.p.m. for 30 min. Supernatants were removed and used to stimulate the U373 MG astrocytoma cells.
Cytokine protein production
Cells were left untreated or stimulated with agonists or vehicle controls, as indicated. In some experiments cells were pre-treated with IL-1R antagonist (IL-1ra, 0.1 μg ml−1) (R&D Systems), or a TLR2-neutralizing antibody TLR2.1 (25 μg ml−1) (Bioquote) or IgG2a (25 μg ml−1) (Bioquote) or CAY10470 (20 nM) (Cayman Chemicals) for 1 h before agonist treatment.
IL-8, IL-6 and MCP-1 protein concentrations in cell supernatants and patient CSF specimens were determined by sandwich ELISA (R&D Systems). Assays were performed at least three times and in duplicate or triplicate on each occasion.
The expression of 174 different cytokines and growth factors in cell supernatants from untreated and PIA-treated U373 MG astrocytoma cells was detected using the RayBio® Human Cytokine Antibody Array C series 2000 kit (RayBiotech), according to the manufacturer's instructions. Signals were detected following exposure to X-ray film (Kodak). Densitometry readings were obtained through illumination on an ultraviolet source (LKB Bromma, 2011 macrovue Transilluminator) and recorded using a gel documentation system (Stratgene Eagle Eye® II).
Collection of CSF specimens
Cerebrospinal fluid was collected from neurosurgical patients with an EVD in situ at Beaumont Hospital, Dublin, by health-care professionals following informed consent and using the appropriate clinical criteria as approved by the hospital ethics committee, over a 3-year period as part of routine care. CSF specimens were examined for sterility or for the presence of S. epidermidis, following growth on Columbia blood agar. S. epidermidis was identified using the API®20 Staph identification system (Biomerieux). CSF specimens were centrifuged at 1000 g for 10 min and cell-free supernatants were immediately transferred into a sterile tube and stored at −80°C until required for experimental analysis. CSF specimens were grouped into the following three defined categories: (i) inflammed CSF, where CSF was sterile and inflammation was due to non-infective cause (n = 1), (ii) normal CSF, where CSF was sterile with no evidence of inflammation as determined by the diagnostic laboratory, i.e. normal glucose, protein and white blood cell count levels (n = 9), and (iii) inflammed and infected CSF, where CSF showed evidence of inflammation, resulting from infection with S. epidermidis (n = 1).
Transfection and reporter gene studies
Transfections were performed according to the methods described previously (Greene et al., 2005). Transfections were performed into cells using TransFast Reagent (Promega) in a 1:1 ratio, according to the manufacturer's instructions, using 100 ng of pRLSV40 constitutive luciferase reporter plasmid (Promega) and 200 ng of either pCDNA3.1 (Invitrogen Life Technologies) or ΔMyD88 expression plasmid (Greene et al., 2005) or pDC304 or Mal P/H expression vector (Carroll et al., 2005). ΔMyD88 contains only a functional TIR domain and lacks the death domain required for downstream signalling. MalP/H is a dominant-negative version of Mal that has a Pro125His point mutation in box 2 of the TIR domain. Cells were supplemented with additional growth media for 24 h at 37°C before being left unstimulated or stimulated as indicated. Cells were lysed with reporter lysis buffer (Promega), and reporter gene activity was quantified by luminometry (PerkinElmer Wallac Victor, 1420 multilabel counter) using the Promega luciferase assay system, according to the manufacturer's instructions.
Laser-scanning cytometry
U373 MG astrocytoma cells and NHA cells were seeded (1 × 105 cells per well) in four-well chamber slides (Nunc, UK), in duplicate. Slides were fixed in methanol (AnalaR) for 5 min, and then labelled indirectly with goat anti-TLR2 or anti-TLR4 (Santa Cruz Biotechnology, USA) and anti-goat FITC, then counter-stained with propidium iodide (PI) (Molecular probes, USA), washed twice in PBS + 1% Tween and allowed to dry. Fluorescence excitation was provided by a 488 nm laser line. Nuclear (PI) and TLR (FITC) fluorescence were measured at 588 ± 10 or 530 ± 20 nm respectively. Antibody-staining cells were then identified and quantified using CompuCyte software on the basis of integrated green fluorescence (Kamentsky et al., 1997).
Data and statistical analysis
Data were analysed using GraphPad Prism 4.0 software package (GraphPad). Data obtained from cytokine array analysis were analysed using the Ray®Bio Analysis Tool (Ray®Biotech). Results are expressed as mean ± SD, and were compared by one-tailed Student's t-test. Differences were considered significant when the P-value was ≤ 0.05.
Acknowledgements
Funding for this project is gratefully acknowledged from the Health Research Board, Ireland. We thank Dr E. Vinogradov, IBS, NRC Canada for the 1H-NMR analysis of PIA and deacetylated PIA.