Mycobacterial infection induces the secretion of high-mobility group box 1 protein
Summary
High-mobility group box protein 1 (HMGB1) is a non-histone nuclear protein that acts as a pro-inflammatory cytokine and is released by monocytes and macrophages. Necrotic cells also release HMGB1 at the site of tissue damage which induces a variety of cellular responses, including the expression of pro-inflammatory mediators. This study investigated the secretion of HMGB1 in mycobacterial infection by macrophages in vitro and in the lungs of infected guinea pigs. We observed that infection by mycobacterium effectively induced HMGB1 release in both macrophage and monocytic cell cultures. Culture filtrate proteins from Mycobacterium tuberculosis induced maximum release of HMGB1 compared with different subcellular fractions of mycobacterium. We demonstrated that HMGB1 is released in lungs during infection of M. tuberculosis in guinea pigs and increased HMGB1 secretion in lungs of guinea pigs was delayed by prior vaccination with Mycobacterium bovis BCG. The secretion of cytokines like tumour necrosis factor alpha (TNF-α) and Interleukin-1β was significantly increased when M. bovis BCG-infected cultures of J774A.1 cells were incubated with HMGB1. Among different mycobacterial toll-like receptor ligands, heat-shock protein 65 (HSP65) was found to be more potent in inducing HMGB1 secretion in RAW 264.7 cells. Pharmacological suppression of p38 or extracellular signal-regulated kinase 1/2 mitogen-activated protein kinases with specific inhibitors failed to inhibit HSP65-induced HMGB1 release, but inhibition of c-Jun NH2-terminal kinase activation attenuated HMGB1 release. Inhibition of the inducible NO synthase and neutralizing antibodies against TNF-α also reduced HMGB1 release stimulated by HSP65. We conclude that HMGB1 is secreted by macrophages during tuberculosis and it may act as a signal of tissue or cellular injury and enhances immune response.
Introduction
Infection with Mycobacterium tuberculosis is accompanied by an intense local inflammatory response which may be critical to the pathogenesis of tuberculosis (TB). TB is a local disease, restricted mostly to the lungs, where granulomas ensure mycobacterial containment at least in the early stages of infection. Latent infection is often lifelong. Increasing numbers of M. tuberculosis and disruption of the granuloma structure result in cavity formation and open disease (Russell, 2007). The activation of host macrophages and induction of pro-inflammatory mediators, such as tumour necrosis factor alpha (TNF-α), occurs early after M. tuberculosis infection and may persist as the organism establishes itself within granulomas (Ulrichs and Kaufmann, 2006).
Pro-inflammatory cytokines [TNF-α, Interleukin (IL)-1 and IL-6] have been implicated in mediating inflammation and have been shown to be responsible for development of tissue injury in animal models of TB (Houben et al., 2006). However, a proper pro-inflammatory response is necessary to contain mycobacterium. In addition, co-ordinated activation of cells is crucial for a productive granuloma to develop, and thus for long-term containment of mycobacteria at distinct sites of infection. The characteristic feature of granulomas is the development of a central, acellular eosinophilic region of caseous necrosis (Ulrichs and Kaufmann, 2006). Both host and mycobacterial factors contribute to caseation. Under intense cytokine and direct cell–cell activation, macrophages and epithelioid cells undergo necrosis and/or apoptosis to form this material. T-cell-derived perforin and granulysin and macrophage-derived reactive oxygen and nitrogen metabolites may also contribute to the necrosis of cells (Houben et al., 2006). Successful containment of the pathogen to the site of the primary lesion results in latent infection, often morphologically seen as calcified granulomatous lesions. In more than 90% of all cases, this well-controlled state can be maintained, thus preventing active TB (Neyrolles et al., 2006).
Recently, it was shown that necrotic cells release the nuclear protein high-mobility group box 1 (HMGB1), an endogenous molecule that possesses inflammatory properties (Rovere-Querini et al., 2004). The release of HMGB1 results in the recruitment of mononuclear cells to the site of tissue damage, where they clear cellular debris and protect against possible disease (Bianchi and Manfredi, 2004). Moreover, it is becoming clear that HMGB1 is actively secreted by cells of the innate immune system in response to microbial and pro-inflammatory stimuli (Scaffidi et al., 2002). Active secretion of HMGB1 mimics the environment created by cell death and promote the recruitment of immune cells (Scaffidi et al., 2002). An important role of HMGB1 in immunopathogenesis is suggested by the demonstration of elevated extracellular levels in disease settings and the effects of anti-HMGB1 in animal models of endotoxin-induced shock and other diseases (Yang et al., 2005). In its extracellular form, HMGB1 can induce a variety of cellular responses, including the expression of pro-inflammatory mediators such as TNF-α, IL-1 and NO, and induction of dendritic cell maturation (Dumitriu et al., 2005). In its role as a cytokine, HMGB1 has a conserved structure (A box, B box and acidic tail), with different regions manifesting opposing actions. Thus, while the HMGB1 B box displays cytokine activity, the A box may antagonize the inflammatory response of HMGB1 (Li et al., 2003).
Monocytes, macrophages, dendritic and natural killer cells secrete HMGB1 in response to toll-like receptor (TLR) ligand interaction, which further acts to enhance both innate and adaptive immune response (Wang et al., 1999). HMGB1 binds receptor for advanced glycation end-products (RAGE), TLR2, possibly TLR4 and other receptors. Activation of RAGE further activates Ras, mitogen-activated protein kinase (MAPK) pathways and, subsequently, NF-κB nuclear translocation (Schmidt et al., 2001). Activation of TLR2 (and/or TLR4) by HMGB1 causes the recruitment of MyD88 and IL-1 receptor-associated kinase, which subsequently activates MAPK pathway and NF-κB translocation, to trigger inflammatory responses (Park et al., 2004). IFN-γ plays an important role in the regulation of HMGB1 release through a TNF-α and Janus kinase 2-dependent mechanism (Rendon-Mitchell et al., 2003).
Multiple pro-inflammatory mediators interact and contribute to granuloma formation in TB; hence we wished to determine if mycobacterial infection caused secretion of HMGB1. For in vitro analysis of mycobacterial infection of macrophages, murine bone marrow-derived macrophages (BMDMs) and cell lines were used. Guinea pigs were used to examine the in vivo secretion of HMGB1 as this model provides pathology similar to that in humans (Orme et al., 2001). We also investigated the effect of HMGB1 on the modulation of macrophage functions against M. tuberculosis and mechanism associated with the active secretion of HMGB1 by the mycobacterial protein heat-shock protein 65 (HSP65).
Results
Mycobacteria-induced HMGB1 release in vitro
To evaluate the release of HMGB1 in mycobacterial infection, BMDM cultures and cultures of macrophage-like J774A.1 cells were stimulated with M. tuberculosis, Mycobacterium bovis BCG, Mycobacterium avium and environmental mycobacteria such as Mycobacterium scrofulaceum. The levels of HMGB1 in the culture medium were subsequently measured by immunoblotting analysis. HMGB1 was detected in the culture medium of BMDMs (Fig. 1A) and J774A.1 cell lines (Fig. 1B) after stimulation with both M. tuberculosis and M. bovis BCG at multiplicity of infection (MOI) 10:1. HMGB1 was not detected in culture medium in the absence of stimuli. The release of HMGB1 was also observed with other less virulent species of mycobacteria, such as M. avium and M. scrofulaceum (Fig. 1C). To determine the effect of MOI of mycobacterium on HMGB1 release, J774A.1 cells were stimulated with M. bovis BCG. HMGB1 was not observed in culture medium at MOI 1:1, but was released maximally at MOI 100:1 (Fig. 1D). Thus, infection of mycobacterium effectively induced HMGB1 release both in macrophage and monocytic cell cultures.
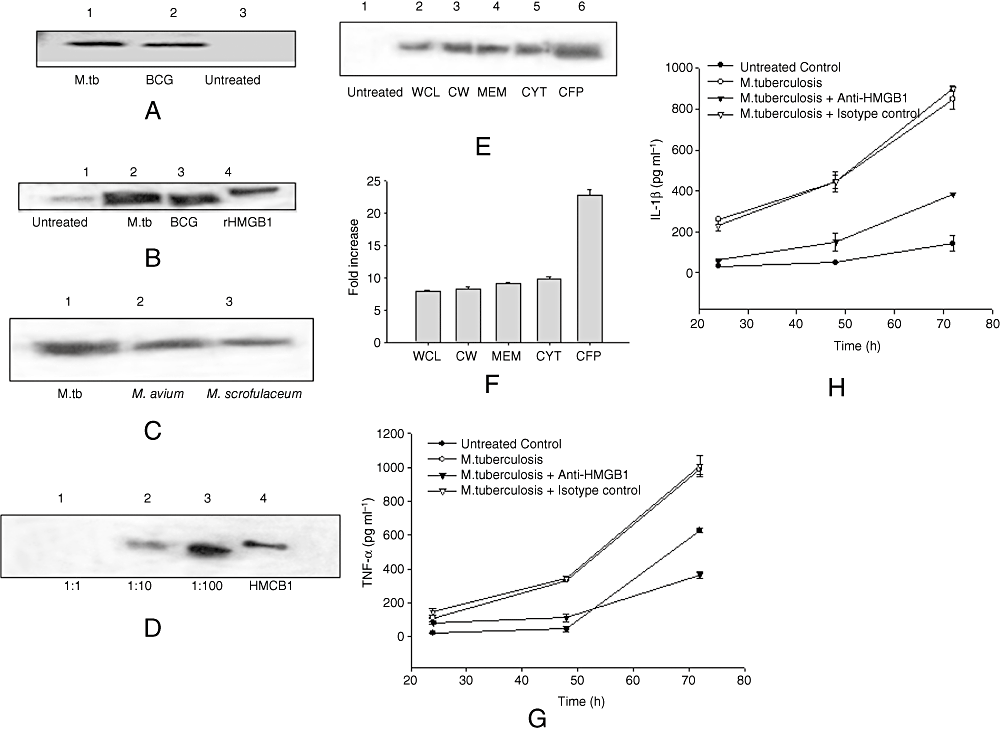
Mycobacteria-induced HMGB1 release in vitro.A. BMDMs from C57BL/6 mice were cultured in the presence of 10:1 MOI of M. tuberculosis and M. bovis BCG for 24 h. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody (Sigma).B. J774A.1 cells were cultured in the presence of 10:1 MOI of M. tuberculosis and M. bovis BCG for 24 h. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.C. RAW 264.7 cells were cultured in the presence of 10:1 MOI of M. tuberculosis, M. avium, M. scrofulaceum for 24 h. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.D. J774A.1 cells were cultured in the presence of 1:1, 10:1 and 100:1 MOI of M. bovis BCG for 24 h. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.E. RAW 264.7 cells were cultured in the presence of different cellular components of mycobacteria for 24 h. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody. WCL, whole-cell lysate; CW, cell wall fraction; MEM, cell membrane fraction; CYT, cytoplasmic fraction.F. Densitometric quantification of Western blot shown in E.G. BMDMs from C57BL/6 mice were first treated with HMGB1 antibody and then cultured with 10:1 MOI of M. tuberculosis. The levels of TNF-α were estimated in culture supernatants by ELISA at various time points after adding M. tuberculosis. The results are representative of three independent experiments.H. BMDMs from C57BL/6 mice were first treated with HMGB1 antibody and then cultured with 10:1 MOI of M. tuberculosis. The levels of IL-1β were estimated in culture supernatants by ELISA at various time points after adding M. tuberculosis. The results are representative of three independent experiments.
We examined the induction of HMGB1 release in J774A.1 cells by different mycobacterial cell components. Whole-cell lysate, cell wall fraction, cell membrane fraction, cytoplasmic fraction and culture filtrate proteins (CFP) induced HMGB1 translocation and extracellular release, as shown by Western blotting (Fig. 1E). CFP induced the maximum amount of HMGB1 release among all cellular fractions, and other cellular components induced similar levels of HMGB1 release (Fig. 1F). To determine whether the mycobacterial cell and its components had a cytotoxic effect on cultured cells, the levels of cytotoxicity in J774A.1 cells was measured using lactate dehydrogenase (LDH) release assay. M. bovis BCG, M. avium and M. scrofulaceum induced a cytotoxicity of 10–15% at MOI of 10:1 whereas M. tuberculosis induced a cytotoxicity of more than 20% at 24 h of incubation (data not shown). All cellular fractions of mycobacterial cell induced a cytotoxicity of less than 5% (data not shown). These observations suggest that these components of the mycobacteria contain TLR ligands that are inducing active secretion of HMGB1 into the extracellular milieu.
In order to understand the role of HMGB1 in mycobacterial infection further, we cultured murine BMDMs with anti-HMGB1 antibody, infected them with M. tuberculosis H37Rv and estimated the levels of pro-inflammatory cytokines such as TNF-α (Fig. 1G) and IL-1β (Fig. 1H). The results of ELISA demonstrated that HMGB1 antibody reduced the TNF-α and IL-1β production induced by M. tuberculosis during time-course of infection, indicating the significance of HMGB1 in inflammatory response during progressive TB. No change was observed in TNF-α and IL-1β biosynthesis when macrophages were incubated with isotype control antibody during M. tuberculosis infection.
To determine whether stimulation with mycobacterium affects HMGB1 cellular localization, J774A.1 infected with M. bovis BCG cultures were immunostained with monoclonal anti-HMGB1 Abs. Quantification of HMGB1 was done by measuring the area either FITC-positive (HMGB1) or -negative in the nucleus and cytoplasm of uninfected or BCG-infected J774 cells. Each compartment (nuclear and cytoplasmic) was outlined using bright field and DAPI fluorescence. Un-stimulated macrophages constitutively expressed HMGB1 and maintained an intracellular pool of HMGB1 in the cytoplasm and nuclear regions (Fig. 2A, panel a). The pattern and localization of HMGB1 staining were noticeably altered as early as 16 h after stimulation with M. bovis BCG (Fig. 2A, panel b). The HMGB1 appeared to be diffusely distributed in both the cytoplasm and nucleus regions of un-stimulated macrophage cultures, but was observed predominantly and uniformly in the cytoplasm of BCG-infected macrophages as numerous aggregated granules with more intense staining (Fig. 2A, panel b). Similar changes of HMGB1 localization have been observed by others for monocytes in response to LPS stimulation (Wang et al., 1999; Chen et al., 2004). Actively multiplying mycobacterial bacilli were observed in the cytoplasm of infected macrophages (Fig. 2A, panel c). A total of 10 cells were counted in each sample for quantitative analysis of HMGB1. Uninfected cells had an average of 45% of the cytoplasm HMGB1-positive, whereas BCG-infected cells reached 65% HMGB1-positive (Fig. 2B). This suggests that macrophages infected with mycobacterium translocate nuclear HMGB1 to the cytoplasm before releasing it into the extracellular milieu. The cellular HMGB1 mRNA levels in cells were determined using real-time polymerase chain reaction (RT-PCR). Consistent with earlier observations (Wang et al., 1999), cultured cells constitutively expressed HMGB1 mRNA and stimulation with LPS, M. bovis BCG and CFP did not significantly change its cellular mRNA levels in macrophage cultures (Fig. 2C).
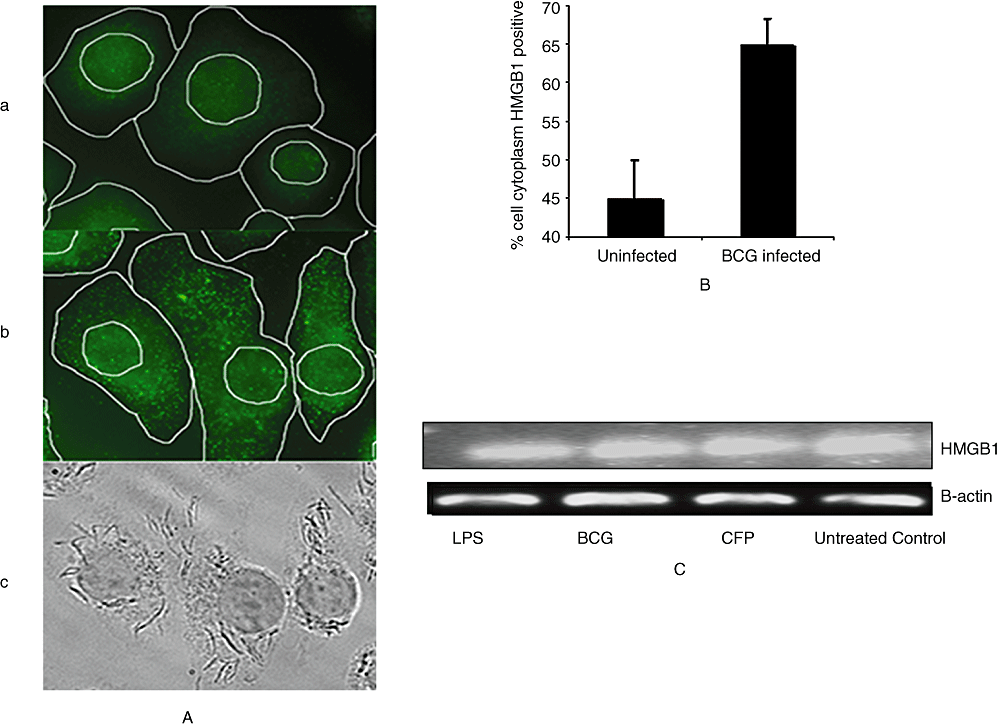
Immunoflourescence staining and RT-PCR of HMGB1 in in vitro cultured monocytes.A. Immunoflourescence staining of J774A.1 cells [control (a) and M. bovis BCG-treated (b)] using HMGB1 antibody (green). Dark field panel of BCG-treated cells (c) is also shown. Each compartment (nuclear and cytoplasmic) was outlined using bright field and DAPI fluorescence. Representative fields from one experiment out of four are shown.B. Quantification of HMGB1 in the cytoplasm of in vitro cultured cells.C. mRNA levels of HMGB1 and β-actin from J774A.1 cells cultured in the presence of LPS, M. bovis BCG and CFP as observed using RT-PCR. The results are representative of at least three independent experiments.
In vivo release of HMGB1 in mycobacterial infection
As HMGB1 acts as a marker of tissue damage and inflammatory response, we hypothesized that vaccination would limit the extent of tissue damage and hence the levels of HMGB1 secretion in a guinea pig model of TB. Guinea pigs were immunized with BCG and infected with low-dose aerosol infection of M. tuberculosis. The levels of HMGB1 in bronchoalveolar lavage (BAL) cells were determined by Western blotting of nuclear and cytoplasmic extracts of cells (Fig. 2A) at day 60 after infection. As shown in Fig. 3A, HMGB1 was found in nuclear extracts of BAL cells of both different groups of guinea pigs. It was detected in very small amount in the cytoplasm of uninfected guinea pigs, and the levels of HMGB1 detected in cytoplasmic extracts of BCG-vaccinated animals were lower than those found to be released in cytoplasm of cells of guinea pigs not vaccinated with BCG. The HMGB1 protein was also detected in BAL fluid of the infected guinea pigs (Fig. 3B). The amount of HMGB1 in BAL fluid of BCG-vaccinated guinea pigs was observed to be much lesser than those found in BAL fluid of unvaccinated guinea pigs.
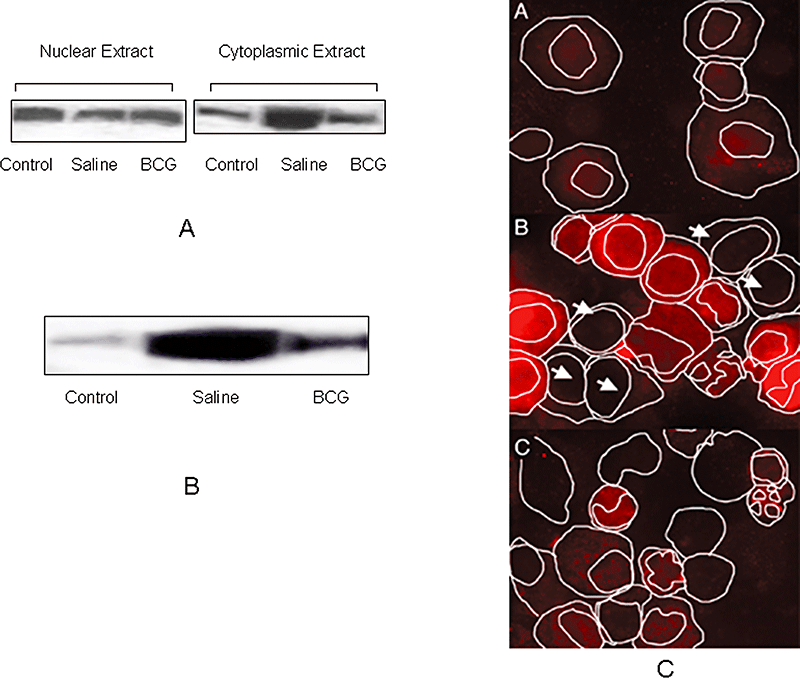
In vivo release of HMGB1 in guinea pig model of TB.A. Guinea pigs (five animals per group) were divided into three groups: uninfected (control), saline-treated and BCG-vaccinated, and then aerosolized with low-dose M. tuberculosis. At day 60 after infection, BAL cells were obtained, nuclear and cytoplasmic extracts of the cells were prepared and immunoblotted with HMGB1 antibody.B. Immunoblot of HMGB1 in the BAL fluid of guinea pigs. The result is a representative of at least three independent experiments.C. Cytospins of the BAL cells from guinea pigs were prepared. Immunoflourescence staining of the cells using HMGB1 antibody (Cy5/red) and DAPI (Blue) was done. Each compartment (nuclear and cytoplasmic) was outlined using bright field and DAPI fluorescence. Representative field from one experiment out of four is shown.
In order to extend these findings, we performed immunofluorescence staining of cytospin preparations of BAL cells using monoclonal anti-HMGB1 antibody. Each compartment (nuclear and cytoplasmic) was outlined using bright field and DAPI fluorescence. HMGB1 staining appeared to be diffusely distributed in the nucleus and cytoplasm of cells of uninfected guinea pigs (Fig. 3C, panel A), but intense staining of HMGB1 was observed in cytoplasm of many cells of infected guinea pigs (Fig. 3C, panel B). Some BAL cells (marked with arrows) in infected guinea pigs were not highly positive for HMGB1 staining, and we assume that it could be either because of absence of infection in those cells or the total release of HMGB1 out of the cells. The predominant staining of HMGB1 in cytoplasm was not observed in cells of animals which were first immunized with BCG and were then infected with M. tuberculosis. (Fig. 3C, panel C). The log10 colony-forming units (cfu) recovered from lungs and spleens of guinea pigs were significantly lower (P ≤ 0.001) in BCG-vaccinated group compared with unvaccinated controls at 60 days post challenge (Fig. 4A). As release of HMGB1 is an indication of tissue injury in lungs, we also estimated expression of cytokines in lungs of infected guinea pigs using RT-PCR in order to detect the level of inflammation. The levels of IFN-γ in lungs of guinea pigs were significantly higher (P ≤ 0.01) in BCG-vaccinated group compared with unvaccinated controls (Fig. 4B). The mRNA levels of TNF-α were found to be significantly higher (P ≤ 0.001) in unvaccinated controls when compared with the BCG-vaccinated group (Fig. 4B). The expression of IL-1β mRNA was found to be significantly higher (P ≤ 0.001) in unvaccinated controls when compared with that of BCG-immunized animals. The levels of TGF-β in lungs of guinea pigs were significantly higher (P ≤ 0.001) in BCG-vaccinated group compared with unvaccinated controls (Fig. 4C). However, no difference was found in gene expression of inducible NO synthase (iNOS) when two experimental groups were compared (Fig. 4C).
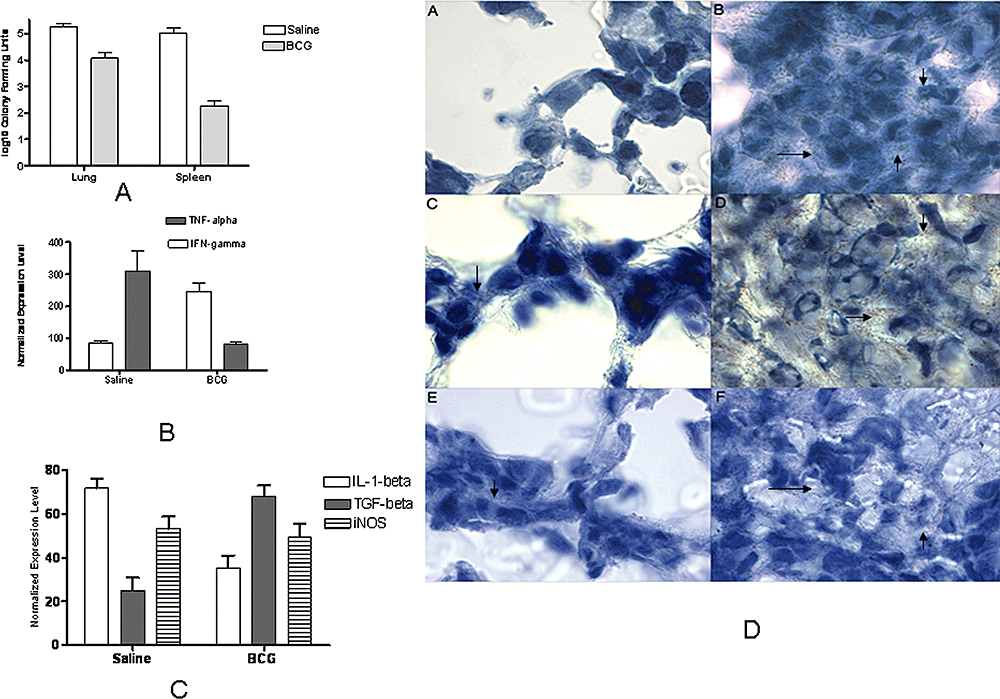
Bacterial count of M. tuberculosis H37Rv, Immunohistochemical analysis and cytokine responses obtained in lungs of guinea pigs at day 60 post infection.A. Log10 cfu of M. tuberculosis obtained from lungs and spleens of infected guinea pigs. ***P < 0.001.B. mRNA levels of cytokines IFN-γ and TNF-α in lungs of guinea pigs as quantified using qPCR. Normalized induction of mRNA was determined from the threshold cycle values normalized for,-actin expression and the normalized to the value derived from healthy animals. **P < 0.01 as compared with saline-treated group ***P < 0.001 as compared with BCG-vaccinated group.C. mRNA levels of IL-1β, TGF-β and iNOS in lungs of guinea pigs as quantified using qPCR. Normalized induction of mRNA was determined from the threshold cycle values normalized for β-actin expression and the normalized to the value derived from healthy animals. ***P < 0.001 as compared with BCG-treated group. +++P < 0.001 as compared with saline-treated group.D. Immunohistochemical staining of HMGB1 (arrows) in lungs of guinea pigs at day 60 post infection. Panel A is from saline-treated control and the section is a representative of the isotype antibody control. Panel B is the granulomatous lung tissue of saline control guinea pig. Panel C is a normal area of pulmonary tissue of saline-treated control guinea pig. Panel D is the central necrotic core of the granuloma from the lungs of saline-treated control guinea pigs. Panels E and F are the representatives of areas of normal lung tissue and lesion, respectively, from a BCG-vaccinated guinea pig. Original magnification 100×.
Immunohistochemcal studies of the lungs from non-vaccinated guinea pigs or those vaccinated with BCG, at day 60 post infection showed that endothelial cells and macrophages stained positively for HMGB1 (Fig. 4D). No staining of HMGB1 was observed in isotype antibody control-treated sections (Fig. 4D, panel A). The histologic appearance and HMGB1 staining were clearly different between the two groups of animals. Lungs from the saline-treated group had extensive areas of pulmonary parenchyma effaced by granulomatous inflammation and coalescing foci of necrosis. The cells in the outer and middle layers of granuloma stained strongly positive for HMGB1 in lungs of saline-treated control animals (Fig. 4D, panel B). The distribution of HMGB1 was found to be less in normal areas of tissue of the lungs in both saline-treated and BCG-vaccinated animals (Fig. 4D, panels C and E). The regions of the granulomas that contained a necrotic core with many dead cells were also areas of strong staining for HMGB1 in lungs of the saline-treated control group (Fig. 4D, panel D). The BCG-treated animals had fewer and much smaller lesions in which mononuclear cells were found to be positively stained for HMGB1 in cytoplasm (Fig. 4D, panel F). However, the inflammatory regions in the lungs of saline-treated control animals were more intensely stained than those of BCG-vaccinated animals.
Effects of HMGB1 on the cytokine release against mycobacterial infection and mycobacterial growth
High-mobility group box protein 1 released by necrotic cells acts as a potent adjuvant in vivo. HMGB1 stimulates further release of TNF-α and other pro-inflammatory cytokines (Kalinina et al., 2004). We hypothesized that HMGB1 may be involved in the modulation of mycobacterium-specific inflammatory responses. J774A.1 cells were stimulated with recombinant HMGB1 (Sigma) and then were infected with M. bovis BCG at MOI of 10:1. The levels of cytokines TNF-α (Fig. 5A) and IL-1β (Fig. 5B) were estimated in culture supernatants by ELISA at various time points after adding M. bovis BCG. Different concentrations of rHMGB1 were tested and optimum concentration of 200 ng ml−1 was used (data not shown). HMGB1 alone did not significantly affect TNF-α or IL-1β production, while BCG alone induced both TNF-α and IL-1β, maximally at 24 h for the former and 72 h for the latter. In combination, the concentrations of TNF-α and IL-1β were significantly increased (P ≤ 0.001), although the kinetics of secretion was not altered. As HMGB1 has been found to have antibacterial activity against the airway pathogen Moraxella catarrhalis and also Escherichia coli and Bacillus megaterium (Zetterstrom et al. 2002; 2006), it was hypothesized that HMGB1 could also kill mycobacteria. J774A.1 cells were cultured in the presence of different concentrations (0.25–1 µM) of rHMGB1 (Sigma) and M. bovis BCG at MOI of 10:1. The cells were lysed at different time points and lysates were plated on Middlebrook 7H11 agar. No difference was found in the growth of BCG in cultured cells in the presence of HMGB1 as observed by log10 cfu of bacteria (Fig. 5C). These observations indicate that HMGB1 does not have anti-mycobacterial activity.
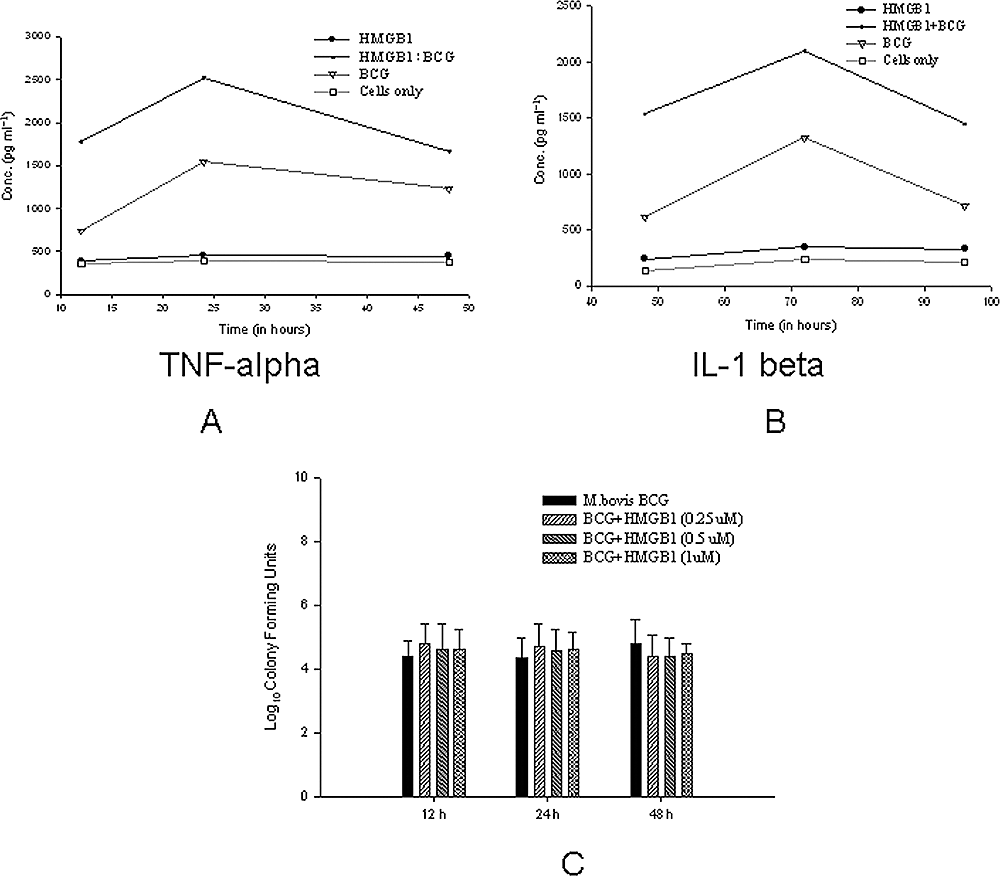
Effects of HMGB1 on the cytokine release against mycobacterial infection and mycobacterial growth.A. The J774A.1 cells were stimulated with recombinant HMGB1 (Sigma) and then were infected with M. bovis BCG at MOI of 10:1. The levels of TNF-α were estimated in culture supernatants by ELISA at various time points after adding M. bovis BCG.B. The levels of IL-1β were estimated in culture supernatants by ELISA at various time points after adding M. bovis BCG.C. J774A.1 cells were cultured in the presence of different concentrations (0.25–1 µM) of rHMGB1 (Sigma) and M. bovis BCG at MOI of 10:1. The cells were lysed at different time points and lysates were plated on Middlebrook 7H11 agar plates. The log10 cfu of M. bovis BCG obtained are shown. The results are representative of at least three independent experiments.
Toll-like receptor ligands of mycobacteria induce active secretion of HMGB1
The release of HMGB1 by cells has been observed using TLR ligands, such as LPS (TLR4), lipoteichoic acid (TLR2) and polyinosinic-polycytidylic acid (TLR3) (Jiang and Pisetsky, 2006). To investigate the release of HMGB1 by mycobacterial ligands, we incubated RAW 264.7 cells with known TLR ligands from mycobacteria such as lipomannan, mannosylated lipoarabinomannan (ManLAM) arabinosylated lipoarabinonannan, HSP65, HSP70 and 38 kDa lipoprotein. It was found that treatment of RAW264.7 cells with these ligands could cause the release of HMGB1 (Fig. 6A). Among these ligands, HSP65 was found to induce the maximum release of HMGB1 (Fig. 6B) after 24 h. This release of HMGB1 was not due to LPS contamination of these components, as levels of endotoxin were found to be lower than 0.005–0.01 ng mg−1 as observed using Limulus amebocyte lysate test (data not shown). This value is much below the toxicity level for the cells cultured in vitro. Purified TLR ligands were not toxic to the cells as less than 2% cytotoxicity was observed using LDH release test during culture of TLR ligands with RAW264.7 cells (Fig. 6C). To further confirm our results, we first treated RAW264.7 cells with TLR antibodies and then cultured them with TLR ligands. As observed in Fig. 6D, the release of HMGB1 by ManLAM and HSP65 was reduced when cells were treated with TLR2 and TLR4 antibodies respectively. This suggests that mycobacterial TLR ligands were also capable of inducing active release of HMGB1 like LPS and viral DNA.
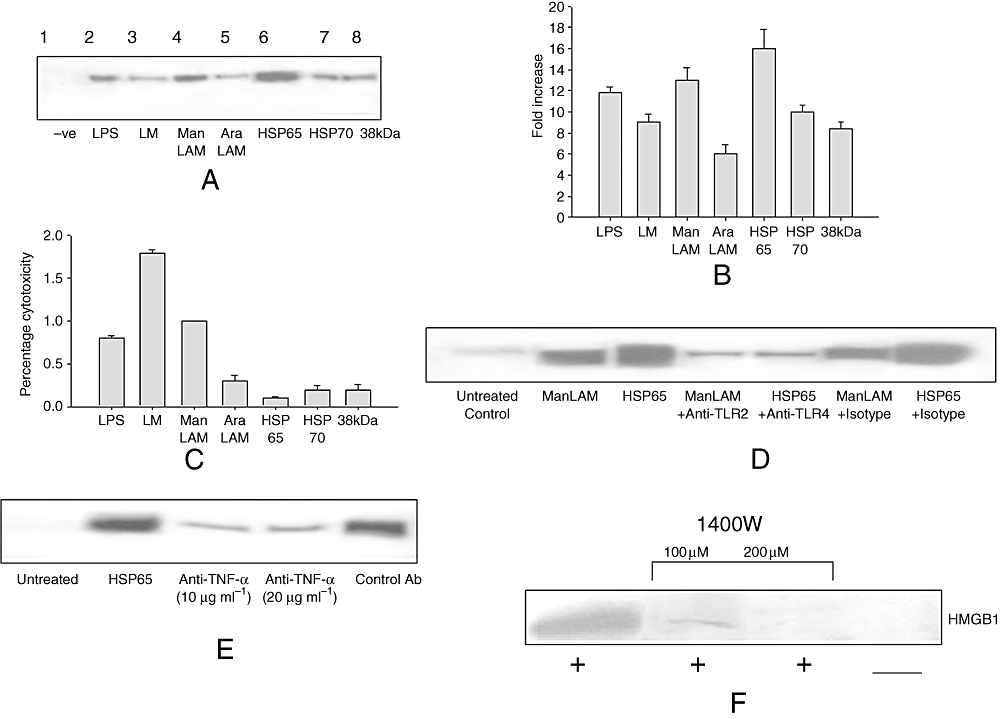
TLR ligands of mycobacteria induce active secretion of HMGB1.A. RAW 264.7 cells were treated with different TLR ligands of mycobacterial cell such as lipomannan (LM), ManLAM, arabinosylated lipoarabinonannan (AraLAM), HSP65, HSP70 and 38 kDa lipoprotein for 24 h. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.B. Densitometric quantification of Western blot shown in A.C. Concentrated cell culture supernatants were subjected to LDH release test. The results expressed as percentage cytotoxicity in comparison with control cells are shown.D. RAW 264.7 cells were treated with neutralizing antibody to TLR2 and TLR4 (R&D systems) receptors and then stimulated with mycobacterial ManLAM and HSP65 respectively. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.E. RAW 264.7 cells were treated with neutralizing antibody to TNF-α and stimulated with mycobacterial HSP65. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.F. RAW 264.7 cells were treated with specific iNOS inhibitor 1400W and stimulated with mycobacterial HSP65. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.
To further investigate the role of pro-inflammatory mediators in HMGB1 release by mycobacterial TLR ligands, we chose HSP65 which induced maximum secretion of HMGB1. Mycobacterial HSP65 protein is known to induce TNF-α and reactive nitrogen intermediates (Bulut et al., 2005); therefore, we investigated the role of TNF-α and nitric oxide in HSP65-induced release of HMGB1. The cells were treated with neutralizing antibody to TNF-α and stimulated with mycobacterial HSP65. As shown in Fig. 6E, the neutralizing antibody to TNF-α inhibited HMGB1 release by RAW 264.7 cells stimulated by HSP65. To evaluate the role of NO in HMGB1 release, the specific iNOS inhibitor 1400W was used. As shown in Fig. 6F, the addition of 1400W in RAW 264.7 cells reduced HMGB1 release by HSP65. 1400W alone did not result in HMGB1 release in RAW 264.7 cells (data not shown).
Inhibition of c-Jun NH2-terminal kinase activation attenuated HMGB1 release induced by HSP65
We further studied the role of signal transduction pathways involved in HSP65-induced HMGB1 release by cells. Because activation of MAPK is required for the production of mediators such as TNF-α, IL-6, IFN-γ and NO, the role of MAPK in the release of HMGB1 was investigated. To demonstrate the inhibition of MAPK activation, RAW 264.7 cells were pre-treated with various specific MAPK inhibitors, followed by stimulation with HSP65 for 24 h. Inhibition of p38 using SB203850 did not inhibit HMGB1 release induced by HSP65 (Fig. 7A). Similarly, other p38-specific inhibitor SB202190 did not reduce HMGB1 release, even at a concentration of 200 nM (Fig. 7B).
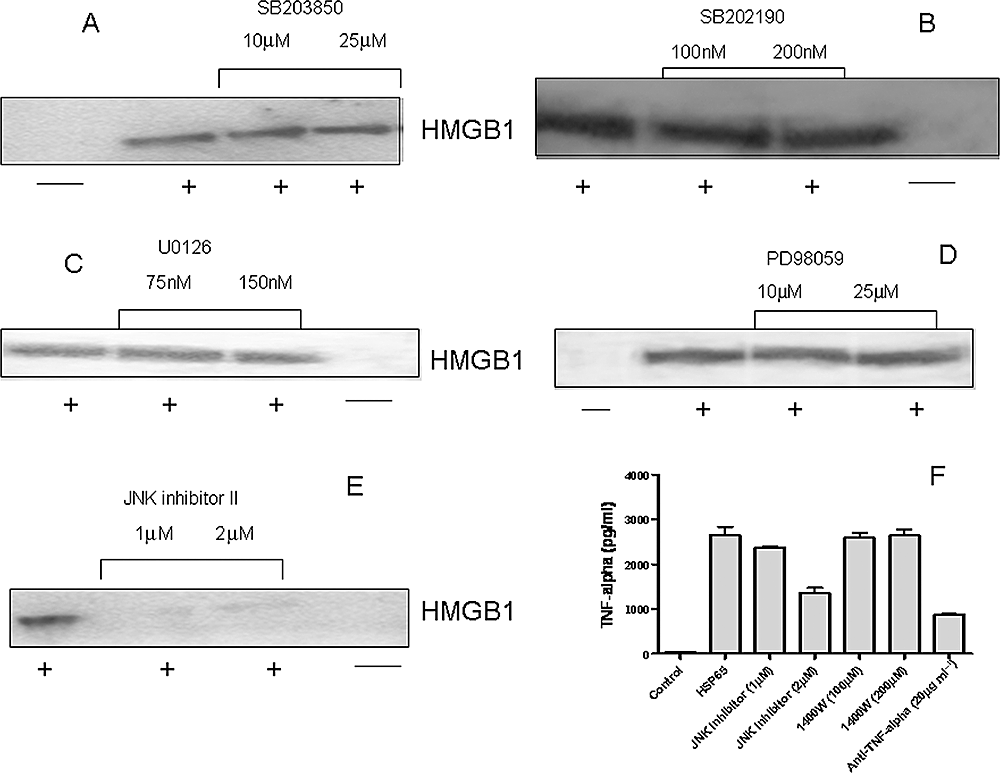
Inhibition of JNK activation attenuated HMGB1 release induced by HSP65.A and B. RAW 264.7 cells were treated with p38-specific inhibitors SB203850 (A) and SB202190 (B) and stimulated with mycobacterial HSP65. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.C and D. RAW 264.7 cells were treated with MEK-specific inhibitors U0126 (C) and PD98059 (D) and stimulated with mycobacterial HSP65. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.E. RAW 264.7 cells were treated with JNK-specific inhibitor and stimulated with mycobacterial HSP65. Concentrated cell culture supernatants were immunoblotted with HMGB1 antibody.F. RAW 264.7 cells in different wells were treated with JNK-specific inhibitor, specific iNOS inhibitor 1400W, neutralizing antibody to TNF-α and stimulated with mycobacterial HSP65 and levels of TNF-α were estimated in culture supernatants using ELISA. The results are responses of culture supernatants from triplicate wells. All results are representative of at least three independent experiments.
To examine the dependence of HMGB1 release on the extracellular signal-regulated kinase (ERK) signalling pathway, specific inhibitors (U0126 and PD98059) of MEK, an upstream activator of ERK1 and ERK2, were used in parallel experiments. U0126, at concentrations up to 150 nM did not affect HSP65-induced HMGB1 release in cultures of RAW 264.7 cells (Fig. 7C). Similarly, PD98059, even at concentrations up to 25 µM, did not affect HSP65-induced HMGB1 release (Fig. 7D), indicating that mycobacterial HSP65 induces HMGB1 release through an ERK1/2-independent mechanism. Inhibition of c-Jun NH2-terminal kinase (JNK) activation using specific inhibitor JNK inhibitor II blocked HMGB1 release induced by HSP65, even at 1 µM concentration (Fig. 7E). To assess the status of TNF-α in relation to HMGB1 secretion pathway, we pre-treated RAW 264.7 with JNK-specific inhibitor and iNOS inhibitor 1400W, stimulated the cells with HSP65 and determined the presence of TNF-α in cell culture supernatants using ELISA (Fig. 7F). JNK-specific inhibitor inhibited the production of TNF-α whereas 1400W did not inhibit the HSP65-induced TNF-α secretion. Inhibition of TNF-α secretion using anti-TNF-α antibody served as positive control. Taken together, our observations suggest that JNK controls the secretion of both HMGB1 and TNF-α induced by mycobacterial HSP65.
Discussion
Recent advances in understanding the cytokine role of HMGB1, a protein thought to be restricted to nuclear and membrane fractions, have placed this protein as a central extracellular mediator in local and systemic responses to necrotic cell death, trauma, sepsis and invasion of pathogens. Different TLR ligands from bacteria and viruses are known to induce release of HMGB1 by cells in vitro (Jiang and Pisetsky, 2006). However, a detailed study on HMGB1 release after bacterial infection is lacking. As mycobacterium is considered to be a most successful pathogen, we focused on the in vitro and in vivo release of HMGB1 during TB. M. tuberculosis, M. bovis BCG and other less virulent species of mycobacteria were found to be capable of inducing secretion of HMGB1 in BMDMs and monocyte cell cultures. Consistent with HMGB1 release associated with LPS exposure (Wang et al., 1999), we did not find any increase in gene expression of HMGB1 after infection with mycobacteria. The release of HMGB1 was found to be dependent on virulence of mycobacterial species. As different species of mycobacteria are known to cause necrotic cell death (Danelishvili et al., 2003) in vitro, HMGB1 release in cultured cell supernatants could be passive in nature. However, we also found that subcellular components of M. tuberculosis, including TLR ligands of bacteria that do not cause cell death in vitro, could also induce release of HMGB1. These results indicate that the HMGB1 release during TB is both passive and active in nature. There is a reciprocal functional relationship between the activities of the early (TNF and IL-1) and late (HMGB1) mediators of inflammatory response (Yang et al., 2005). In this regard, our results also show that HMGB1 can participate in ‘cross-talk’ for the propagation and amplification of downstream pro-inflammatory responses in TB.
Guinea pigs exposed to very small numbers of virulent tubercle bacilli by the respiratory route develop a disease which mimics many of the important features of the pathogenesis of human TB, including the expression of strong protective immunity following vaccination with BCG (Orme et al., 2001). Therefore, we selected the guinea pig model of TB to study HMGB1 release in vivo and the associated inflammatory response. The translocation of HMGB1 protein from the nucleus to the cytoplasm was observed in the BAL cells of infected guinea pigs. These observations indicate that HMGB1 released by lung macrophages could be an important mediator of the inflammatory response, and may help in secretion of pro-inflammatory cytokines (TNF-α and IL-1β) and release of nitric oxide observed during pulmonary TB (Ulrichs and Kaufmann, 2006). The balance between anti-inflammatory (e.g. TGF-β) and pro-inflammatory cytokines regulates the destructive process and inflammation in tissues (Feldmann et al., 1996). We found a greater inflammatory response (HMGB1 and TNF-α) in unvaccinated guinea pigs than in BCG-vaccinated guinea pigs after infection with M. tuberculosis. However, levels of TGF-β mRNA were found to be higher in BCG-treated guinea pigs, indicating an anti-inflammatory response was induced by the protective BCG vaccine.
Pre-clinical trials of candidate TB vaccines in animal models rely on BCG vaccine as a ‘gold standard’ to compare the immunological responses and protective efficacy of a novel TB vaccine (Skeiky and Sadoff, 2006). BCG-vaccinated guinea pigs have approximately 2–3 log10 cfu reduction in their lung and spleen when compared with PBS-treated guinea pigs (Izzo et al., 2005), confirming that BCG is currently the most successful TB vaccine in animal models. An important observation of this study was that BCG vaccination could restrict the release of HMGB1 in BAL cells in guinea pig model of pulmonary TB. BCG-vaccinated guinea pigs have been shown to have fewer granulomatous lesions, absence of central necrosis in lungs and decreased levels of TNF-α as compared with unvaccinated guinea pigs (Izzo et al., 2005; McMurray et al., 2005). As HMGB1 release in the cytoplasm was associated with a greater inflammatory response and necrotic cell death, this may explain our results that HMGB1 was either absent or found to be less concentrated in cytoplasm of BAL cells of BCG-vaccinated guinea pigs. Guinea pigs immunized with M. tuberculosis Ag85B protein-based subunit vaccine were found to have comparable levels of HMGB1 protein in cytoplasm of BAL cells with that of unvaccinated animals (data not shown). In this regard, whether HMGB1 release in cytoplasm of lung cells can be a marker to predict the protective efficacy of a candidate TB vaccine in animal models of TB is at this point still a debatable issue.
High-mobility group box protein 1 has been found to be capable of binding bacterial substances, especially lipids (Rouhiainen et al., 2007), and the mycobacterial cell wall is rich in complex lipids. We used commercially available recombinant HMGB1 produced in E. coli in our experiments and this may explain why we found much less TNF-α and IL-1β activity in J774A.1 cells alone. HMGB1 in combination with M. bovis BCG significantly increased the secretion of these cytokines when compared with BCG alone, suggesting that a combination of M. bovis BCG and HMGB1 may increase the secretion of cytokines by cells in vitro possibly because of interaction of HMGB1 with mycobacterial cell lipids. Binding of HMGB1 to substances derived from microbes might create complexes that upregulate innate immune responses. Several highly charged recombinant proteins, such as heat shock proteins produced in bacterial expression systems (Gao and Tsan, 2003), have been shown to induce immune cell activation through binding to microbe-derived substances. As heat shock proteins have been used as adjuvants for TB vaccines in animal models (Vordermeier et al., 2003), and it may be useful to test HMGB1 as an adjuvant for TB vaccines.
Stimulation of macrophages by TLR ligands leads to upregulation of iNOS expression and production of NO (MacMicking et al., 1997). NO displays a host of activities in inflammatory response, and mycobacterial heat shock proteins are known to induce NO (Bulut et al., 2005). In this study, the kinetics of NO production and HMGB1 release in vitro (i.e. late appearance of both) suggests a role for NO in HSP65-induced HMGB1 release. Further, inhibiting iNOS attenuates HMGB1 release induced by HSP65. It is possible that HMGB1 acts as a downstream effector of NO, with selective inhibition of iNOS attenuating the HMGB1 release. In addition to NO, other mediators induced by mycobacterial heat shock proteins such as TNF-α may induce HMGB1 release. In our study, in agreement with a previous report (Jiang and Pisetsky, 2006), neutralizing M. tuberculosis induced TNF-α-inhibited HMGB1 release from the cells. JNK activation plays an important role in iNOS expression (Chan and Riches, 2001) and mycobacterial components are known to produce nitric oxide by JNK activation (Chan et al., 2001). Although JNK inhibition blocked HMGB1 release induced by HSP65, blocking p38 and ERK activation did not inhibit this process. These results suggest that p38 and ERK1/2 may not be involved in HMGB1 release induced by HSP65; this finding is in agreement with a previous study on HMGB1 release induced by LPS (Chen et al., 2004). Our observation that JNK pathway also controls the secretion of HSP65-induced TNF-α implies that the JNK controls HMGB1 secretion via both nitric oxide and TNF-α-mediated pathway. The attenuation of NO synthesis did not affect the secretion of TNF-α in agreement with a previous study (Tucker et al., 1991), but secretion of HMGB1 was inhibited by nitric oxide inhibitor, indicating that TNF-α and NO activate different signalling cascades in HSP65-induced HMGB1 secretion.
Our results that the HSP65-induced HMGB1 secretion is partly dependent on TNF-α suggest that HMGB1 exert its inflammatory action downstream of TNF-α. This finding is particularly important because the secreted HMGB1 may in turn induce further secretion of TNF-α as shown by our study and others (Gardella et al., 2002), raising the possibility that a pro-inflammatory loop exists between TNF-α and HMGB1. Because HMGB1 lacks a hydrophobic signal sequence, it is secreted via a vesicle-mediated secretory pathway analogous to that used by IL-1β (Gardella et al., 2002). The secretion of IL-1β is also dependent on TNF-α and is mediated via vesicle-mediated secretory pathway (Gardella et al., 2001). As HMGB1 lacks secretory signal sequence and also is secreted by non-classical secretory pathway, there is the possibility that secretion of both HMGB1 and IL-1β is controlled by TNF-α by similar mechanisms.
To conclude, these results indicate that binding of mycobacterial ligands such as HSP65 to TLR4 on macrophages activates JNK and leads to production of NO and TNF-α that further causes the release of HMGB1. HMGB1 is critically required for proper transcriptional control by different transcription factors and thus HMGB1 knockout mice cannot survive because of this reason (Calogero et al., 1999). Our observations could not provide deep insight into relationship between HMGB1 and TB pathogenesis in vivo as a result of non-viability of HMGB1 knockout mice. In addition, HMGB1, being an intracellular protein, cannot be totally inhibited using antibody depletion methods in vivo. Finally, our work establishes the fact that HMGB1 release acts as a signal for the inflammatory response and tissue injury during pulmonary TB. It will be of interest to extend these studies possibly by using conditional knockout mice to determine if there is an effect on the pathology of the infection.
Experimental procedures
Cells and animals
J774A.1 and RAW 264.7 cells were purchased from American Type Culture Collection and cultured in DMEM medium supplemented with 10% FBS. M. tuberculosis H37Rv, M. bovis BCG Pasteur, M. avium and M. scrofulaceum were grown from low-passage seed lots in Proskauer–Beck liquid media containing 0.05% Tween 80 to early mid-log phase. Cultures were aliquoted into 1 ml tubes and frozen at −70°C until used. Mycobacterial cell components were provided by TB Vaccine Testing and Research Materials Contract at Colorado State University. To obtain bone marrow-derived macrophages (BMDM), femurs from C57BL/6 mice (The Charles River Laboratory) were dissected free of connective tissue and flushed with DMEM medium. Bone marrow cells were seeded at 1 × 106 cells ml−1 in the presence of 30% conditioned medium from L929 cells. On day 7 of culture, non-adherent cells were removed by vigorous washing with DMEM medium. The adherent BMDMs were cultured in Opti-MEM (Invitrogen Life Technologies) medium without FBS for experiments. Specific pathogen-free, outbred, Hartley strain guinea pigs (Charles River Breeding Laboratories, Wilmington, MA) were individually housed, fed commercial guinea pig chow and maintained in a temperature and humidity-conditioned environment on a 12 h light/dark cycle. Each animal was randomly assigned to a vaccination treatment group. All protocols for animal use were approved by the Animal Care and Use Committee of the Colorado State University.
Cell culture
J774A.1 and RAW 264.7 cells (3 × 106 cells) were plated in 6-well culture plates in Opti-MEM medium without FBS for 4 h and washed twice with Opti-MEM medium. Cells were then stimulated with M. tuberculosis, M. bovis BCG, M. tuberculosis cell components, M. avium, M. scrofulaceum and mycobacterial components for 24 h, and supernatants were collected for cytokine and HMGB1 assay. Similar experimental conditions were used in experiments with BMDMs.
BCG vaccination and AEROSOL challenge
Guinea pigs were vaccinated with 103 cfu of M. bovis BCG (BCG Pasteur) intradermally. Non-vaccinated guinea pigs received an equal volume of pyrogen-free saline. The guinea pigs were rested for 8 weeks before being infected. A Madison chamber aerosol generation device was used to expose the animals to an aerosol of M. tuberculosis which was calibrated to deliver approximately 20–30 bacilli into the lungs. Guinea pigs from non-vaccinated and BCG-vaccinated groups were euthanized at day 60 after aerosol infection and the lungs were removed aseptically. To assess bacterial loads, individual organ homogenates were plated on nutrient Middlebrook 7H11 Bacto agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.). Bacterial colonies were counted after 2–3 weeks of incubation at 37°C.
Preparation of lung lavage cells
To obtain the BAL cell population, BAL was performed by instilling ice-cold 2% heparin in phosphate-buffered saline (pH 7.4) into the lungs of euthanized guinea pigs. Lavage cells were washed twice in DMEM medium with 10% FBS by centrifugation at 320 g for 10 min at 4°C. After the second wash, the cell pellet was resuspended in DMEM medium supplemented with 2 µM l-glutamine, 100 U of penicillin ml−1 and 10% FBS. Viable BAL cells were enumerated by trypan blue exclusion. An aliquot (70 µl) of the BAL cell population (105 cells) was spun for 5 min at 140 g in a cytospin centrifuge, the slides were air-dried, fixed in 4% paraformaldehyde and stained for immunoflourescence microscopy. Nuclear and cytoplasmic extracts of lung lavage cells were prepared using CeLytic NuCLEAR Extraction Kit (Sigma) according to manufacturer's instructions.
Immunohistochemical analysis
Lungs of guinea pigs were infused with 30% OCT (Tissue-Tek) in PBS through the trachea. Once removed from the pulmonary cavity, lungs were embedded in OCT, frozen in a bath of liquid nitrogen for a few seconds, and then stored at −80°C. Serial sections, 9 µm thick, from each lung, were cut on a cryostat (Leica; CM 1850) using the Instrumedics tape transfer system, fixed in cold acetone, and air-dried. The sections were washed, and unspecific Ab binding was blocked with 3% BSA-PBS solution. Thereafter, the sections were incubated overnight at 4°C with HMGB1 or Isotyoe control antibody. All sections were washed three times in PBS and incubated with the secondary detection antibody conjugated to HRP (Jackson Immunoresearch). Finally, the reaction was developed using aminoethylcarbazole (BioGenex) as substrate. The sections were counterstained with Meyer's hematoxylin and thereafter mounted with crystal/mount (Biomedia).
Biochemical and immunochemical assays
The levels of TNF-α and IL-1β in supernatants were measured from the cultured J774A.1 cells using OptEIA ELISA kits (BD Biosciences), and assays were performed in accordance with manufacturer's instructions. For Western blotting of HMGB1, supernatants of cultured cells collected after 20–24 h of culture, lavage fluids, nuclear and cytoplasmic extracts were concentrated using Amicon ultra-4 centrifugal filter (Millipore) as described previously (Rendon-Mitchell et al., 2003). Protein concentration of concentrated supernatants, lavage fluids, nuclear and cytoplasmic extracts was determined by the BCA method (Pierce BCA kit) to ensure equal loading, and 25 µg of each sample was resolved on 12% Tris-SDS polyacrylamide gel. Protein was transferred to nitrocellular membranes, blocked with 5% bovine serum albumin in PBS-Tween, and blotted with mouse anti-HMGB1 monoclonal antibody (Sigma). The membrane was then incubated with HRP-conjugated anti-mouse IgG (Jacksonimmuno Research), and developed using ECL chemiluminiscence kit (Pierce). The relative levels of HMGB1 in culture supernatants were quantified using Quantity One software (Bio-rad) after capturing Western blot images by a charge-coupled device camera.
RNA isolation and RT-PCR
Total RNA was isolated from J774A.1 cells using the Trizol Method (Invitrogen) as per manufacturer's instructions. To the RNA, 4 µl of a 10 mM dNTP mix (Invitrogen), 2 µl of 10× first-strand buffer (500 mM Tris–HCl, pH 8.3, 750 mM KCl, 30 mM MgCl2, 50 mM dithiothreitol), 2 µl of Random Decamer primers (50 µM), 100 U Moloney Murine Leukemia Virus–Reverse Transcriptase (100 U ml−1, Invitrogen) and nuclease-free water were added to yield a final reaction volume of 25 µl. Reaction mixtures were incubated at 42°C for 1 h and at 95°C for 10 min to inactivate the reverse transcriptase. cDNA (5 ng) was amplified with a standard reaction mix containing 1× PCR buffer (10 mM Tris–HCl, pH 8.3, 50 mM KCl), 1.5 mM MgCl2, 0.125 mM each dNTP, 0.4 µM HMGB1 primers and 1.25 U AmpliTaq Gold DNA polymerase (5 U ml−1) (Applied Biosystems). Nuclease-free water was added to yield the final reaction volume of 25 µl. A 648 bp open reading frame of HMGB1 was amplified using HMGB1 primers described earlier (Wang et al., 1999).
Measurement of LDH release from J774A. 1 cell lines
Estimation of necrosis in cultured cells was determined by measurement of LDH release using the CytoTox96 non-radioactive cytotoxicity assay (Promega). Background release of LDH was obtained with untreated control cells, and maximum release of LDH with lysed uninfected cells. Percentage cytotoxicity was calculated by the formula: [release of LDH from infected cells (OD490)/maximum LDH release (OD490)] × 100.
Immunofluorescence microscopy
J774A.1 cells (1 × 103 cells) were cultured in 8-well chamber slides (Nunc) and stimulated with M. bovis BCG for 18–20 h. The cells were fixed with paraformaldehyde and washed three times with PBS. J774A.1 cells and guinea pig lung lavage cytospin cells were permeabilized with 0.05% Triton X-1000 solution for 60 min at room temperature. The cells were then incubated with mouse anti-HMGB1 monoclonal antibody (Sigma) for 60 min at room temperature and again washed three times with PBS. After washing, the cells were incubated with FITC-conjugated anti-mouse IgG1 antibody/Alexa-fluor 488 conjugated anti-mouse IgG1 (Invitrogen) for 60 min at room temperature in dark. Finally, the cells were washed with PBS and mounted with 3:1 Vectashield : DAPI (Vector Laboratories) and photographed with a cooled Princeton change-coupled device camera mounted on an Olympus BX-60 fluorescence microscope.
Real-time polymerase chain reaction
Total RNA was isolated from guinea pig lung homogenates using the Trizol Method (Invitrogen) as per manufacturer's instructions. The cDNA was prepared by reverse transcription and RT-PCR, performed by using SYBR Green I double-stranded DNA binding dye (Applied Biosystems). The real-time primers used for guinea pig IFN-γ, TNF-α, TGF-β1, IL-1β and iNOS were published earlier (Allen and McMurray, 2003). The level of induction of mRNA was determined from the threshold cycle values normalized to the β-actin gene and then normalized to the value derived from healthy TB-free animals.
Statistical analysis
Two-way comparison between test and control group was performed using Student's t-test. The data are given as means ± standard error of the mean.
Acknowledgements
This work was supported by the NIH AI40091 Funding (A.A.I.).




