The Vibrio cholerae cytolysin promotes activation of mast cell (T helper 2) cytokine production
Summary
Many strains of Vibrio cholerae produce a cytolysin (VCC) that forms oligomeric transmembrane pores responsible for vacuolization of several cell types in culture. Here we suggest that VCC could contribute to the T helper 2 (Th2) response seen in the natural infection; acting through TLR2, VCC enhances mast cells secretion of IL-4, IL-6 and TNF-α by 330-, 290- and 550-fold respectively. Moreover, VCC-induced cytokine production is dependent on increased cytosolic Ca2+ and on the presence of the Src family kinases Lyn and Fyn, known to be required for FcεRI-dependent activation of mast cells. These findings strongly suggest that VCC has a pro-inflammatory activity promoting a Th2-type immune profile.
Introduction
Cholera has long been considered a classic paradigm of a non-inflammatory toxigenic diarrhoea until Vibrio cholerae infection was found to induce a T helper 2 (Th2)-type of immune profile and the recruitment of inflammatory cells in the intestinal mucosa (Mathan et al., 1995; Qadri et al., 2000). More recently, a significant accumulation of mucosal mast cells in the crypt and villus lamina propria of adult cholera patients has been reported (Qadri et al., 2004).
Enteropathogenic V. cholerae can elaborate different exotoxins: cholera toxin (CT), zonula occludens toxin and a membrane-damaging toxin, referred to as haemolysin or V. cholerae cytolysin (VCC). VCC is a water-soluble toxin secreted as a 79 kDa inactive pro-haemolysin (Alm et al., 1988; Yamamoto et al., 1990), which is proteolytically cleaved within its N-terminal part (Nagamune et al., 1997) to generate the mature toxin of 63 kDa. In cholesterol- and ceramides-rich membranes (Zitzer et al., 1999) VCC forms heptameric channels, with a moderate anion preference, responsible for vacuolization and eventual lysis of several cell types in culture (Coelho et al., 2000; Figueroa-Arredondo et al., 2001; Moschioni et al., 2002; Pantano and Montecucco, 2006). VCC is believed to contribute to the development of cholera diarrhoea (Hichinose et al., 1987).
Cholera toxin affects several steps in the induction of a mucosal immune response, which alone or in combination might explain its strong adjuvant action after oral immunization. It has been claimed that CT primarily induces Th2 type immune responses characterized by CD4+ T cells producing IL-4, IL-5, IL-6 and IL-10 and by the production of IgA, IgG1 and IgE antibodies (Holmgren et al., 2003). The immune modulatory property of CT is primarily directed to APC cells which are induced to mature and efficiently present to T lymphocytes, whereas no strong evidence is reported on a possible role in activating mast cells: indeed, just one report described the ability of CT in inducing peritoneal mast cells to release IL-6 (Leal-Berumen et al., 1996).
Considering the recent demonstration of the abundance of mast cells in cholera patients and on the basis that no other bacterial factors have been identified as immune modulator so far, we decided to address the possibility that VCC could be such a factor, by evaluating its ability in activating mucosal mast cells. Here we show that VCC stimulates mast cells to produce IL-4, IL-5 and IL-6, cytokines documented to direct Th2 development (Mowen and Glimcher, 2004); this effect depends on a cytosolic calcium increase and on the two Src kinases Fyn and Lyn. Taken together, these results support the idea that VCC may play a major role in the induction of the Th2 response seen upon V. cholerae infection, by acting on mast cells.
Results
Recent reports indicate increased levels of inflammatory mediators in patients infected by V. cholerae that occur in parallel with recruitment of innate immune cells, including mast cells and neutrophils, in the intestinal mucosa (Qadri et al., 2004). In addition to CT (Leal-Berumen et al., 1996; Rocha et al., 2003), other bacterial factors are likely to be involved. In order to assess whether VCC is one of these factors, we examined its effect on mouse bone marrow-derived mast cells (BMMCs) (Gilead et al., 1990). As shown in Table 1, treatment of BMMCs with 500 pm VCC for 4 h led to the production and secretion of substantial amounts of IL-4, IL-5, IL-6 and TNF-α. VCC may induce cell lysis at concentration < 100 pm (Moschioni et al., 2002); however, we found no effect on cell viability under the present experimental conditions (data not shown).
| Treatment | IL-4 (pg/106 cells) (mean ± SEM) | IL-5 (pg/106 cells) (mean ± SEM) | IL-6 (pg/106 cells) (mean ± SEM) | TNF-α (pg/106 cells) (mean ± SEM) |
|---|---|---|---|---|
| Saline | 0.141 ± 0.040 | 0.349 ± 0.134 | 3.559 ± 0.885 | 1.273 ± 0.551 |
| VCC | 46.466 ± 9.467*** | 1.101 ± 0.174** | 1055.2 ± 188.9*** | 693 ± 85.586*** |
- Bone marrow-derived mast cells were incubated for 4 h with 500 pm VCC and cytokine secretion was measured in the culture supernatants with Bio-Plex™ cytokine assay. Data represent the average of three independent experiments with triplicate samples. Significance of VCC-treated versus control was determined by Student's t-test (** P < 0.005;*** P < 0.0005).
The above findings suggested that VCC treatment caused mast cell activation. IgE-dependent activation of both human and rodent mast cells is characterized by an influx of extracellular Ca2+, which is essential for subsequent release of both preformed (granule-derived) mediators and newly generated eicosanoids and cytokines (Bradding and Conley, 2002). Moreover, a similar toxin, the cytotoxin VacA of Helicobacter pylori, stimulates mast cells to produce and secrete cytokines following the induction of cytosolic Ca2+ increase (Molinari et al., 1998; de Bernard et al., 2005). Figure 1 shows that 500 pm VCC induces a rapid increase in cytosolic Ca2+ concentration in Fura2-AM-loaded BMMCs. The bimodal response suggested that VCC might induce Ca2+ release from intracellular stores followed by a more sustained ion influx possibly through store-operated Ca2+ channels (SOCs). In mast cells, the release of Ca2+ from intracellular stores is primarily controlled by endoplasmic reticulum (ER) inositol 1,4,5-trisphosphate receptors which deplete intracellular stores and activate Ca2+ influx through plasma membrane channels (capacitative entry) (Rivera and Gilfillan, 2006). To test whether VCC triggered this signalling pathway, VCC-treated cells were incubated with the Ca2+ chelator EGTA or with the PLCγ inhibitor U73122, which prevents IP3 synthesis. As shown in Fig. 2A, the presence of EGTA in the extracellular medium impaired the late phase increase of cytosolic Ca2+, but did not abolish the initial release of Ca2+ from intracellular stores. In contrast, U73122 inhibited the initial release of Ca2+ from intracellular stores (Fig. 2B). These results support the hypothesis that VCC-induced Ca2+ mobilization involves both intracellular stores and capacitative currents.
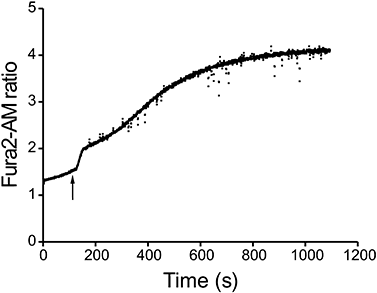
Cytosolic Ca2+ increase induced by VCC. BMMCs were loaded with Fura2-AM, and the fluorescence ratio (340 nm/380 nm) was measured with a dual-wavelength modular fluorimeter as detailed in Experimental procedures. Arrow indicates the VCC addition (final concentration 500 pm). The experiment shown is representative of at least five individual experiments.
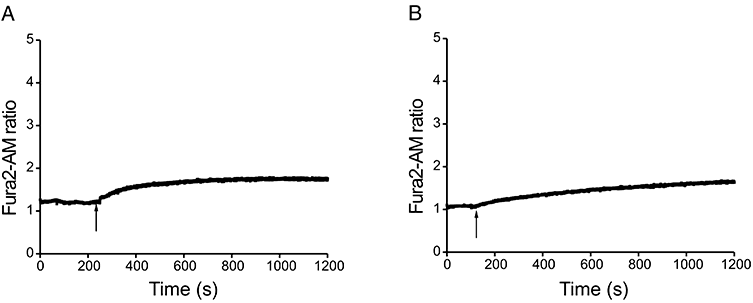
Pharmacological characterization of the Ca2+ response. BMMCs, loaded with Fura2-AM, were either incubated with 5 mM EGTA just before starting the fluorescence recording (A), or pre-incubated for 10 min at 37°C with 30 μm U73122 (B). Arrows indicate VCC addition (final concentration 500 pm). Each panel shows one representative experiment from a minimum of three.
To test whether the intracellular Ca2+ increase evoked by VCC is involved in cytokine synthesis and secretion, mast cells were treated with the intracellular Ca2+ chelator BAPTA-AM (10 μm) before stimulation with VCC. As shown in Fig. 3, the chelation of intracellular Ca2+ almost completely abolished the VCC-induced cytokine secretion. Same results were obtained when the experiment was carried on in low-Ca2+ medium, thus permitting to exclude that the effects observed with BAPTA-AM would be a consequence of the toxicity of the chelator by-products. Similarly, a marked decrease was also observed after the inhibition of IP3 synthesis with U73122 (Fig. 3).
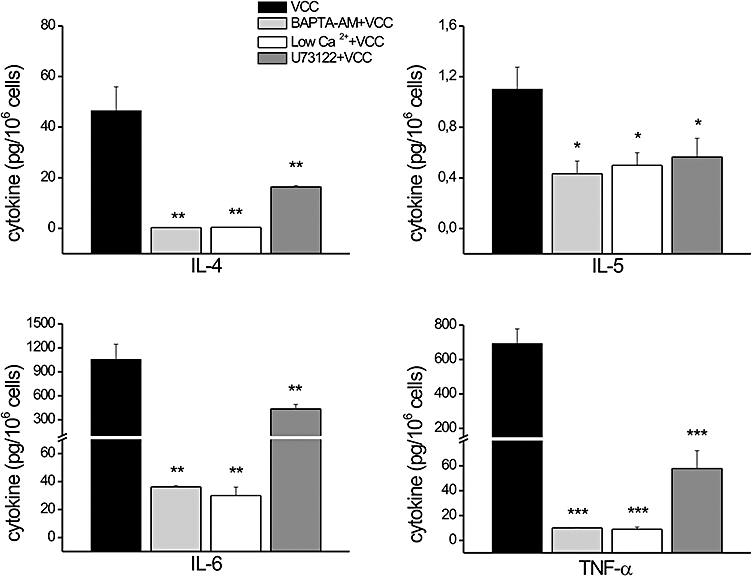
Role of Ca2+ on VCC-induced cytokine release. BMMCs were incubated for 4 h with 500 pm VCC and cytokines secretion was measured in the culture supernatants with Bio-Plex™ cytokine assay. BAPTA-AM (10 μm) and U73122 (30 μm) were pre-administrated for 30 min and 10 min, respectively, before adding the toxin. Data represent the average of three independent experiments with triplicate samples. Significance was determined by Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005, versus VCC).
Cells were harvested after 30 min and processed for determination of cytokine mRNA for IL-4, IL-5, TNF-α and IL-6. Compared with cells treated only with VCC, the expression of cytokine mRNA decreased dramatically after BAPTA-AM treatment or in low Ca2+ conditions, and was also impaired by treatment with the PLCγ inhibitor U73122 (data not shown). Intracellular calcium increase is essential for subsequent release of both preformed (granule-derived) mediators and newly generated cytokines. However, when we tested whether VCC administration triggered granule exocytosis, we did not observe any β-hexosaminidase release (data not shown).
The flow of ions, such as Cl-, is likely to play an important role in mast cell activation through their effects on membrane potential and therefore Ca2+ influx. SOCs, that carry Ca2+ into cells, conduct larger currents at negative membrane potentials (Hoth and Penner, 1992). In order to determine whether Cl- flux was required for VCC-induced BMMC activation and cytokine release, we monitored cells pretreated with DIDS (100 μm) before the exposure to VCC. As illustrated in Fig. 4, cytokine release was markedly reduced; concomitantly mRNA production was impaired as well (data not shown).
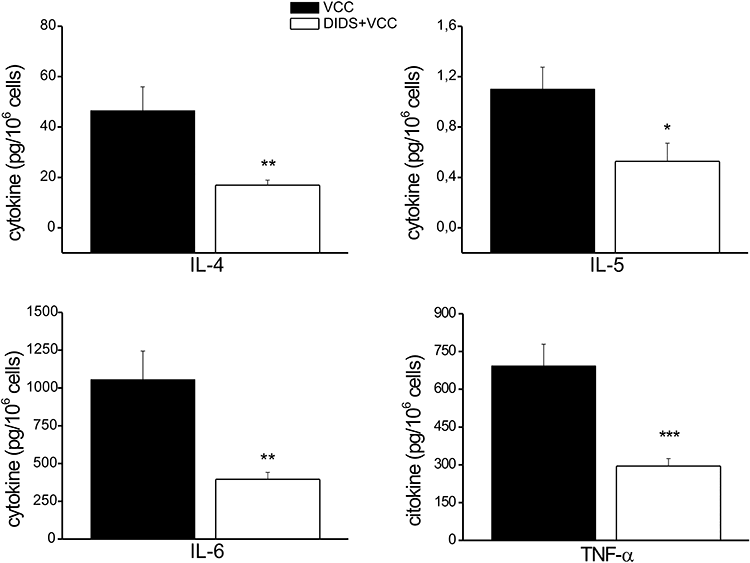
Effect of the chloride channel inhibitor on VCC-induced cytokine release. BMMCs were pre-incubated or not with 100 μm DIDS for 50 min before being exposed to 500 pm VCC. After 4 h of incubation cytokines secretion was measured in the culture supernatants with Bio-Plex™ cytokine assay. Data represent the average of four independent experiments with triplicate samples. Significance was determined by Student's t-test (*P < 0.05; **P < 0.005; ***P < 0.0005, versus VCC).
The VCC-induced Ca2+ mobilization from intracellular stores in an IP3-dependent manner, suggested that its mast cell activation is, at least in part, a consequence of a membrane receptor engagement. Thus, once the receptor is activated, phospholipase C would generate IP3 and the Ca2+ released from intracellular stores would trigger extracellular Ca2+ entry, sustained by chloride influx.
To study the issue of VCC-mediated Ca2+ fluxes as dependent on receptor engagement, we took the approach of determining if known early mast cell signalling events were required for the VCC-induced cytokine responses. Drugs that increase the intracellular Ca2+ concentrations by transiting Ca2+ across the plasma membrane, like ionomycin or the Ca2+ ionophore A23187, can induce expression of cytokine genes in a manner independent from early signalling events (Plaut et al., 1989). Thus, a requirement for the Src family protein tyrosine kinases Fyn or Lyn would provide a strong argument for a receptor-mediated event; indeed, these kinases are key regulators of phosphatidylinositides and Ca2+ responses in BMMCs activated through the high affinity IgE receptor (FcεRI) (Blank and Rivera, 2004). As shown in Fig. 5, the absence of Lyn in BMMCs caused a marked impairment of VCC-mediated cytokine production, at variance from previous reports showing that a IgE-dependent stimulation of Lyn-deficient BMMCs resulted in an unaltered or enhanced cytokine response relative to wild-type cells (Nishizumi and Yamamoto, 1997; Kawakami et al., 2000; Hernandez-Hansen et al., 2005). Additionally, the absence of Fyn showed an even more dramatic impairment of VCC-mediated cytokine release from these cells. A reduction in the VCC-induced cytokine mRNAs in both mutant cells, with respect to the wild-type cells, was also observed (data not shown). Impaired cytokine release from Fyn-deficient BMMCs was also previously observed after stimulation via FcεRI (Gomez et al., 2005). While Fyn and Lyn were suggested to have a predominant positive and negative regulatory role, respectively, in mast cell effector responses (Parravicini et al., 2002; Odom et al., 2004), a recent report has demonstrated a cooperative role between Fyn and Lyn in regulating the activation of sphingosine kinases (Olivera et al., 2006). Thus the results shown in Fig. 5 identify another point of cooperation for these two kinases in VCC-mediated cytokine responses. The requirement for both Fyn and Lyn in VCC-mediated BMMC cytokine production supports the requirement of early signalling events that impact on both phosphatidylinositol metabolism and Ca2+ responses and suggests a receptor-mediated activation by VCC.
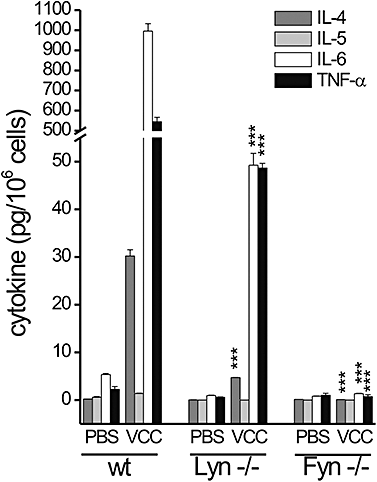
Cytokine profile of BMMCs from Lyn−/− and Fyn−/− mice after VCC treatment. BMMCs purified from wild-type and Lyn- or Fyn-null mice, were incubated for 4 h with or without 500 pm VCC. Cytokines secretion was measured in the culture supernatants with Bio-Plex™ cytokine assay. The data shown are from five experiments conducted with different cell cultures derived from at least three different mice for each genotype. Significance of mutant versus wild-type BMMCs was determined by Student's t-test (***P < 0.0005).
The presence of Toll-like receptors (TLRs) on mast cells (Marshall, 2004) and the recent demonstration of a link between Src kinases and TLRs (Chang et al., 2004; Chun and Prince, 2006; Kannan et al., 2006), prompted us to investigate the possibility that VCC activated mast cells by acting as agonist of a TLR. In order to address this point we used human embryonic kidney (HEK) 293 cells transfected with plasmids encoding distinct human TLRs. HEK293 cell lines lack expression of endogenous TLRs, although their TLR signalling machinery is fully functional. The common pathway leading to NF-κB activation requires the phosphorylation and degradation of the cytosolic inhibitor of NF-κB, IκB-α. The engagement of a specific TLR, expressed on HEK293, was monitored by evaluating the phosphorylation of IκB-α after exposure to VCC. As shown in Fig. 6 phosphorylation of IκB-α was observed only in cells expressing TLR2, whereas no activation was observed in cells expressing either TLR3 or TLR4. These results, suggesting that VCC is an agonist of TLR2, were confirmed by the cytokine response observed in BMMCs derived from TLR2 KO mice: Table 2 clearly shows that the absence of the receptor results in the complete abrogation of the VCC-induced cytokine mRNA.
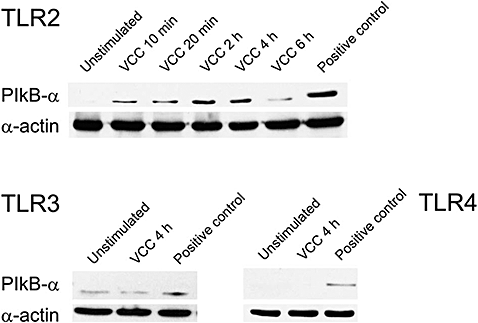
VCC-induced phosphorylation of IκBα in HEK293 cells transfected with plasmid encoding distinct human TLRs. HEK293 cells expressing the indicated TLR were incubated for different times either with VCC or with the specific agonists (indicated as positive control). After different times, cell lysates were subjected to SDS-PAGE immunoblotting with anti-phospho IκBα. Blot with α-actin antibody was used as a control for equal loading.
| IL-4 (AU) (mean ± SEM) | IL-5 (AU) (mean ± SEM) | IL-6 (AU) (mean ± SEM) | TNF-α (AU) (mean ± SEM) | ||||
|---|---|---|---|---|---|---|---|
| wt | tlr2 −/− | wt | tlr2 −/− | wt | tlr2 −/− | wt | tlr2 −/− |
| 41.55 ± 0.58 | 1.49 ± 0.02*** | 6.27 ± 0.34 | 1.04 ± 0.06* | 19.88 ± 0.19 | 1.14 ± 0.02** | 18.51 ± 0.94 | 5.00 ± 0.19*** |
- Bone marrow-derived mast cells were incubated for 30 min with 500 pm VCC and cytokine mRNA expression was evaluated by real-time PCR analysis. Data represent the average of three independent experiments with triplicate samples. Significance of VCC-treated tlr2-/−BMMCs versus VCC-treated wt BMMCs was determined by Student's t-test ( * P < 0.05;** P < 0.005;*** P < 0.0005).
Discussion
This study addressed the possibility that VCC has a role in promoting the inflammation associated with the V. cholerae infection. VCC was found to efficiently induce BMMCs to produce IL-4, IL-5 and IL-6, all cytokines that can contribute to the Th2 response observed in the natural infection (Marinaro et al., 1995; Qadri et al., 2000). The fact that the released amount of IL-5 was minimal, when compared with that of the other cytokines, is in agreement with the evidence that murine mast cells produce small amount of this cytokine with respect to the human counterpart (Bischoff, 2007). Moreover, no Th-1 polarizing cytokines, such as IL-12, were found to be induced by the toxin (data not shown). VCC was able not only to stimulate the secretion of cytokines but also to increase their mRNA level, in a Ca2+-dependent manner. The fact that the prevention of the IP3 synthesis did not completely abrogate the cytokine release might be a reflection of the triggering of other TLR2-elicited signals, such as MyD88/IRAK, which less depend on calcium. Accordingly, there is reported evidence on TLR9 that the MyD88 and Src kinases pathways are distinct but cooperate to achieve full receptor function (Sanjuan et al., 2006). The rise in intracellular Ca2+, which occurred within a few seconds after VCC administration, showed a bimodal behaviour: the first phase of VCC-induced Ca2+ increase was attributable to the ion release from ER (in a PLCγ-dependent manner) and the second phase to the entry of extracellular Ca2+. Although cytosolic Ca2+ increase is expected to trigger mast cell degranulation, we did not observe any appreciable β-hexosaminidase release after VCC administration (data not shown); this is in agreement with recent evidence showing that mast cells can discriminate between the signals required for cytokine production and those required for the exocytosis of preformed granules (Rivera, 2006). The inhibitory effect on cytokine synthesis and release following DIDS treatment can be interpreted by considering that SOCs, which carry Ca2+ into cells, conduct larger currents at negative membrane potentials (Hoth and Penner, 1992). Thus, the inhibition of the plasma membrane Cl- channels would limit the massive entry of Ca2+ required for the activation of the mast cells. Obviously, we cannot exclude that VCC might also contribute to the Cl- entry by forming pores on the BMMC membranes and the addition of DIDS would inhibit this contribution. However, our finding that VCC can activate BMMCs through the engagement of a receptor strongly supports a pore-independent SOCs-mediated mechanism. Accordingly, a key finding of this study is that VCC-induced cytokine response is completely abrogated in tlr2−/− BMMCs suggesting the essential role of the TLR2 in the cytolysin-mediated mast cells activation. The link between TLRs and Src family kinase is also reasonably well established in various systems; for example, it was recently demonstrated, in airway epithelial cells, that in response to bacterial ligands, c-Src initiates TLR2-associated signalling, followed by recruitment of phosphatidil inositol 3 kinase (PI3K) and PLCγ to affect the release of Ca2+ from intracellular stores. The latter is important for the downstream activation of pro-inflammatory genes transcription (Chun and Prince, 2006). In mast cells, Fyn has been demonstrated to be important for de novo production of various cytokines (such as IL-4, IL-6 and TNF-α) after FcεRI activation; this Src kinase is also essential for IκB-α phosphorylation and degradation, events required for NF-κB nuclear translocation and gene transcription (Gomez et al., 2005). Therefore, we propose that both Fyn and Lyn are activated upon the engagement of the TLR2 by VCC, a model similar to that of antigen receptors, which merits further investigation. Moreover, while the link between Lyn and TLR2 has already been demonstrated (Kannan et al., 2006), we provide here the first evidence for the role of Fyn and its downstream signalling events in VCC-mediated TLR2 activation.
In summary, our report provides new insights into the V. cholerae-induced inflammation by showing the relevance of another virulence factor in promoting Th2 responses besides CT. We are aware of the fact that mast cells are quite heterogeneous, and that BMMCs are not identical to mice jejunum mast cells and even less to human intestinal mast cells; thus, it remains to be determined if an in vivo differentiated population of mast cells responds to VCC similarly to BMMCs.
Experimental procedures
Reagents
Vibrio cholerae cytolysin was purified from culture supernatants of V. cholerae O1 El Tor 8731 (Hall and Drasar, 1990). The cleaved and active form of VCC was obtained by ethanol precipitation (final concentration, 40%), preparative isoelectric focusing in a sucrose density gradient, and hydroxyapatite chromatography (Zitzer et al., 1997; 1999). Cell culture media, FBS and HBSS buffer were from Invitrogen/Gibco. Fura2-AM and pluronic acid were from Molecular Probes; Sulfinpyrazone, U73122, EGTA, 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (disodium salt) (DIDS), were from Sigma. BAPTA-AM was from Calbiochem.
Generation of BMMCs
Mice fyn−/− and lyn−/− were obtained as described (Odom et al., 2004); mice tlr2−/− (Takeuchi et al., 1999) were a kind gift of Professor Zychlinsky. Bone marrow was isolated from 5- to 6-week-old wild-type and gene-disrupted mice as previously described (Saitoh et al., 2000). BMMCs were grown in RPMI media supplemented with FBS, stem cell factor (SCF), and IL-3 as previously described (Razin et al., 1984; Saitoh et al., 2000; Odom et al., 2004). For BMMC cultures, FcεRI receptor expression was monitored weekly as previously described (Saitoh et al., 2000) and cells were used for experiments when > 95% of the population were FcεRI+. All the experiments were carried on in complete RPMI, as above, without SCF and IL-3.
Intracellular Ca2+ measurement
A total of 106 BMMCs were incubated with 5 μm Fura-2 acetylmethyl ester (Fura2-AM), 250 μm sulfinpyrazone and 32 μm pluronic acid in HBSS for 40 min at room temperature before Ca2+ measurements. Cells, washed and suspended in 1.6 ml HBSS, were transferred to a cuvette. Measurements of the Fura-2 fluorescence were performed in a RF-5301PC spectrofluorophotometer (Shimadzu, Germany) equipped with a thermostat controlled cell holder and a stirring device. When indicated, immediately before addition of VCC (500 pm final concentration), 5 mM EGTA was added to chelate extracellular Ca2+. In the case of U73122 treatment, cells were pre-incubated for 10 min with the drug (30 μm) at 37°C before starting fluorescence recording. In all experiments, toxin was added directly to the cuvette. Cells were excited at λ 340 and 380 and emitted light was collected using a 520 nm band-pass filter. Data are expressed as fluorimetric ratios considering that available calibration procedures of the cytosolic [Ca2+] provide very different values.
Detection of cytokines in culture supernatants
Supernatants were collected 4 h after the incubation with the stimuli and the amount of IL-4, IL-5, IL-6, TNF-α and IL-12 protein was quantified by Bioplex Cytokine Assays (Bio-Rad Laboratories), according to the manufacturer's protocol. For BAPTA-AM, U73122 and DIDS treatment, 106 cells ml−1 were pre-incubated at 37°C for 30 min, 10 min and 50 min, respectively, before VCC intoxication. In the case of low Ca2+ condition, VCC intoxication was performed in RPMI plus 5 mM EGTA. Control cells were incubated with PBS (indicated as saline in Table 1).
Real-time PCR analysis
Total RNA was isolated from 2 × 106 cells using SV Total RNA Isolation System (Promega Corporation) according to the manufacturer's instructions. RNA was reverse-transcribed and amplified with the following primers: 5′-GTCATCCTGCTCTTCTTTCTCG-3′ and 5′-TGTGGTGTTCTTCGTTGCTGTG-3′ for IL-4; 5′-AAGAGAAGTGTGGCGAGGAG-3′ and 5′-CAGTTTTGTGGGGTTTTTGC-3′ for IL-5; 5′-TTCTGCAAGTGCATCATCGT-3′ and 5′-CCGGAGAGGAGACTTCACAG-3′ for IL-6; 5′-TACTGAACTTCGGGGTGATCGGTCC-3′ and 5′-CAGCCTTGTCCCTTGAAGAGAACC-3′ or TNF-α; 5′-GATTACTGCTCTGGCTCCTA-3′ and 5′-TCGTACTCCTGCTTGCTGAT-3′ for β-actin. After amplification, data analysis was performed using the second derivative method algorithm. For each sample the amount of cytokine mRNA was expressed as n-fold of the normalized amount of mRNA from untreated cells [1 AU = mRNA cytokine concentration (fmol µl−1)/mRNA β-actin (fmol µl−1)].
MTS assay
MTS assay was done in 96-well plates using a CellTiter 96® kit (Promega). Briefly, 105 cells were plated in 96-well plates the day before experiment. At the end of treatment, culture medium was removed and replaced by 100 μl of complete medium without phenol red plus 20 μl of MTS tetrazolium to each well. During an incubation period of 1.5 h at 37°C, the MTS salt is metabolically reduced only by viable cells into an insoluble coloured formazan; the absorbance/optical density was read and recorded by a microplate reader at wavelength of 485 nm. Values are expressed as per cent of viable cells compared with untreated cells, considered as 100%. This assay was performed in parallel with all the cell experiments to exclude any artefact resulting from differences in cell viability.
Assay of β-hexosaminidase release
A total of 2 × 106 BMMCs ml−1 were incubated for 1 h at 37°C in Hepes-Tyrode buffer (137 mM NaCl, 5.6 mM glucose, 5 mM KCl, 0.5 mM NaH2PO4, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, 0.1% BSA, pH 7.4) with VCC (500 pm). After collecting the supernatants, cells were dissolved in 0.2% Triton X-100 in PBS. The β-hexosaminidase content of supernatants and cells was determined as described (Schwartz et al., 1979) and the net percentage of the β-hexosaminidase released was calculated as it follows: β-hexosaminidase in supernatant/(β-hexosaminidase in supernatant + β-hexosaminidase in cells) × 100.
TLR screening
Human embryonic kidney 293 cells constitutively expressing TLR2/CD14 (HEK293-TLR2/CD14), or TLR3 (HEK293-TLR3) or TLR4/CD14/mD2 (HEK293-TLR4/CD14/mD2) (Invivogen, San Diego, CA, USA) were grown in low-glucose Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal calf serum. Before the experiments, HEK293 cells were plated in 24-well tissue culture plates at a density of 5 × 105 cells ml−1. Adherent cells were collected at various times after the addition of 25 μg ml−1 Poly (I:C) (as positive control ligand of TLR3) or 100 ng ml−1 lipopolysaccharide (as positive control ligand of TLR4) or 1 μmHelicobacter pylori HP-NAP (as positive control ligand of TLR2) (Amedei et al., 2006) or 500 pm VCC. Monolayers were washed with ice-cold PBS. Cells were lysed in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100 and 1 μg ml−1 each of sodium ortovanadate, PMSF, leupeptin, pepstatin and aprotinin) by incubation on ice for 5 min. Lysates were cleared by centrifugation and proteins were separated in SDS-PAGE and transferred to nitrocellulose membranes; after being blocked with TBS-T (150 mM NaCl, 50 mM Tris-HCl pH 7.4, 0.02% Tween-20) supplemented with 3% milk, membranes were blotted with anti-phospho-IκBα antibody (Cell Signaling Technology, Danvers, MA, USA). After stripping blots were re-probed with an anti-α actin antibody (Amersham Pharmacia Italia, Milan, Italy) used as a control for equal loading.
Statistical analyses
Data are means ± SEM. Student's t-test was used for statistical analysis of differences between experimental groups. A P-value equal or below 0.05 was defined as a significant difference.
Acknowledgements
This work was supported by the following grants: AIRC Regional grant proposal 2005 Veneto to C.M.; the Italian Ministry of University and Research (MIUR) to M.d.B and to C.P.; ASI, OSMA, Ministero della Sanità Ricerca Finalizzata 2004 to C.P.; AIRC to C.T.B. The research of S.O. and J.R. was supported by the Intramural Research Program of the National Institute of Arthritis and Musculskeletal and Skin Diseases of the National Institutes of Health. The authors wish to thank Dr M.M. D'Elios for helpful discussion and critical reading of the manuscript.




