Apicomplexan UCHL3 retains dual specificity for ubiquitin and Nedd8 throughout evolution
Summary
Post-translational modification of proteins by ubiquitin or ubiquitin-like polypeptides such as Nedd8 controls cellular functions including protein degradation, the cell cycle and transcription. Here we have used an activity-based chemical probe that covalently labels ubiquitin hydrolases. We identify four such enzymes from Toxoplasma gondii by mass spectrometry. The homologue of mammalian UCHL3 was cloned from both T. gondii and Plasmodium falciparum and we show that both enzymes possess deubiquitinating as well as deNeddylating activity. A phylogenetic analysis of the UCHL3 amino acid sequences from several eukaryotes suggests that dual specificity for ubiquitin and Nedd8 was present in the ancestral eukaryotic UCHL3 and has been conserved throughout evolution. Finally, the structural characterization of UCHL3 from T. gondii shows a unique insertion at the surface of this enzyme, which may be involved in novel interactions with other proteins. The characterization of these apicomplexan UCHL3s adds to our understanding of the ubiquitin and Nedd8 pathways in these parasites.
Introduction
The ubiquitin-proteasome system is conserved throughout evolution, with ubiquitin (Ub) itself being one of the most conserved proteins in eukaryotes, differing by only four amino acids across plants, yeast and mammals (Catic and Ploegh, 2005). In addition to Ub, there is a family of small post-translational protein modifiers known as ubiquitin-like proteins (Ubls). These include Nedd8, SUMO, ISG15 and Atg8, among others. The Ubl tags modify other proteins important for degradation, transcription, DNA repair, signal transduction, autophagy and cell cycle control. The mode of Ubl attachment to proteins always proceeds via the C-terminal glycine residue of the Ubl, whose carboxyl group forms an amide bond with a lysine side-chain of the target protein (or a primary amine in the case of Atg8) (Kerscher et al., 2006). The reverse reaction (deubiquitination) is catalysed by a diverse group of enzymes, collectively known as deubiquitinating enzymes (DUBs). This group contains several protein families, including ubiquitin C-terminal hydrolases (UCHs), ubiquitin-specific proteases (USPs), SUMO-specific proteases (SENPs), autophagins, otubains, ataxin-3/josephin ubiquitin proteases and JAMM isopeptidases (Amerik and Hochstrasser, 2004). The fact that all of these families, with the exception of JAMMs, belong to the class of cysteine proteases allowed us to develop a series of ubiquitin and ubiquitin-like derivatized probes that covalently target DUBs with excellent specificity (Borodovsky et al., 2002; Hemelaar et al., 2004).
Proteins with deubiquitinating activity have been identified in both viruses and bacteria (Balakirev et al., 2002; Barretto et al., 2005; Kattenhorn et al., 2005; Lindner et al., 2005; Schlieker et al., 2005) and are hypothesized to have immune evasive functions. Surprisingly, Ubl pathways have been given little attention in eukaryotic pathogens. In addition to expressing DUBs that target host functions, like viruses and bacteria, parasites also possess Ubl pathways of their own. Given the necessity of Ubls in all aspects of cell life and the success of ubiquitin-based therapies in cancer (Voorhees and Orlowski, 2006), interference with pathogen Ubls could lead to novel therapeutic targets. A better understanding of the function of these versatile post-translational modifiers within eukaryotic pathogens necessitates an inventory of the pathway components. To this end, we previously made use of ubiquitin-based electrophilic probes to identify DUBs in Plasmodium falciparum. We characterized PfUCH54 as a deubiquitinating enzyme (Artavanis-Tsakonas et al., 2006), but despite this enzyme's homology to mammalian UCH37, PfUCH54 was found to have additional deNeddylating activity and a long poly asparagine insertion. These findings highlight the importance of carefully identifying and characterizing members of this pathway in eukaryotic pathogens. Even though ubiquitin and its regulators are highly conserved, this by no means precludes additional and different ways of directing Ubl-mediated cellular functions in ways unique to eukaryotic pathogens.
Here, we sought to explore deubiquitinating activity in other pathogenic apicomplexans and therefore examined the parasite Toxoplasma gondii. Toxoplasma is widespread, infecting almost all warm-blooded vertebrates (Wong and Remington, 1993), with typical human infection rates of 10–30% in the USA (Jones et al., 2001) and up to 80% in other geographical areas (Montoya and Liesenfeld, 2004). T. gondii causes a wide range of symptoms determined mainly by the immune status of the host (Montoya and Liesenfeld, 2004). In immunocompetent individuals, the invasive tachyzoite stage converts to the slowly replicating bradyzoite stage. These reside in quiescent tissue cysts of the brain, and cause a chronic, but usually asymptomatic infection. However, serious cases of toxoplasmosis can occur following immunosupression of adults, and are commonly associated with organ transplantation or AIDS. Congenital transmission of the parasite to the foetus can cause critical complications in pregnancy (Montoya and Liesenfeld, 2004).
We identify four DUBs from T. gondii by their reactivity with an Ub activity-based probe. One of the newly identified enzymes is orthologous to mammalian UCHL3. We cloned this DUB from T. gondii and P. falciparum. Both apicomplexan UCHL3s react with ubiquitin and Nedd8, a feature that is shared by multiple orthologous enzymes, including human UCHL3 (HsUCHL3). We performed a phylogenetic analysis of multiple UCHL3 orthologous sequences from species ranging from plants to mammals. The results of this analysis suggest that the dual specificity of the current UCHL3 enzymes has been inherited from an ancestor that also possessed this feature. Finally, we created a homology model of the tertiary structure of UCHL3 from T. gondii. This model shows a unique 18-amino-acid insertion that may play a role in the interaction of this enzyme with other proteins.
Results
Ub protease activity is present in T. gondii-infected human foreskin fibroblasts
Enzymes that remove the post-translational modifiers Ub, Nedd8, SUMO and ISG15 can be assayed in cell lysates by using epitope-tagged electrophilic derivatives of these proteins (Borodovsky et al., 2001; Hemelaar et al., 2004). This strategy was used to identify proteases from a variety of sources (Borodovsky et al., 2002; Hemelaar et al., 2004; Kattenhorn et al., 2005; Artavanis-Tsakonas et al., 2006; Misaghi et al., 2006). To generate the probe, these small-protein modifiers are equipped with an electrophile such as vinylmethylester (VME) or vinylsulfone (VS) at their C-terminus. When a DUB attacks the modified Ubl, a thioether bond is formed between the active-site cysteine of the target protease and the probe. The epitope tag on the probe can be used to detect these interactions immunochemically or can be employed for retrieval of the target protease. The probes used in this study are based on the mouse sequences of Ub, Nedd8 and SUMO1. Mouse and T. gondii Ub differ in only one amino acid (E16D) while Nedd8 and SUMO exhibit approximately 50% identity between the two species.
To determine if T. gondii-infected human foreskin fibroblasts (HFFs) contain non-host proteins either contained within or secreted from T. gondii with deubiquitinating activity, we prepared lysates of HFFs 48 h after infection with RH strain T. gondii tachyzoites and reacted the lysates with HA-tagged Ub-VME. Immunoblotting revealed the presence of several bands unique to the infected sample, the most prominent being a polypeptide of about 40 kDa as well as a number of polypeptides with a molecular mass greater than 150 kDa (Fig. 1A). The presence of these specific bands prompted their identification.
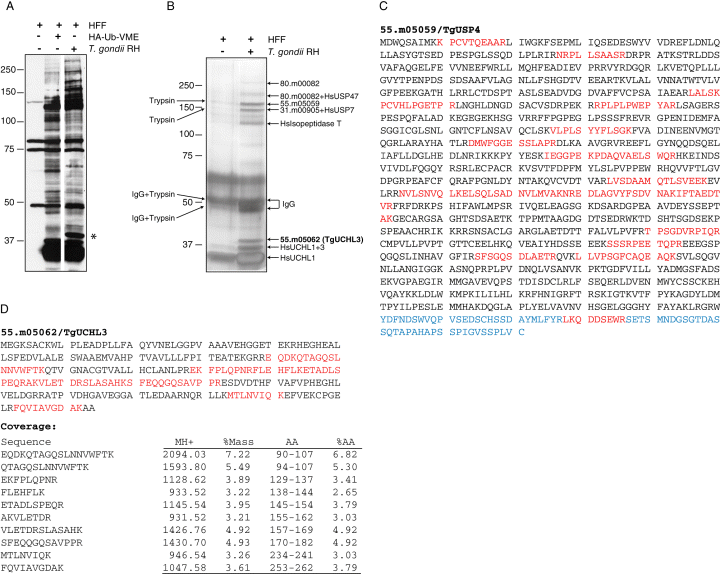
Identification of deubiquitinating enzymes in T. gondii-infected human foreskin fibroblasts (HFFs).A. Lysates of HFFs either uninfected or infected with T. gondii RH were reacted with HA-Ub-VME, separated by 8% SDS-PAGE and then immunoblotted with anti-HA-HRP. Specific bands to the T. gondii-infected HFFs could stem from parasite-internal or secreted DUBs. Molecular weights in kDa are indicated on the left. The reactive polypeptide later identified as TgUCHL3 is marked by an asterisk.B. Preparative scale lysates derived from 3 × 108 HFFs were reacted with HA-Ub-VME, immunoprecipitated with anti-HA resin, SDS-PAGE separated, and polypeptides indicated by the arrows were excised, trypsin digested and subjected to tandem mass spectrometry. Four T. gondii-specific DUBs were identified and their accession numbers for the Toxoplasma Database are indicated. Some bands contained multiple peptides from different proteins or from Homo sapiens polypeptides.C. Polypeptides identified by mass spectrometry for the T. gondii DUB with the Accession No. 55.m05059 are shown in red in the sequence of the protein. The 71 amino acids at the very C-terminus of the protein were not annotated in ToxoDB and are shown in blue. The identified peptide in that stretch is marked in red.D. Polypeptides identified by mass spectrometry for TgUCHL3 are shown in red for the sequence of the protein. Mass spectrometry data for the peptides identified are shown. MH+ is the protonated mass of each peptide, %Mass indicates the per cent of the total protein mass occupied by each peptide, and %AA identifies the per cent of amino acid coverage.
Identification of Ub-VME-reactive proteins from T. gondii
We scaled up the DUB labelling reaction by using cell lysates from 3 × 108 HFFs 48 h after infection with T. gondii RH at a multiplicity of infection (moi) of 5, followed by incubation with HA-Ub-VME. We chose not to separate parasites from cells so that DUBs potentially secreted by the parasites into the host cell could also be detected (Misaghi et al., 2006). The reacted material was immunoprecipitated, separated by SDS-PAGE and the polypeptides were visualized by silver staining (Fig. 1B). Polypeptides specific to the T. gondii-infected sample were excised, trypsinized and subjected to tandem mass spectrometry (MS/MS). Four T. gondii-specific polypeptides were identified, all of which corresponded to annotated putative C-terminal Ub hydrolases with ToxoDB Accession No. 80.m00082, 55.m05059, 31.m00905 and 55.m05062 (Fig. 1B). blast searches revealed the closest mammalian homologues for these enzymes to be USP7, USP4, UBP5 and UCH-L3 respectively. All genes contain multiple introns. USP7, USP4 and UBP5 have ambiguous initiation and termination sites as extracted from the available database. RACE PCR analysis of the USP4 homologue (55.m05059) revealed a misannotation in ToxoDB. The C-terminal 71 amino acids, highlighted in blue in Fig. 1C, are absent from the predicted open reading frame (ORF) for this protein. A blast search of the sequence of our 3′ RACE PCR product against the Toxoplasma genome reveals that this stretch includes two exons on chromosome VIIb, 14725 base pairs downstream of the predicted termination of the protein. The correct sequence is shown in Fig. 1C, together with the extent of peptide coverage obtained by MS/MS. Moreover, we find a peptide that maps to this missing stretch of the protein by mass spectrometry. Sequence alignment with HsUSP4 shows a 27% identity and 40% similarity between the proteins, with all catalytic residues conserved (data not shown). The UBP5 and USP7 sequences have not been verified and are shown in their currently annotated state in Fig. S1.
We decided to further investigate the UCH-L3 homologue TgUCHL3 (Fig. 1D), as the mammalian enzyme has been well characterized (Johnston et al., 1999; Misaghi et al., 2005) and provided us with a well-defined backdrop against which to describe its parasite counterpart. We were particularly interested in the ability of UCH-L3 to both deubiquitinate and deNeddylate target proteins (Wada et al., 1998; Hemelaar et al., 2004) and to determine whether this feature has been conserved throughout evolution.
UCHL3s from T. gondii and P. falciparum also have deNeddylating activity
To verify the specificity of the deubiquitinating activity, as well as test for deNeddylating and possible deSUMOylating activity of TgUCHL3, we cloned the ORF of TgUCHL3 from T. gondii cDNA. We further generated a catalytically inactive mutant by substituting the putative active-site cysteine with an alanine. Both constructs were translated in vitro and reacted with Ub-VME, Nedd8-VS and SUMO-VS.
TgUCHL3 reacted with both the Ub and the Nedd8 probes, as seen from the 9 kDa shift in electrophoretic mobility (Fig. 2A), but failed to be modified by the SUMO probe (data not shown). Nedd8 and Ub reactivities were abolished by pre-incubation of extracts and of TgUCHL3 with the alkylating agent N-ethylmaleimide (NEM), confirming the nature of this enzyme as a cysteine protease. This was further supported by the inability of the cysteine to alanine mutant to be modified by either probe. Interestingly, T. gondii Nedd8 and SUMO both share a similar degree of conservation to their human counterparts. Thus, we conclude that adduct formation is not an occurrence that applies across the board to all electrophilic Ubl derivatives.
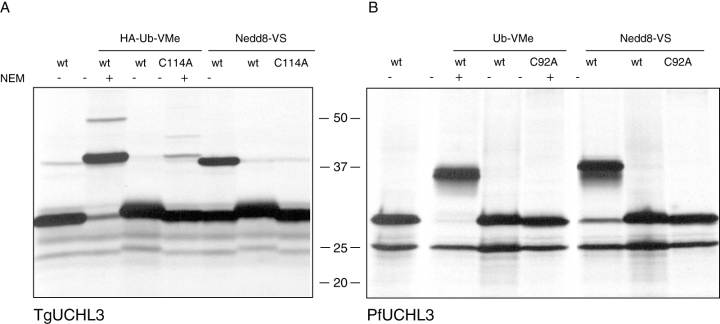
Evidence for deubiquitinating and deNeddylating activity of T. gondii and P. falciparum UCHL3. In vitro transcription/translation using TgUCHL3 (A) and PfUCHL3 (B) both as either the wild-type or the catalytically inactive cysteine to alanine mutant was conducted in the presence of 35S-methionine. Translation products were reacted with the probes indicated, separated by SDS-PAGE and analysed by autoradiography which revealed the dual specificity towards Ub and Nedd8 for both wild-type UCHL3 variants. Reactivity is abolished by pre-incubation with the alkylating agent NEM or with the catalytically inactivated variants demonstrating the necessity of the active-site cysteine.
To determine whether the homologous gene product from P. falciparum also reacts with both Ub-VME and Nedd8-VS, we cloned the ORF of PfUCHL3 (NP_702465) from total 3D7 mRNA by reverse transcription polymerase chain reaction (RT-PCR), as this gene contains multiple introns. We noted a difference in the annotated sequence from the Plasmodium database versus the gene product we cloned. Comparison of the intron donor/acceptor sites with the genomic sequence of this gene validates our cloned sequence, which is further supported by sequence alignment with other UCHL3 homologues (Fig. 3A). PfUCHL3 was in vitro translated and reactivity with both the Ub as well as the Nedd8 probes was demonstrated (Fig. 2B). PfUCHL3 also failed to react with a SUMO probe (data not shown). Again, inclusion of NEM and mutation of the catalytic cysteine to an alanine abolished probe modification. We concluded that both UCHL3s from T. gondii and P. falciparum, which are only 32% identical and 42% similar, retain dual specificity towards Ub and Nedd8, as described for human UCHL3 (Hemelaar et al., 2004).
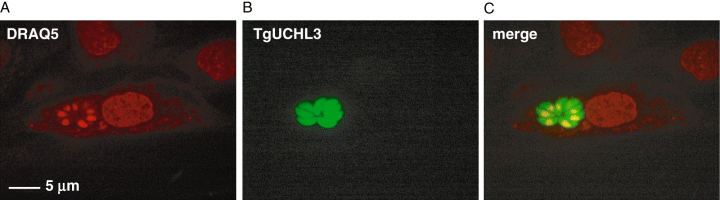
In vivo localization of TgUCHL3. HFFs were infected with transgenic T. gondii expressing TgUCHL3-YFP. Following a 20 h infection, cells and parasites were exposed to the nuclear dye DRAQ5 immediately prior to imaging. A HFF containing multiple T. gondii tachyzoites is shown.A. The nuclear stain DRAQ5 (false coloured red).B. The distribution of TgUCHL3 within the parasites (false coloured green).C. The merged image.
TgUCHL3 localizes to both the nucleus and cytoplasm of T. gondii
We next wanted to investigate the subcellular localization of TgUCHL3 in the tachyzoite stage of T. gondii. To this end, we infected HFFs with T. gondii RH stably expressing TgUCHL3 C-terminally fused to YFP and incubated infected cells with a nuclear dye immediately before imaging. Using a spinning-disk confocal microscope we obtained z-stack images of HFFs infected with parasites. In some cases a single parasite was present within the parasitophorous vacuole, whereas in others the parasites had multiplied. An example of the latter is shown in Fig. 3. We found that TgUCHL3 localized to both the nucleus and the cytoplasm of T. gondii and was not secreted into the parasitophorous vacuolar space, This distribution is not surprising, as other DUBs have been localized both nuclearly and cytoplasmically (Soboleva et al., 2005). Furthermore, the function of TgUCHL3 as a deNeddylating enzyme, which likely regulates degradation of cell cycle factors as seem in mammals (Chiba and Tanaka, 2004), supports a nuclear localization.
DeNeddylating activity of UCHL3 is conserved throughout evolution
To gain insight into the origin of the dual specificity of UCH-L3, we performed a phylogenetic analysis of the sequence of UCHL3 in several species. Notably, we were unable to find any UCHL3 orthologues in species like Theileria parva, Entamoeba histolytica and Babesia bovis, whereas we only found two UCHL3-like pseudogenes in Tetrahymena thermophila. We aligned the amino acid sequences of UCHL3 in multiple organisms, ranging from parasites to mammals (Fig. 4A). This alignment shows the presence of two similar large-amino-acid insertions in the sequences of T. gondii and wild cabbage Brassica oleracea UCHL3 (BoUCHL3). We next deduced the phylogenetic relationship of the aligned UCHL3 sequences by maximum likelihood and parsimony analyses. As shown in Fig. 3B, the results of both analyses are consistent with the phylogenetic relationship of the species under study. Notably, the sequence of T. gondii UCHL3 clusters only loosely with the sequences of fellow apicomplexan Cryptosporidium parvum and P. falciparum UCHL3, in spite of the close phylogenetic relationship between T. gondii and C. parvum. On the other hand, the sequence of BoUCHL3 robustly clusters with that of Arabidopsis thaliana UCHL3 (AtUCHL3), also a plant, which suggests that the insertions in TgUCHL3 and BoUCHL3 are not phylogenetically related. Finally, we created a homology model of the structure of TgUCHL3 using HsUCHL3 as a template (Misaghi et al., 2005). This structure shows that the large insertion in the sequence of TgUCHL3 maps to the surface of the protein and does not affect the interaction site with ubiquitin (Fig. 5). However, an analysis of allowed conformations suggests that this insertion may interact with ubiquitin outside the canonical interaction site (Fig. 5).
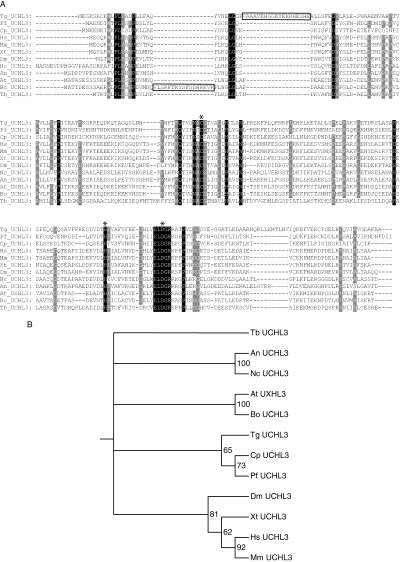
Phylogenetic analysis of UCHL3.A. The amino acid alignment of UCHL3 from multiple organisms is shown. Residues common to all of the sequences are highlighted in black. Residues conserved in more than 80% of the sequences are highlighted in grey. Large insertions in Toxoplasma gondii and Brassica oleracea are boxed. Asterisks mark the residues of the catalytic triad. Tg, T. gondii; Pf, Plasmodium falciparum; Cp, Cryptosporidium parvum; Hs, Homo sapiens; Mm, Mus musculus; Xt, Xenopus tropicalis; Dm, Drosophila melanogaster; Nc, Neurospora crassa; An, Aspergillus nidulans; At, Arabidopsis thaliana; Bo, B. oleracea; Tb, Trypanosoma brucei.B. The phylogenetic relationship of UCHL3s from multiple species is shown. The alignment in (A) was used to calculate the phylogenetic relationship between the different sequences by maximum likelihood (ML) and parsimony. Both methods gave equivalent results, with slight differences in robustness, as measured by a bootstrap analysis. The ML tree is shown. A threshold bootstrap value of 60% was required to display a branch. Bootstrap values are shown for each branch.
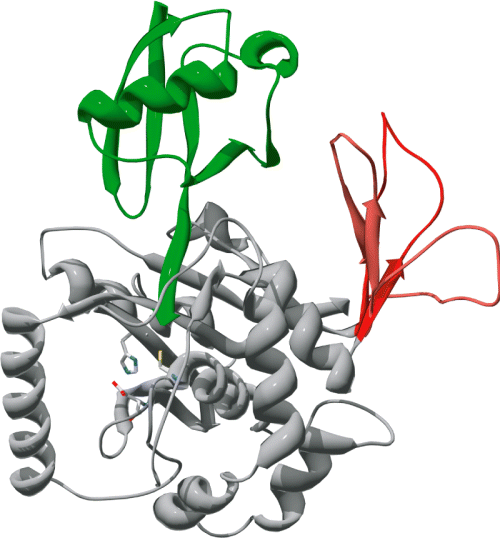
Predicted structure of TgUCHL3. A ribbon representation of the homology model of TgUCHL3 (grey) bound to ubiquitin (green) is shown. The TgUCHL3 sequence was manually threaded over the crystal structure of human UCHL3. The 18-amino-acid insertion in TgUCHL3 is displayed in three of the allowed conformations in red.
Discussion
Ubiquitin-proteasome and other ubiquitin-like pathways have been conserved throughout evolution, a fact that speaks to the role of these dynamic modifications in many cellular processes. We recently identified PfUCH54 from P. falciparum as a deubiquitinating and deNeddylating enzyme. PfUCH54 displays several traits unique to malarial gene products, such as insertions of long tracts of Asn residues, likely a consequence of the high genomic AT content in Plasmodium (Singh et al., 2004). We now characterize an additional member of this family of enzymes in P. falciparum, PfUCHL3, and we show that its homologue in T. gondii (32% identical to PfUCHL3) is also expressed and retains a similar activity profile in both parasites. Although the dual reactivity of UCHL3 with Ub and Nedd8 is conserved between these apicomplexans, the extent of the sequence identity with their host is sufficiently different to consider this enzyme a possible drug target. To further bolster this argument, the only target proteins for Neddylation found so far are cullins, which are involved in cell cycle control (Chiba and Tanaka, 2004). The partial localization of TgUCHL3 to the nucleus in live parasites also supports its potential function as a regulator of cell cycles and transcription factors. Therefore, interfering with a regulator of this modifier may successfully disrupt parasite growth and maturation.
In the mammalian system, a small group of deNeddylating enzymes have been identified to date. These include CSN5 (Lyapina et al., 2001; Schwechheimer et al., 2001), DEN1/NEDP1/SENP8 (Gan-Erdene et al., 2003; Mendoza et al., 2003; Hemelaar et al., 2004), USP21 (Gong et al., 2000) and UCH-L3 (Wada et al., 1998; Hemelaar et al., 2004). In T. gondii we were able to identify homologues of all four aforementioned proteins, whereas in P. falciparum we have thus far identified only three, with no candidates for SENP8 homologues as of yet. Our previous finding that the P. falciparum UCH37 homologue has deNeddylating activity, unlike its mammalian counterpart, suggests that parasites may have evolved different sets of proteins to regulate UBL attachment and removal.
Through the use of the Ub-VME probe, we identified four DUBs in T. gondii. Given the scope of the DUB family with more than 80 members in the mammalian and at least 17 in yeast (Borodovsky et al., 2001), these four DUBs are unlikely to represent this class of enzymes in T. gondii in its entirety. Previous experience with this probe suggests a successful identification rate of approximately 20–30% (Borodovsky et al., 2001; 2002). Therefore, we assume that the DUB family in T. gondii may encompass at least a dozen members. At present ToxoDB identifies 13 putative UCHs and PlasmoDB reveals 14 members of this family (Artavanis-Tsakonas et al., 2006). Possible reasons for incomplete retrieval might include expression below the level of detection for the scale of these experiments, life cycle-dependent expression, as well as a limited reactivity range for the VME elecrophilic group. Indeed, conjugation of different electrophiles to the ubiquitin moiety results in unique DUB profiling patterns for each reactive group (Borodovsky et al., 2002). A proteomics approach will be central not only in elucidating which ORFs are actually expressed functionally, but also in fine-tuning gene annotations in currently available genome databases. In this study alone, we demonstrate two examples of misannotated genes.
Using the corrected amino acid sequence for PfUCHL3, we performed a phylogenetic analysis of UCHL3 from a wide variety of species to investigate the evolutionary origin of the dual specificity of this enzyme. Interestingly, we could not find a UCHL3 homologue in T. parva, E. histolytica or B. bovis. These absences suggest that UCHL3 was lost in Piroplasmida and other eukaryotes. Although we cannot rule out gaps in the genomic sequences of these organisms as responsible for this result, the presence of two UCHL3-like pseudogenes in T. thermophila suggests that UCHL3 gene losses indeed occurred through diverse mechanisms. TgUCHL3 shows an 18-amino-acid insertion when aligned with the other sequences. Only B. oleracea UCHL3 contains a similar insertion, although at a slightly different position. We then inferred a phylogenetic tree from the amino acid alignment of UCHL3s by parsimony and maximum likelihood analysis. Both methods gave equivalent results, with slight differences in the bootstrap score of the branches. The resulting tree is consistent with a model of vertical evolution, in which an ancestral eukaryotic UCHL3 gene gave rise to all of the current UCHL3 genes by mutation and selection events. All UCHL3s investigated to date – yeast (Johnston et al., 1999), human (Wada et al., 1998; Hemelaar et al., 2004) and now apicomplexans – share dual specificity for Ub and Nedd8. Therefore, the dual specificity towards ubiquitin and Nedd8 is likely to have been a feature of the ancestral eukaryotic UCHL3.
According to this model, the insertion in TgUCHL3 appeared after the separation of T. gondii and C. parvum lineages, whereas the insertion in BoUCHL3 appeared after the separation of B. oleracea and A. thaliana lineages. Therefore, both large insertions arose independently. A striking feature of the UCHL3 phylogenetic tree is the location of TgUCHL3, which constitutes the only inconsistency with the vertical evolution model. Independent taxonomic evidence clusters T. gondii with C. parvum, whereas TgUCHL3 shows an early divergence from the apicomplexan branch, which in turn prompts the incorrect clustering of CpUCHL3 with PfUCHL3. This divergence is not due to the large insertion in TgUCHL3, as parsimony analysis does not take this sequence stretch into account. Moreover, the conservation of the key catalytic and structural residues, along with the functional data presented here, suggests that the enhanced sequence variability of TgUCHL3 is unlikely to affect its activity. We performed a homology model to check the structural consequences of the 18-amino-acid insertion in TgUCHL3. This insertion is located on the protein surface, and does not involve the interface between TgUCHL3 and ubiquitin. Therefore, this large insertion might be involved in the interaction of TgUCHL3 with novel cofactors or deubiquitination substrates.
Taken together, these data suggest that the dual specificity of UCHL3 for ubiquitin and Nedd8 is a feature conserved throughout evolution. Moreover, UCHL3 from T. gondii diverges from other species more than expected. This divergence might well affect UCHL3's capacity to bind other proteins and might of course extend to other components of the Ub-proteasome pathway. The ability to interfere with the apicomplexan life cycle is an important goal from a public health perspective and we are currently developing methods with which to study the Nedd8 pathway in apicomplexans more thoroughly.
Experimental procedures
Cells and parasites
RH strain T. gondii were grown in confluent HFFs maintained in DMEM, 10% fetal calf serum (FCS), penicillin/streptomycin and fungizone (Roos et al., 1994). To generate T. gondii RH that stably express TgUCHL3 as a C-terminal YFP fusion protein, tubTgUCHL3YFP/sagCAT (see below) was transfected into the tachyzoites by electroporation as previously described (Roos et al., 1994) and selected with 2 μM chloramphenicol.
Labelling and immunoblot
Human foreskin fibroblasts were cultured in DMEM with 10% FCS, 4 mM glutamine, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin. Forty-eight hours after infection with T. gondii strain RH at an moi of 5, the cells were lysed in 100 mM Tris/HCl pH 7.4, 20 mM NaCl, 70 mM KCl, 12 mM MgCl2, 1 mM phenylmethylsulfonylfluoride, 1 mM dithiothreitol, 0.5% NP-40 for 20 min at 4°C. Cell debris was removed by centrifugation and 25 μg of total lysate protein was diluted with 2 volumes 50 mM Tris/HCl pH 8, 100 mM NaCl and reacted with 0.2 μg of HA-Ub-VME for 1 h at room temperature. To block reactive sulfhydryl groups of cysteines, the lysate was pre-incubated with 10 mM NEM for 15 min at room temperature. Lysates were separated by gel electrophoresis and proteins were transferred to a PVDF membrane, blocked with 5% milk before being probed with 1:1000 anti-HA-HRP antibody (Roche, Mannheim, Germany). After six successive washes in PBS-Tween proteins were visualized by chemiluminescence.
Identification of labelled DUBs using mass spectrometry
Human foreskin fibroblasts (3 × 108) were lysed 48 h after infection with T. gondii strain RH at an moi of 5. The samples were processed as described above except that 4 mg of total lysate protein was reacted with 40 μg of Ub-VME. Gel bands were excised and digested with trypsin. The resulting peptides were analysed by liquid chromatography/MS/MS and the data correlated against the Tg10x_31-Draft3_TIGR database using SEQUEST. All proteins reported were identified by the presence of two or more peptides.
Cloning and mutagenesis
Toxoplasma gondii total RNA was isolated from unsynchronized tachyzoite RH culture using Trizol reagent. cDNA was prepared using the SMART RACE cDNA amplification kit (Clontech, Mountain View, CA). TgUCHL3 coding cDNA (55.m05062) was amplified by PCR using ToxoDB to design the primers TgUCHL3For: 5′-GAGATCTGGATCCCCATGGAGGGGAAGAGC and TgUCHL3Rev: 5′-GCTCTAGAGCCTACGCGGCCTTGGCGTC. The resulting PCR products were digested with BamHI and XbaI and ligated into the multicloning site of pcDNA3.1(+) to yield pcDNA3TgUCHL3. This construct was used as a template to generate the cysteine to alanine mutant of TgUCHL3 using Stratagene's QuikChange II site-directed mutagenesis kit (La Jolla, CA) and the primers 5′-GTCGGTAATGCAGCCGGCACTGTGGCCCTGCTCC and 5′-GGAGCAGGGCCACAGTGCCGGCTGCATTACCGAC. In order to generate TgUCHL3 C-terminally fused to YFP the gene was re-cloned into the BglII and AvrII sites of tubYFP-YFP/sagCAT (Gubbels et al., 2003). This yielded the vector tubTgUCHL3YFP/sagCAT.
Total mRNA from 3D7 P. falciparum was prepared as described in Kyes et al. (1999). Using the PlasmoDB prediction for the coding sequence of Pf14-0576, primers were designed against the 5′-CGGAATTCATGGCAAAGAATGATATTTGGAC and 3′-CCGCTGAGTATAATATCAAAGTTATCGTTTGG ends with EcoRI and XhoI linkers respectively, for directional cloning. Following Pfu amplification, the product was digested and cloned into pcDNA 3.1(+) in frame with a C-terminal HA epitope tag. In order to generate the cysteine to alanine mutant the same strategy as described above was used with the primers 5′-TACATTCCAAACTCAGCTGGAACCATAGCCTTG and 5′-CAAGGCTATGGTTCCAGCTGAGTTTGGAATGTA.
In all cases competent Escherichia coli were transformed with the plasmids and resulting colonies were grown for DNA isolation and sequence verification. Sequencing of PfUCH24 revealed a slightly different coding sequence than that predicted by PlasmoDB. The cloned amino acid sequence is shown in Fig. 3A.
Both 5′ and 3′ cDNA ends for TgUSP4 were amplified by RACE PCR using the Invitrogen GeneRacer kit and primers 5′-GAAGCCAGACGCGCAAGTGGCTGAGC and 3′-CCCCAGCTGCGGCTCTCACTTCTGC. Nested RACE products (using the same gene-specific primers but nested universal primers) amplified with TripleMaster polymerase mix (Eppendorf) were TOPO cloned into plasmid pCR2.1 (Invitrogen) and sequenced.
In vitro translation and labelling
Template plasmid DNA (200–300 ng) of either TgUCHL3 or PfUCHL3 as the wild type or the cysteine to alanine variant was added to 25 μl of rabbit reticulocyte lysate TNT T7 Quick Coupled Translation/Transcription Sytem mastermix (Promega, Madison, WI). The reactions were supplemented with 1 μCi of 35S-methionine (Perkin Elmer) each and incubated at 30°C for 45 min. Each sample mixture was then reacted with 0.2 μg of the probe as described above, subjected to SDS-PAGE (10% polyacrylamide) and analysed by autoradiography.
Live microscopy
Human foreskin fibroblasts were infected with T. gondii RH stably expressing tubTgUCHL3YFP/sagCAT. Twenty hours after infection 1 μM DRAQ5 (Alexis Biochemicals, San Diego, CA) was added for 5 min and the cultures were imaged on a spinning disk confocal microscope (Perkin Elmer Ultraview RS, Boston, MA) with a Prairie 3 watt laser with AOTF (Prairie Technologies, Middleton, WI). The system incorporated a Nikon TE2000-U inverted microscope using a Nikon 100× 1.4NA DIC lens and Nikon type A immersion oil (Melville, NY). Cells were maintained at 37°C with 5% supplemental CO2 in room air using a Solent scientific chamber (Segensworth, UK), which fully enclosed the microscope stage area. The camera used in these experiments was the Hamamatsu Orca ER camera (model number C4742-95-12ERG). Metamorph software (Molecular Devices, Sunnyvale, CA) was used for acquisition.
Phylogenetic analysis
The sequences of UCHL3 from 12 organisms including metazoans, fungi, plants, apicomplexans and Trypanosoma brucei were aligned automatically with clustalx version 1.8 (http://bips.u-strasbg.fr/en/Documentation/ClustalX/) and then manually with genedoc version 2.6. (http://www.psc.edu/biomed/genedoc). The programs used for phylogenetic inference are included in the Phylip package version 3.6 (http://evolution.genetics.washington.edu/phylip/getme.html). After producing 100 bootstrapped replicates of the original alignment with the bootstrap program, the most parsimonious tree was calculated with the protpars program. A second tree was constructed by maximum likelihood analysis with the proml program. The consense tree was calculated after both analyses with the consense program, using the maximum likelihood algorithm and forcing a 60% minimum bootstrap score to display a branch. TbUCHL3 was used to root the trees.
Homology modelling
The sequence of TgUCHL3 was manually threaded over the structure of HsUCHL3 (Misaghi, 2005) using DeepView v3.7. The resulting alignment was used to build a model of the tertiary structure of TgUCHL3 with Swiss-Model (http://swissmodel.expasy.org/). Residues 30–48 of the resulting model, corresponding to a large insertion in TgUCHL3 sequence, were rebuilt with DeepView. We analysed multiple possible conformations of this insertion according to similar sequences in proteins of known structure.
Acknowledgements
We thank Christy Comeaux and Manoj Duraisingh for providing P. falciparum 3D7 RNA. E.-M.F. is supported by the Swiss National Science Foundation, K.A.-T. is a National Institutes of Health National Research Service Award Fellow (1F32CA105862-01), V.Q. is supported by the Ministerio de Educación y Ciencia of Spain and M.-J.G. by a Scientist Development Grant from the American Heart Association.