Role of lipids in killing mycobacteria by macrophages: evidence for NF-κB-dependent and -independent killing induced by different lipids
Summary
We have shown that several lipids can modulate the macrophage innate immune response against mycobacteria and enhance their killing. Since NF-κB is required for mycobacterial killing, we tested the ability of lipids to activate NF-κB in uninfected macrophages and those infected with mycobacteria. In uninfected cells, sphingomyelin (SM), phosphatidylinositol-4-phosphate (PIP) and arachidonic acid (AA) enhanced NF-κB activation and the cell surface expression of CD69, a macrophage activation marker regulated by NF-κB. Sphingosine (Sph), sphingosine-1-phosphate (S1P), diacylglycerol (DAG), eicosapentanoic acid (EPA) and phosphatidyl choline (PC) failed to activate either NF-κB or CD69. Ceramide (Cer) activated CD69 expression without activating NF-κB. In Mycobacterium smegmatis-infected cells, NF-κB was transiently activated in a manner that was enhanced by SM, PIP and AA. In contrast Mycobacterium avium mostly repressed NF-κB activation and only SM and AA could induce its partial activation. While lipids that activate NF-κB in uninfected cells tend to kill mycobacteria in macrophages Sph and S1P failed to activate NF-κB under most conditions but nevertheless enhanced killing of M. smegmatis, M. avium and M. tuberculosis H37Rv. Our results argue that both NF-κB-dependent and -independent mechanisms are involved in macrophage killing of mycobacteria and that both mechanisms can be enhanced by selected lipids.
Introduction
Pathogenic mycobacteria such as Mycobacterium tuberculosis and M. avium are known to be contained in macrophages within phagosomes that are unable to fuse with late endosomes and lysosomes. Consequently, these pathogenic mycobacteria survive and grow in macrophages (Russell, 2001). In contrast, non-pathogenic mycobacteria such us Mycobacterium smegmatis reside in phagosomes that fully mature by fusing with late endocytic compartments, a process that facilitates the killing of these bacteria (Anes et al., 2006; Jordao et al., 2008).
We have previously shown that several lipids can modulate the macrophage innate immune response against mycobacteria (Anes et al., 2003). Some of those lipids were able to influence the innate immune response in macrophages by stimulating at least four different mechanisms: (i) the ability of phagosomes to nucleate actin de novo, (ii) the fusion of phagosomes with late endosomes/lysosomes, (iii) lowering the pH of phagosomal compartments and (iv) the killing of mycobacteria inside macrophages.
Isolated phagosomes enclosing latex beads or M. smegmatis can assemble actin in vitro whereas those containing M. avium or M. tuberculosis cannot. However, a number of lipids could enhance actin assembly by phagosomes containing M. tuberculosis or M. avium in vitro. In addition, when these lipids were added to macrophages infected with these microorganisms they enhanced the association of actin with phagosomes. In agreement, this association correlates well with the extent of phago-lysosomal fusion and mycobacterial killing. In contrast, some lipids such as the polyunsaturated fatty acids (PUFAs), eicosapentanoic acid (EPA) or docosohexanoic acid (DHA), as well as phosphatidylcholine (PC) induced growth of pathogens and inhibited actin assembly from phagosomes (Anes et al., 2003; Treede et al., 2007).
The omega-3 lipids are well established to have anti-inflammatory properties (Calder, 2007). In contrast, the n-6 PUFAs such as arachidonic acid (AA) are known to induce a strong pro-inflammatory response (Calder, 2006a,b). These observations argue that different lipids can stimulate or inhibit certain processes related to the innate immune response in macrophages.
The role of lipids in the inflammatory process has been extensively analysed (Pettus et al., 2004; Calder, 2006b). It is known that lipids can elicit cellular responses via many different molecular mechanisms (Calder, 2006b; Oskeritzian et al., 2007). For example, they can act as intracellular signalling molecules that can activate some G protein-coupled receptors (Wymann and Schneiter, 2008). Most lipids when added to cells are likely to be incorporated into cellular membranes, where they induce a plethora of signalling events. In addition, sphingosine-1-phosphate (S1P) and lysophosphatidic acid (LPA) also have receptors at the plasma membrane (Futerman and Hannun, 2004).
The development of an inflammatory response is a key event during mycobacterial infections (Russell, 2007). Lipids can be both pro- and anti-inflammatory (Pettus et al., 2004; Oskeritzian et al., 2007). The interplay between inflammation and the development of chronic tuberculosis can be affected by differences in the lipid composition in the granuloma (Thormar and Hilmarsson, 2007).
NF-κB is a critical regulator of genes involved in inflammation (Caamano and Hunter, 2002). This transcription factor consists of two subfamilies: the ‘NF-κB’ proteins and the ‘Rel’ proteins which are present in the cytoplasm as heterodimers, most commonly of p65 and p50 subunits in a complex with an inhibitor, IκB (Karin and Lin, 2002; Delhalle et al., 2004). When pro-inflammatory signalling occurs via activation of cell surface receptors such as the Toll-like receptors, IκB becomes phosphorylated and degraded by the proteosomal system. This releases the active subunits that enter the nucleus, where they upregulate the transcription of hundreds of genes, a reflection of the complexity of this part of the inflammatory response (Natoli et al., 2005). In a recent study we showed that NF-κB is transiently activated in macrophages early after infection with M. smegmatis. This activation is essential for mycobacterial killing since when NF-κB is blocked M. smegmatis survives. One role of NF-κB in this system is to induce synthesis of a family of lysosomal enzymes and potential regulators of phago-lysosome fusion (Gutierrez et al., 2008).
Many links have been described between lipids and NF-κB activation in different systems. For example, in Caco-2 cells AA activated NF-κB whereas EPA had no effect (Ramakers et al., 2007). Moreover, in the same cells PC, which inhibits LBP actin assembly, inhibited NF-κB activation induced by TNF-α (Treede et al., 2007). Ceramide (Cer) has also been shown to inhibit NF-κB activation in LPS-treated macrophages (Hsu et al., 2001).
However, the effect of lipids on activation of NF-κB in the context of mycobacterial infections is not known. In addition, the signalling mechanisms that lipids activate in macrophages during the response to mycobacteria are partially understood. Here, we systematically analysed 10 different lipids for their ability to activate NF-κB in J774 macrophages. We also monitored the upregulation and cell surface expression of the macrophage activation marker CD69 that is downstream of NF-κB (Blazquez et al., 1999). One goal of this study was to test the hypothesis that lipids that have been shown to stimulate membrane-dependent assembly of actin by phagosomes in vitro, and mycobacterial killing in macrophages, are activators of the pro-inflammatory response involving NF-κB. Conversely, the inhibitory lipids that inhibit the in vitro phagosome actin nucleation and tend to increase mycobacterial growth in macrophages were hypothesized to inhibit NF-κB activation. With the exception of Cer, sphingosine (Sph) and S1P our data are consistent with these hypotheses. Cer, Sph and S1P are potently mycobacteriocidal but are only able to activate NF-κB under some limited conditions.
Results
Effect of some lipids on macrophage activation
NF-κB is one of the key transcription factors activated during the inflammatory process. Therefore, we first tested the effects of different lipids for their ability to activate NF-κB in uninfected mouse J774 macrophages. To monitor NF-κB activation we analysed p65 intracellular labelling by immunofluorescence microscopy. The presence of the NF-κB subunit p65 in the nucleus has been shown to be a reliable estimator of NF-κB activation (Nelson et al., 2002; Gutierrez et al., 2008). In parallel, we labelled F-actin with rhodamine-phalloidin. We were especially interested in visualizing any obvious morphological changes to the actin cytoskeleton. We also evaluated the CD69 surface expression as another marker of macrophage activation that is downstream of NF-κB activation (Marzio et al., 1997).
Under control conditions, cells displayed a cytoplasmic pattern of p65 distribution and actin was localized in the cortex and some intracellular punctate structures (Fig. 1A). We first tested LPS, a potent stimulator of NF-κB that served as a reference for macrophage activation. Within 1 h of LPS treatment, p65 was transported to the nuclei of J774 cells (Fig. 1B). In the same cells, there was a strong surface staining of CD69 that was undetectable in non-stimulated cells (Fig. 1A). In addition, the CD69-positive membrane filopodia could be seen to be strongly colocalized with rhodamine-phalloidin. The differences between stimulated and non-stimulated cells were dramatic, and have been described previously (Huang et al., 2004).
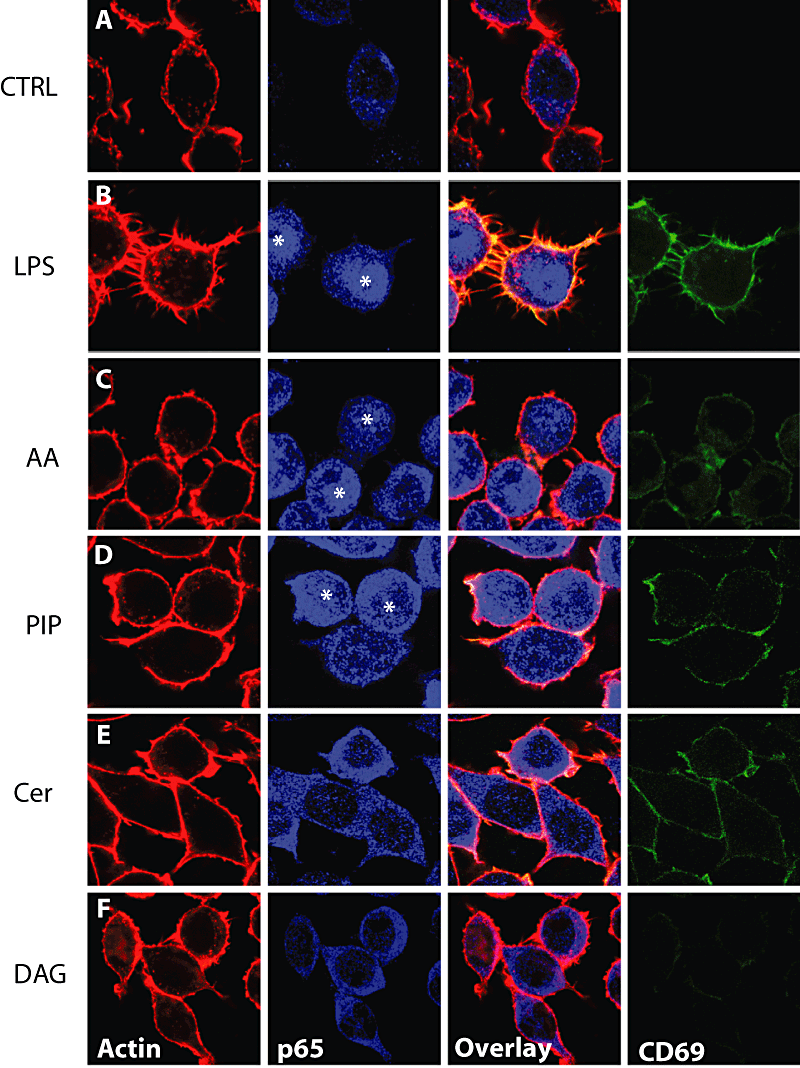
Macrophage activation status in response to lipids. J774 macrophages were treated with the indicated lipids or LPS (10 μg ml−1) for 1 h and cells were fixed with 3% PFA and incubated with anti-CD69 FITC-coupled. Cells were subsequently processed for immunofluorescence against p65 (blue) and rhodamin-phalloidin labelling. Representative confocal images are depicted. Asterisks indicate nuclei positive for p65 indicating nuclear translocation. AA, arachidonic acid, PIP, phosphatidylinositol-1-phosphate; Cer, C6-ceramides; DAG, diacylglycerol.
In previous studies we optimized the concentration of each lipid as well as the solvent to be used for their application to cells. With small exceptions, the conditions used for each lipid were the same as that determined empirically in the LBP in vitro actin assembly assay (Anes et al., 2003). It should be noted that the optimal concentrations (the minimum concentration needed for the above effects) varies widely from 10–100 nM for S1P to 50–125 μM for AA (see Experimental procedures).
We first tested AA and phosphatidylinositol-4-phosphate (PIP), two strongly stimulatory lipids in the LBP actin assay (abbreviated as ‘+’ lipids). After this treatment there was a higher fraction of cells in which p65 translocated into the nucleus (Fig. 1C and D). In parallel, CD69 was present on the plasma membrane of these lipid-treated cells (Fig. 1C and D). In both cases CD69 was expressed in a fraction of cells where it tended to colocalize with actin, but the surface appearance of these lipid-treated macrophages was different from LPS-treated cells. No prominent surface spicules/filipodia, highly evident after LPS treatment, was seen with any of the lipids. In contrast, Cer and diacylglycerol (DAG) did not significantly activate NF-κB after 1 h incubation. However, we observed that the surface expression of CD69 increased in Cer-treated cells (Fig. 3A). EPA and PC had no effect on NF-κB activation or CD69 surface expression (see below). Therefore, our observations indicate that these lipids, with known anti-inflammatory effects, had no effect on NF-κB activation.
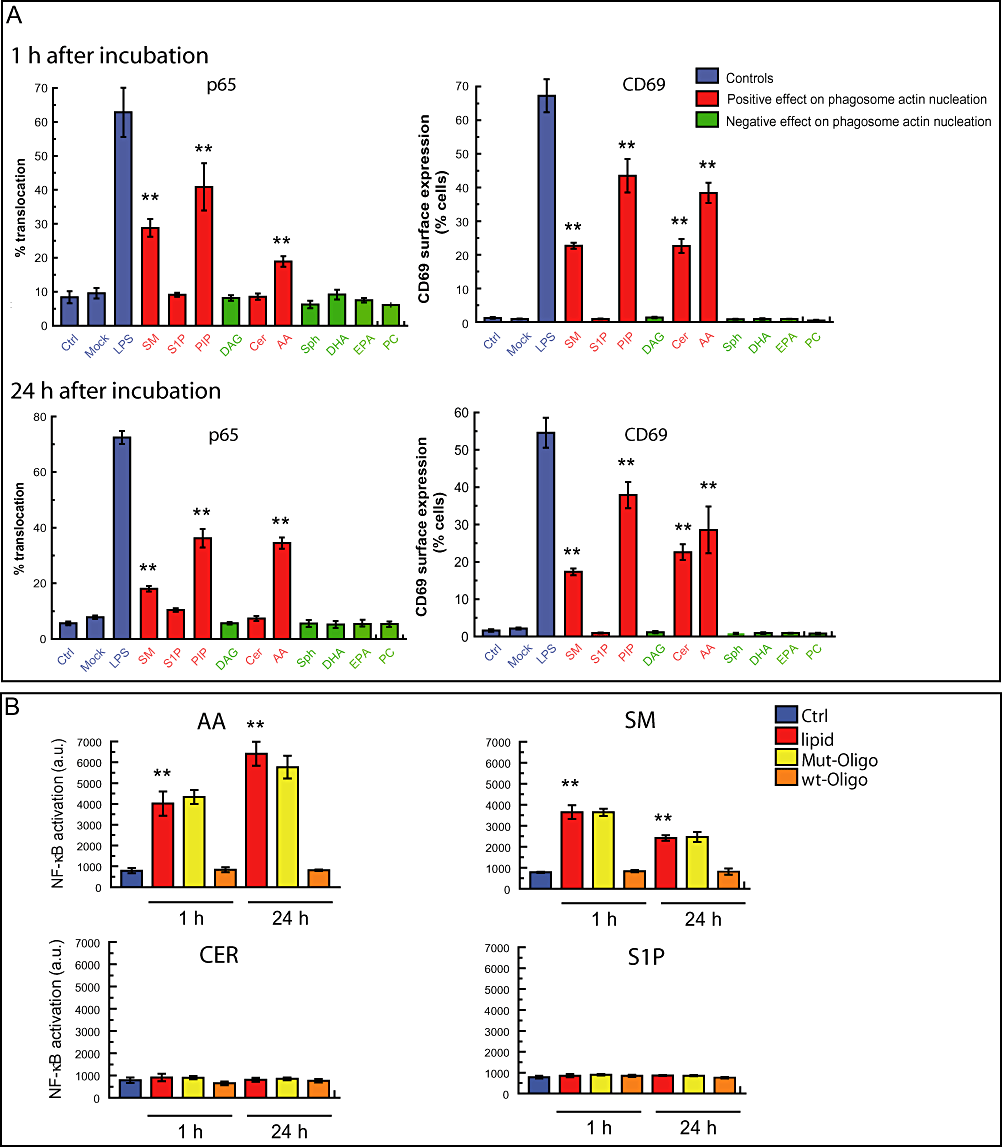
Pro-inflammatory lipids induce NF-κB activation in J774 macrophages.A. Quantitative analysis of macrophage activation in the presence of 10 different lipids. J774 macrophages were treated with the indicated lipids or LPS during 1 or 24 h. Cells were fixed and processed as indicated in Fig. 1. One hundred cells were counted from three independent experiments to asses the nuclear localization of p65 and surface expression of CD69. Mock represents cells treated only with the solvent used to dissolve the lipids. Results are shown as means ± SEM of three independent experiments. **P ≤ 0.001 relative to control in the same conditions. SM, sphingomyelin; S1P, sphingosine-1-phosphate; PIP, phosphatidilinositol-4-phosphate; DAG, diacylglycerol; Cer, C6-ceramide; AA, arachidonic acid; Sph, sphingosine; DHA, docosohexanoic acid; EPA, eicosapentanoic acid; PC, phosphatidylcoline.B. NF-κB activation in lipid-treated macrophages measured by ELISA. J774 cells were treated with lipids at the indicated time points. Nuclear fractions were isolated and DNA-binding assay of induced NF-κB activation determined. NF-κB-binding activity was effectively competed for by the wild-type consensus oligonucleotide but not by the mutated oligonucleotide. Results are shown as means ± SEM of three independent experiments. **P ≤ 0.001 relative to control.
After treatment with either Sph or its downstream metabolite S1P, the intracellular distribution of p65 changed. As shown in Fig. 2A, although these lipids failed to induce translocation of NF-κB into the nucleus they induced an altered pattern of localization in the cytoplasm whereby p65 was strongly associated with the cortical actin. A partial colocalization between p65 and actin was observed in macrophages with both lipids (Fig. 2B, insets). Although the precise implications of these observations remain to be elucidated other studies have observed a link between NF-κB and the actin cytoskeleton (Are et al., 2000; Nemeth et al., 2004).
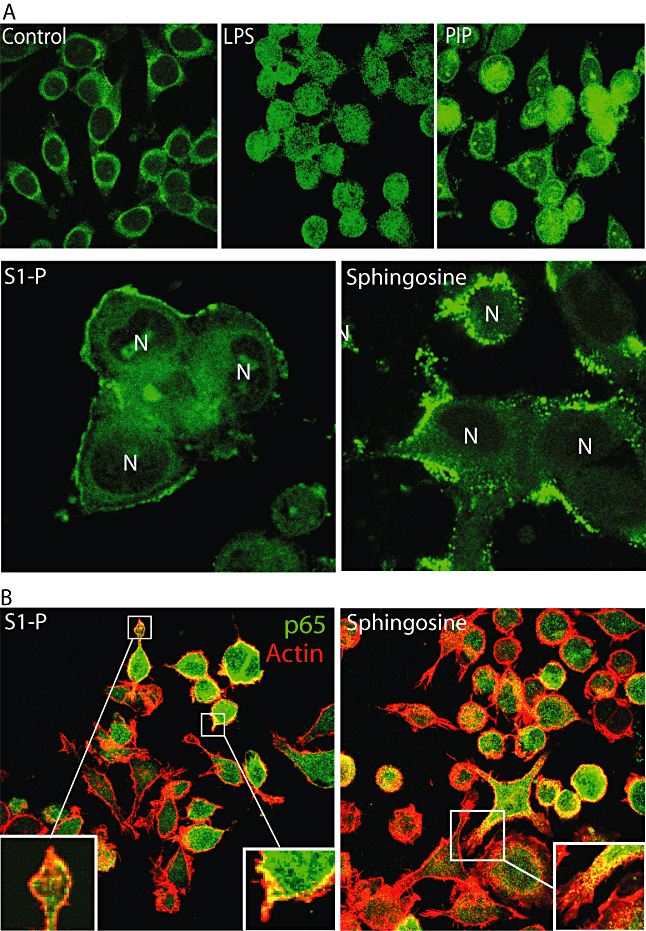
Effect of sphingosine (Sph) and sphingosine-1P (S1P) on actin distribution.A. Macrophages were treated with LPS, PIP, Sph or S1P or left untreated (control) for 1 h and subsequently fixed and processed for p65 detection (green). Note the differential distribution of p65. N, nucleus.B. Macrophages were treated with Sph or S1P and double detection of p65 (green) and actin (rhodamine-phalloidin) was performed. Note the colocalization between actin and p65 in the presence of the lipids (insets).
Quantitative analysis of the effect of 10 lipids on the activation state of macrophages
The ability of some, but not other lipids to induce NF-κB activation prompted us to extend our analysis to more lipids. We quantified the p65 nuclear localization and cell surface expression of CD69 and estimated the percentage of positive cells at 1 and 24 h after adding 10 different lipids. In Fig. 3A the bars are colour coded to indicate the lipids that are stimulatory (+) in the latex bead phagosome (LBP) actin assembly assay at physiological ATP (red bars, Anes et al., 2003) whereas the green bars indicate the inhibitory (−) lipids. A basal level of NF-κB activation is reflected in a small fraction of activated cells (between 5% and 10%) under non-stimulating condition, as previously reported (Birbach et al., 2002). Three (+) lipids significantly enhanced transport of p65 into the nucleus, namely AA, SM, and most potently PIP, although the percentage of positive cells was significantly less than with LPS (Fig. 3A). One (+) lipid, Cer, had no effect while all five (−) lipids also showed no effect on NF-κB activation. The observed pattern was maintained from 1 to 24 h, with some quantitative differences. Whereas AA was more potent after 24 h than at 1 h, SM was a stronger activator of NF-κB at the 1 h time point.
To validate these observations we tested some of the lipids by ELISA. This approach allows the detection of the p65 phosphorylated form in nuclei isolated from cells. In agreement with the immunofluorescence data, in cells treated with AA and SM, p65 was not only translocated to the nucleus but was able to bind its target DNA. We also confirmed by this approach that Cer and S1P did not significantly activate NF-κB (Fig. 3B). Taken together, these results indicate that AA, PIP and SM can elicit NF-κB activation in macrophages whereas S1P, DAG, Cer, Sph, DHA, EPA and PC had not significant effect.
When we analysed the surface expression of CD69 in macrophages treated with lipids, the same three (+) lipids AA, SM and PIP that activated NF-κB were found to enhance cell surface labelling for CD69. However, Cer, a (+) lipid that failed to activate NF-κB, induced a significant expression of CD69 in the plasma membrane at 1 and 24 h, relative to untreated cells (Fig. 3A). This suggests that treatment with Cer can induce synthesis and intracellular transport of CD69 to the plasma membrane by a mechanism that is independent of the NF-κB activation.
To provide an independent assessment of the effect of lipids on cell surface expression of CD69, we also performed FACS analysis. Using this technique we were able to confirm that treatment of macrophages with AA, Cer, PIP and SM for 24 h significantly increased the number of CD69-positive macrophages whereas Sph, DHA, EPA, S1P and DAG had no significant effect under the same conditions (Fig. S1).
It is important to remark that none of the observed effects of any of the lipids was due to endotoxin contamination of the lipid stocks since the endotoxin content was lower than 1 EU ml−1 in all samples tested (Table S1).
Interplay between M. smegmatis infection, NF-κB activation and lipid treatment
Macrophages infected with M. smegmatis without lipid supplements are able to activate NF-κB by 1 h after infection. However, at 24 h the NF-κB activation is reversed to the basal levels (Gutierrez et al., 2008). This activation during the first hour is crucial for the macrophage to kill M. smegmatis since when NF-κB activation is blocked the bacteria grow in J774 and bone marrow macrophages (Gutierrez et al., 2008). We have shown previously that many (+) lipids enhance the macrophage innate immune response leading to bacterial killing while the (−) lipids tend to lower the resistance of the macrophage and allow mycobacterial growth (Anes et al., 2003).
With this background, we next addressed NF-κB activation in the context of mycobacterial infection. For this, we tested the lipids on p65 distribution (blue channel) and F-actin localization (red channel) in J774 cells infected with GFP-M. smegmatis (green). As shown on Fig. 4, EPA was able to reverse the activation induced by M. smegmatis infection at 1 h. Moreover, AA activated NF-κB in M. smegmatis-infected cells at 24 h, a time when NF-κB was not activated in infected cells without lipid treatment (Fig. 4 and Gutierrez et al., 2008). These observations argue that NF-κB activation induced by M. smegmatis can be counter-balanced by some lipid treatments.
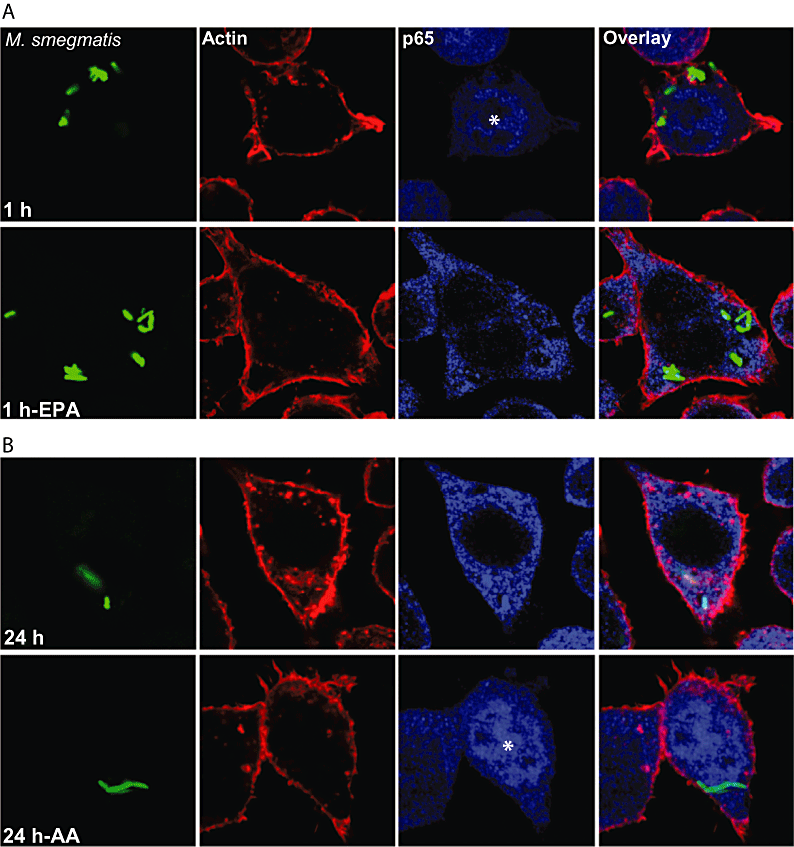
M. smegmatis-infected macrophages. NF-κB activation can be modulated by lipids.A. J774 macrophages were infected with GFP-M. smegmatis (green) and treated or not with EPA for 1 h. Afterwards, cells were fixed and subsequently processed for immunofluorescence against p65 (blue) and rhodamin-phalloidin labelling (red). Representative confocal images are depicted.B. J774 macrophages were infected with GFP-M. smegmatis for 24 h in the presence or absence of AA. Cells were processed as indicated for (A). EPA, eicosapentanoic acid; AA, arachidonic acid. Asterisks indicate nuclear positive staining for p65 (nuclear translocation).
Quantitative analysis for nine lipids revealed a number of interesting results. First, the same three lipids SM, PIP and AA that stimulated the NF-κB system in uninfected cells now boosted the system, after 1 h infection, significantly above the levels of nuclear p65 seen with M. smegmatis without lipids (Fig. 5). Second, an additional (+) lipid, S1P, that had no effect on NF-κB activation in uninfected cells, or cells infected for 1 h with M. smegmatis, showed a striking capacity to enhance the system after 24 h. The third interesting observation was that in M. smegmatis-infected cells some of the (−) lipids (Sph and DAG) did not prevent the normal activation of NF-κB by M. smegmatis. This argues that under this condition these lipids are more neutral than anti-inflammatory. Alternatively, the induction of NF-κB activation by M. smegmatis might be more potent than the anti-inflammatory effect of those lipids. On the other hand EPA and DHA were able to slightly but significantly reverse the NF-κB activation induced by M. smegmatis at 1 h. This is in agreement with the striking ability of these lipids to increase the survival in M. tuberculosis-infected cells, even after 2 weeks of infection (Anes et al., 2003). We also performed ELISA of p65 to confirm the effect of selected lipids during infection with M. smegmatis. Both AA and S1P were able to activate NF-κB in M. smegmatis-infected cells while Sph and Cer had no effect in agreement with the fluorescence data (Fig. S2A).
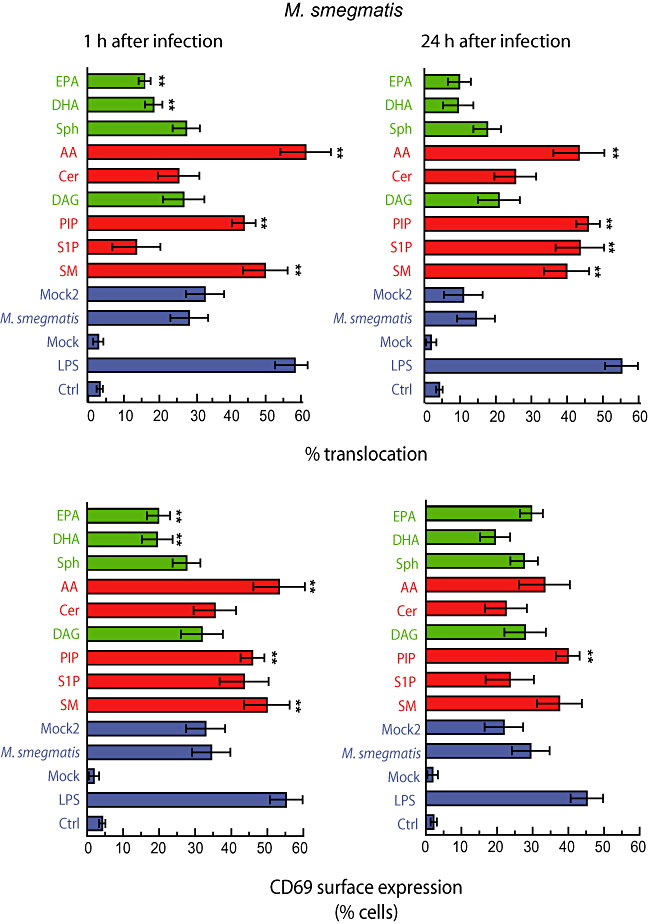
Quantitative analysis of M. smegmatis in macrophages treated with different lipids. J774 macrophages were infected with M. smegmatis-GFP in the presence or absence of the indicated lipids or LPS (10 μg ml−1) during 1 or 24 h. Cells were fixed and processed for detection of p65 distribution or CD69 surface expression. One hundred cells were counted from three independent experiments to asses the nuclear localization of p65 and surface expression of CD69. Mock represents cells treated with the solvents used to dissolve the lipids. Mock2 represents cells infected with M. smegmatis in the presence of the solvent. Results are shown as means ± SEM of three independent experiments. **P ≤ 0.001 relative to infected cells in the same conditions. SM, sphingomyelin; S1P, sphingosine-1-phosphate; PIP, phosphatidylinositol-phosphate; DAG, diacylglycerol; Cer, C6-ceramide; AA, arachidonic acid; Sph, sphingosine; DHA, docosohexanoic acid; EPA, eicosapentanoic acid.
The analysis of CD69 surface expression showed similar patterns to NF-κB activation. As for NF-κB, AA, PIP and SM treatment significantly increased the fraction of cells expressing CD69 on their surface. At 24 h the effects of these lipids was less prominent. Here, S1P also tended to increase the CD69 surface expression after 1 h, but not after 24 h. Clearly, when one compares Fig. 3 (uninfected cells) with Fig. 5 (infected cells), it is evident that the presence of M. smegmatis by itself leads to a significant stimulation of CD69 surface expression while the lipids have at best a modest stimulatory (AA, SIP, SM, AA) or little (EPA, DHA) effect on the system. Using FACS analysis, we confirmed that infection of macrophages with M. smegmatis induced CD69 surface expression. Moreover, PIP was able to enhance the CD69 expression in M. smegmatis-infected cells and DHA had no effect (Fig. S3). Altogether, these results indicate that lipids can modulate both the pro-inflammatory response and the activation state induced by M. smegmatis in macrophages.
Interplay between NF-κB activation, lipid treatment and M. avium infection
Quantitative analysis of the localization of p65 in M. avium-infected cells showed that this pathogen is less effective than the non-pathogenic M. smegmatis in inducing NF-κB activation (Lee and Schorey, 2005; Gutierrez et al., 2008) (Fig. 6). We had observed that by 1 h, the earliest time selected for all the experiments here, the levels of cells with nuclear localization of p65 were close to the basal levels seen in uninfected cells (Gutierrez et al., 2008). When the lipids were added the most striking effect was the low levels of activation induced by any lipid after the infection (Fig. 6). However, two of the three (+) lipids that activated NF-κB in M. smegmatis-infected cells, SM and AA, still induced a small, but significant activation of the system at 1 and 24 h after infection with M. avium (Fig. 6). A small but significant increase was also observed with Cer and even with a (−) lipid Sph after 24 h (Fig. 6). In agreement with these data, activation of NF-κB, as monitored by ELISA, was also observed with Cer and Sph in M. avium-infected cells whereas S1P had no effect (Fig. S2B). Overall these data argue that M. avium can potently inhibit the NF-κB activation while some of the lipids are able to overcome this inhibition to some extent.
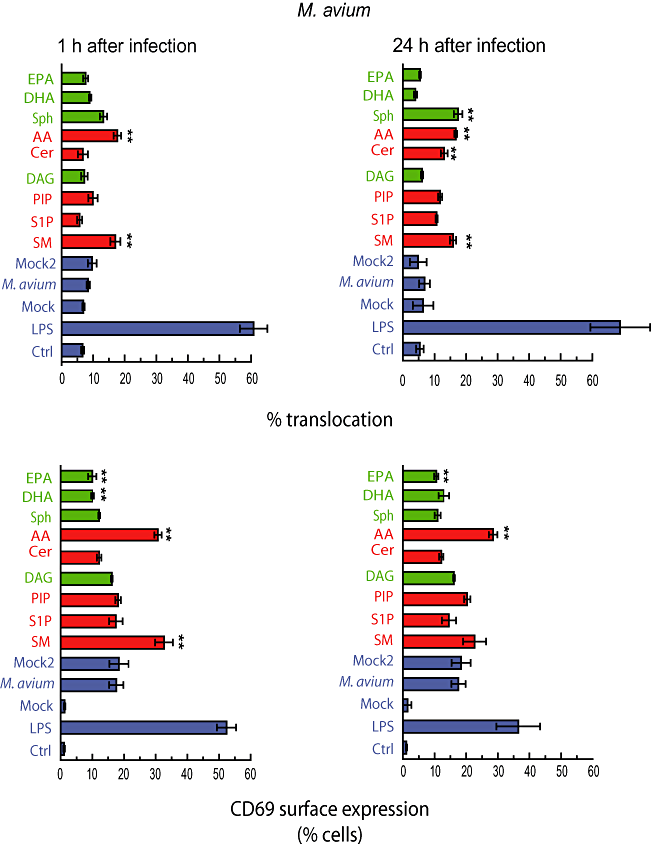
Quantitative analysis of M. avium in macrophages treated with different lipids. J774 macrophages were infected with M. avium-rhodamine in the presence or absence of the indicated lipids or LPS during 1 or 24 h. Cells were fixed and processed for detection of p65 distribution or CD69 surface expression. One hundred cells were counted from three independent experiments to asses the nuclear localization of p65 and surface expression of CD69. Mock represents cells treated with the solvent used to dissolve lipids. Mock2 represents cells infected with M. avium in the presence of the solvent. Results are shown as means ± SEM of three independent experiments. **P ≤ 0.001 relative to infected cells in the same conditions. SM, sphingomyelin; S1P; Sphngosine-1-phosphate; PIP, phosphatidylinositol-phosphate; DAG, diacylglycerol; Cer, C6-ceramide; AA, arachidonic acid; Sph, sphingosine; DHA, docosohexanoic acid; EPA, eicosapentanoic acid.
When CD69 surface expression was analysed, we observed that M. avium induced CD69 expression, but at low levels (∼20% of cells) compared with either M. smegmatis (30–40%) or LPS (> 50%) (Fig. 6). We also observed a low expression of CD69 in M. avium-infected macrophages compared with M. smegmatis-infected macrophages by FACS analysis (Fig. S3). Only two (+) lipids, AA and SM, could stimulate the CD69 surface expression in M. avium-infected cells after 1 h, while only AA significantly stimulated CD69 after 24 h (Fig. 6). In agreement with these data, AA also increased the CD69 expression in infected cells compared with non-infected cells by FACS (Fig. S3). On the other hand, inhibitory lipids such us EPA and DHA reduced the number of macrophages positive for CD69 at the plasma membrane at 1 h and EPA at 24 h. EPA also showed a modest effect in inhibiting the surface expression of CD69 by FACS analysis (Fig. S3). These results show that the presence of the pathogenic mycobacteria in macrophages inhibits both NF-κB activation and CD69 surface expression while AA and SM are able to partially overcome this block. In addition, lipids such as EPA and DHA were able to enhance the inhibition of NF-κB activation.
Effect of Sph and S1P on M. smegmatis, M. avium and M. tuberculosis survival
In recent studies we used the intracellular survival of mycobacteria as a sensitive bioassay of the potency of selected lipids. In general, lipids that stimulate phagosome actin assembly (AA, SM, Cer and S1P) enhance mycobacterial killing whereas those that inhibit this process (DAG, PC and Sph) stimulate mycobacterial growth. Given that we had tested AA, SM, Cer, DAG, PC, but not Sph and S1P, in some detail for their ability to kill non-pathogenic and pathogenic mycobacteria (Anes et al., 2003; Treede et al., 2007; Table 1), we decided to test the role of Sph and S1P on the survival of M. smegmatis, M. avium and M. tuberculosis (H37Rv).
| Lipid | Actin nucleation on phagosomes | p65 activation | CD69 surface expression | MS-p65 activationa | MS-CD69 surface expressiona | MA-p65 activationb | MA-CD69 surface expressionb | Mycobacterial killing |
|---|---|---|---|---|---|---|---|---|
| SM | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Killingc (1) |
| S1P | (+) | 0 | 0 | (+) | 0 | 0 | 0 | Killingd (this study and 2) |
| PIP | (+) | (+) | (+) | (+) | (+) | 0 | 0 | ND |
| Cer | (+) | 0 | (+) | 0 | 0 | (+) | 0 | Killinge (1) |
| AA | (+) | (+) | (+) | (+) | (+) | (+) | (+) | Killingf (1) |
| Sph | (−) | 0 | 0 | 0 | 0 | (+) | 0 | Killingg (this study) |
| DAG | (−) | 0 | 0 | 0 | 0 | 0 | 0 | No significant |
| DHA | (−) | 0 | 0 | (−) | (−) | 0 | (−) | Growthh (1) |
| EPA | (−) | 0 | 0 | (−) | (−) | 0 | (−) | Growthi (1) |
| PC | (−) | 0 | 0 | ND | ND | ND | ND | Growthj (3) |
- a. Compared with M. smegmatis-infected cells – activation either 1 or 24 h.
- b. Compared with M. avium-infected cells – activation either 1 or 24 h.
- c. H37Ra, M. avium ATCC 25291.
- d. H37Rv, M. smegmatis, M. avium MAC101.
- e. H37Ra, M avium ATCC 25291.
- f. M. tuberculosis H37Ra, M. tuberculosis H37Rv, M. tuberculosis strain 575, M. tuberculosis strain 781, M. avium ATCC 25291.
- g. M. tuberculosis H37Ra, M. tuberculosis H37Rv, M. smegmatis, M. avium ATCC 25291, M. avium MAC101.
- h. M. tuberculosis H37Ra, M. tuberculosis H37Rv, M. tuberculosis strain 575, M. tuberculosis strain 781.
- i. M. tuberculosis H37Ra, M. tuberculosis H37Rv, M. tuberculosis strain 575, M. tuberculosis strain 781.
- j. M. tuberculosis H37Rv.
- (1) Anes et al. (2003).
- (2) Garg et al. (2004).
- (3) Treede et al. (2007).
- ND, no data; MS, M. smegmatis-infected; MA, M. avium-infected.
As shown in Fig. 7A, the addition of Sph or S1P at 0.5 μM to M. smegmatis-infected cells at the start of the infection had no significant effect on the survival of the bacteria until 8 h post infection in J774 cells. Thus, the first killing period in this system (0–4 h) and the growth phase between 4 and 8 h are not affected by either lipid. However, the striking second phase of killing of M. smegmatis between 8 and 12 h was strongly enhanced by both Sph and S1P. When 5 μM lipids were tested S1P had no effect at 24 h, although it did stimulate more killing by 48 h. Sph induced a bactericidal effect at both time points (24 h and 48 h) and both concentrations tested.

Survival of M. smegmatis, M. avium MAC101 and M. tuberculosis H37Rv in the presence of Sph and S1P.A. Survival of M. smegmatis. J774 cells were infected with M. smegmatis as described in Experimental procedures and colony-forming units (cfu) were determined. Sph and S1P (0.5 and 5 μM final concentration) were added for time of infection.B. Survival of M. avium. J774 cells were infected with M. avium as described in Experimental procedures and cfu were determined. Sph and S1P were added for the time of infection.C. Survival of M. tuberculosis H37Rv. J774 cells were infected with M. tuberculosis H37Rv as described in Experimental procedures and cfu were determined. Sph and S1P were added for the time of infection.*P ≤ 0.001 for Sph and S1P relative to control in same conditions.
When Sph or S1P were added to cells infected with M. avium there was no significant effect of either lipid, at 0.5 or 5 μM between 3 and 24 h, when the bacteria tend to be killed. However, by days 3 and 5 after infection the lipids showed bactericidal effects that were more prominent at the higher concentration of the lipids (Fig. 7B). A similar pattern of Sph- and S1P-induced killing was also seen with M. tuberculosis H37Rv (Fig. 7C).
It should be noted that S1P was unable to activate NF-κB in uninfected cells or in cells infected with M. avium (although it did enhance NF-κB activation, but not CD69 in M. smegmatis-infected cells at 24 h, Fig. 5). Similarly, Sph was unable to activate NF-κB in either uninfected orM. smegmatis-infected cells (although it did show some stimulation of NF-κB but not CD69 in M. avium-infected cells, Fig. 6). Collectively these data argue that Sph and S1P can enhance macrophage killing of mycobacteria under some conditions that do not involve NF-κB activation.
Discussion
This study was initiated by the hypothesis that the assembly of actin by phagosomes, and likely by other membranes such as the plasma membrane, is part of the cellular pro-inflammatory response. According to this view, conditions that favour membrane-dependent actin assembly tend to be pro-inflammatory whereas conditions that inhibit actin assembly are anti-inflammatory. In agreement with this hypothesis was the observation that many lipids that activated LBP actin assembly also enhanced the killing of mycobacteria in macrophages whereas lipids that inhibited LBP actin assembly tended to increase intracellular growth of mycobacteria (Anes et al., 2003). We therefore systematically tested 10 lipids for their ability to stimulate NF-κB and its downstream protein CD69 in uninfected J774 cells and in cells infected with M. smegmatis and M. avium.
For most of the tested lipids our results were consistent with the hypothesis that actin assembly is indeed part of the inflammatory response and that lipid signalling is crucial for pro- and anti-inflammatory responses of macrophages (Table 1). In uninfected cells three (+) lipids (indicating stimulation in the LBP actin assay) SM, PIP and AA activated NF-κB and surface expression of CD69, while the (−) lipids Cer, DAG, Sph, DHA, EPA and PC failed to activate these processes. These lipids therefore behaved according to the above hypothesis. The (−) lipid Cer did not fit into this pattern since it activated CD69, but not NF-κB. Since Cer has mycobactericidal properties (Anes et al., 2003) this provided the first evidence in our study of a lipid inducing macrophages to kill mycobacteria in the absence of NF-κB activation.
Two other lipids Sph and S1P also behaved in ways which were in part inconsistent with our hypothesis. The (−) lipid Sph failed to activate NF-κB or CD69 in uninfected cells, consistent with our hypothesis, but it also stimulate killing of M. smegmatis, M. avium and M. tuberculosis H37Rv, which goes against the hypothesis. Similarly, the (+) lipid S1P was unable to activate NF-κB under all conditions we tested (except after 24 h infection with M. smegmatis) but it nevertheless also enhanced killing of M. smegmatis, M. avium and M. tuberculosis. The mycobacteriocidal effects of Sph were also observed before (Anes et al., 2003) while the ability of S1P to enhance killing of M. tuberculosis was described by Garg et al. (2004). This ability of Sph and S1P to behave similarly in killing mycobacteria is surprising given that these lipids usually have opposite effects in cells (Spiegel and Milstien, 2003; Hannun and Obeid, 2008), as they do in the LBP actin assembly assay (Anes et al., 2003).
Both Sph and S1P clearly signal inside cells, presumably at the cytoplasmic side of membranes (Spiegel and Milstien, 2003; Hannun and Obeid, 2008). The two isoforms of sphingosine kinase that convert Sph to S1P are cytoplasmic enzymes. It is important to point out that S1P is also a potent extracellular ligand that binds to five different receptors at the plasma membrane (S1PR-1–5) (Rosen and Goetzl, 2005). In this study we presume that the main effects we observed with S1P occurred via signalling from plasma membrane S1P receptors while Sph, which has no known surface receptors, must presumably enter the cells for its effects. Despite this difference it was striking that the addition of both lipids (with mycobactericidal effect) led to a similar and dramatic re-localization of the NF-κB subunit p65 from a general cytoplasmic pattern to one that colocalized strongly with the macrophage cortical actin. This association of p65 with actin has been described earlier in rat fibroblasts (Are et al., 2000). Interaction of p65 with the actin cortex may be a mechanism to prevent NF-κB from being activated, as suggested before (Zhu et al., 1998). However, other studies have suggested that an actin-dependent mechanism may even facilitate nuclear import of p65 in endothelial cells (Fazal et al., 2007).
Infection with M. smegmatis even without lipid treatment is able to significantly activate NF-κB within 1 h infection. As pointed out in Introduction this activation of NF-κB is essential for macrophages to kill M. smegmatis (Gutierrez et al., 2008). The ability of the pathogens M. avium, and likely M. tuberculosis to partially block NF-κB activation (Lee and Schorey, 2005; Pathak et al., 2005; 2007) is likely to be an important survival mechanism for these pathogens. Pathogenic mycobacteria are also known to produce significantly less pro-inflammatory molecules, such as cytokines, compared with cells infected with non-pathogenic mycobacteria (Falcone et al., 1994; Beltan et al., 2000). Our results here are consistent with the notion that two lipids that facilitate killing of mycobacterial pathogens, SM and AA, may be bactericidal at least in part as a consequence of activating NF-κB; both lipids enhance NF-κB activation and CD69 expression under all conditions in M. smegmatis- and M. avium-infected macrophages. In contrast, of the (−) lipids (which inhibit LBP actin assembly), of which all tested so far enhanced mycobacterial growth in macrophages, none was able to activate NF-κB. Moreover, there was a tendency for EPA and DHA to reduce both the level of activation of NF-κB and CD69 surface expression in M. smegmatis-infected macrophages (Table 1). These observations are consistent with their well-documented anti-inflammatory properties (Calder, 2007).
While our results point to an NF-κB-independent ability of Cer, Sph and S1P (under most conditions) to kill mycobacteria they leave open the possible mechanisms by which such killing might occur. One intriguing speculation stems from recent data showing that Cer and S1P have been shown to stimulate autophagy under some conditions (Lavieu et al., 2006; 2008). Since it is now established that autophagy is a mechanism by which pathogenic mycobacteria can be eliminated by macrophages (Gutierrez et al., 2004), it is tempting to suggest that these lipids may be mycobactericidal via this process.
In summary, this study has shown that SM, AA and PIP are potent activators of the pro-inflammatory response via NF-κB. Since NF-κB activation leads to an increase in the rate of phagosome maturation and is required for macrophages to kill M. smegmatis (Gutierrez et al., 2008), this study provides a potential mechanism to explain how these lipids may activate mechanisms that kill mycobacterial pathogens. Consistent with this notion, the mycobactericidal lipids facilitate phagosome maturation (Anes et al., 2003). The lipids EPA, DHA, PC and DAG fail to activate NF-κB and either have no effect (DAG) or actually enhance mycobacterial growth in macrophages (Anes et al., 2003; Treede et al., 2007). Cer, Sph and S1P fall into a different category of lipids that are unable to activate NF-κB but nevertheless have the ability to enhance killing of mycobacteria. This argues that lipids can enhance mycobacterial killing via both NF-κB-dependent and NF-κB-independent mechanisms.
Experimental procedures
Reagents
The following antibodies were used: polyclonal rabbit anti-p65 (Santa Cruz, CA, USA). Mouse monoclonal anti-CD69 FITC-labelled was purchased from BD Biosciences, Germany. Secondary antibodies anti-rabbit were conjugated with Alexa555 and Alexa488 (Molecular Probes). Rhodamine-phalloidin was from Molecular Probes.
The following concentrations were used: 50 μM AA in ethanol (Sigma, St Louis, MO); 100 nM Sph in ethanol (Calbiochem); 100 nM S1P in methanol (Calbiochem); 1 μM C6-ceramide in methanol (Sigma, St Louis MO); 50 μM PIP in methanol : chloroform 1:1 (Calbiochem); 10 μM DAG in DMSO (C8 form-Calbiochem); 1 μM PC mixture in ethanol (Sigma, St Louis, MO); 15 μM DHA in ethanol (Sigma, St Louis, MO); 50 μM sphingomyelin in methanol : chloroform 1:1 (Sigma, St Louis, MO); and 15 μM EPA in ethanol (Sigma, St Louis, MO). Solvents without lipids were routinely tested, referred to in the figures as the (mock) control.
Cell lines and bacterial culture conditions
The mouse macrophage cell lines J774A.1 were cultured as described previously (Anes et al., 2006). M. smegmatis mc2155 harbouring a p19-(long-lived) EGFP plasmid and M. avium MAC 101 were grown as previously described (Anes et al., 2003). M. avium MAC 101 strain was kindly provided by Dr Ulrich Schaible (London School of Hygiene and Tropical Medicine, London). Briefly, M. smegmatis mc2155 was grown in medium containing Middlebrook's 7H9 broth Medium (Difco), Nutrient broth (Difco) supplemented with 0.5% glucose and 0.05% Tween 80 at 37°C on a shaker at 220 r.p.m. Bacteria were subcultured every day in fresh medium for 7–10 days before use in infection studies. M. tuberculosis H37Rv was grown in Middlebrock 7H9 medium (Difco) and was maintained as described before (Anes et al., 2003).
Macrophage infection
Bacterial cultures in exponential growth phase were pelleted, washed in PBS and re-suspended in DMEM medium to a reach a multiplicity of infection (moi) of 10. Clumps of bacteria were removed by ultrasonic treatment of bacteria suspensions in an ultrasonic water bath for 15 min followed by a low-speed centrifugation for 2 min. Cells were seeded onto 24-well tissue culture plates at 70% confluence. In each experiment, after 1 h infection cells were washed with PBS and gentamicin (10 μg ml−1) in DMEM was added to kill extracellular bacteria.
Indirect Immunofluorescence
Cells were fixed with 3% paraformaldehyde solution in PBS for 10 min and quenched by incubating with PBS 50 mM NH4Cl. Subsequently, cells were permeabilized with 0.05% saponin in PBS containing 0.2% BSA, and then incubated with the primary and secondary antibodies. Cells were mounted with Dako mounting media and analysed by confocal microscopy (Zeiss LSM510).
ELISA
This was assayed using a multiwell assay TransAM NF-κB Kit (Active Motif, Belgium). Briefly, J774-infected macrophages were scraped and nuclear extracts isolated. Five micrograms of total protein from each sample was incubated in 96-well plates coated with NF-κB consensus oligonucleotide sequence (5′-AGTTGAGGGGACTTTCCCAGGC-3′) for 1 h and then with primary anti-NF-κB antibody and subsequently with secondary HRP-conjugated antibody. After a chemioluminiscent reaction, the luminescence produced was recorded. Competition experiments were conducted with the 22 bp double-stranded DNA, either wild type (see above) or mutated: 5′-AGTTGAGCTCA CTTTCCCAGGC.
Colony-forming unit assay
Macrophages were plated in a 24-wells plate and infected with M. smegmatis, M. avium or M. tuberculosis H37Rv at different time points. Cells were washed with PBS and lysed with sterilized water. Quantitative cultures for M. smegmatis, M. avium or M. tuberculosis H37Rv were performed by 10-fold serial dilutions inoculated on 7H10 agar plates. Five microlitres were plated by triplicate and the number of colonies was counted after 48 h (M. smegmatis), 3 days (M. avium) or 3 weeks (M. tuberculosis H37Rv) and referred as number of colonies (colony-forming unit) per millilitre.
Statistical analysis
Data are presented as means ± SEM of at least three independent experiments; P-values (anova, two ways) are relative to the control.
Acknowledgements
We are grateful to David Liebl for critical reading of this manuscript. We would also like to thank Stefan Terjung at the Advanced Light Microscopy Facility and Andy Riddell at the Flow Cytometry Facility (EMBL, Heidelberg) for excellent technical assistance and advice. M.G.G. was supported by a Research Fellowship from the Alexander von Humboldt Foundation and is currently funded by an EMBO Fellowship. G.G. was partially supported by DFG Sphingolipid Network Grant SPP-1267. E.A. was supported by Fundação para a Ciência e a tecnologia (FCT) Grant PPCDT/BIA-BCM/55327/2007.