Interleukin-18 secretion and Th1-like cytokine responses in human peripheral blood mononuclear cells under the influence of the toll-like receptor-5 ligand flagellin
Summary
Flagellin is the major protein component of the flagella from motile bacteria and was identified as the ligand for toll-like receptor (TLR)-5. Whereas its effects on epithelial cells have been studied in detail, activation of human peripheral blood mononuclear cells (PBMC) by flagellin is characterized only partially. By using the recombinant protein of Salmonella muenchen we confirm the proinflammatory nature of flagellin as detected by nuclear factor-κB activation and interleukin (IL)-8 production. Aim of the current study was to elucidate in PBMC effects of flagellin on IL-18 and Th1-like cytokine responses. We report that flagellin in pathophysiologically relevant concentrations augmented release of mature IL-18 by THP-1 monocytes, PBMC, and whole blood stimulated with nigericin or by ATP-mediated P2X7 purinergic receptor activation. Further key functions of the IL-18/IL-12/interferon-γ (IFNγ) pathway were upregulated by flagellin. Flagellin synergized with IL-12 for production of IFN-γ and augmented secretion of interferon-inducible protein-10, a CXC-chemokine that is key to the generation of Th1-type responses. In contrast, neither IL-18-binding protein nor IL-4 was affected. Taken together, the present data demonstrate for the first time that flagellin at concentrations that are detectable in the blood compartment during sepsis efficiently enhances the IL-18/IL-12/IFNγ pathway and thus Th1-like cytokine responses in PBMC.
Introduction
Interleukin (IL)-18 is a member of the IL-1 family of cytokines which efficiently promotes interferon-γ (IFNγ) production by T cells and NK cells thereby shaping immunity towards a Th1-like phenotype (Tsutsui et al., 2000; Dinarello and Fantuzzi, 2003; Mühl and Pfeilschifter, 2004). Moreover, a proinflammatory cytokine cascade is initiated in peripheral blood mononuclear cells (PBMC) by IL-18 that is associated with production of key inflammatory molecules including tumour necrosis factor-α (TNFα), IL-1β, IL-8 (Puren et al., 1998) and matrix metalloprotease (MMP)-9 (Nold et al., 2003). However, a most distinctive characteristic of IL-18 is its constitutive expression in a diverse array of human cell types among others monocytes (Pirhonen et al., 1999), PBMC (Puren et al., 1999), synoviocytes (Möller et al., 2001), colon carcinoma cells (Paulukat et al., 2001) and keratinocytes (Kämpfer et al., 2000). This aspect of its biology separates IL-18 from other proinflammatory cytokines such as TNFα and IL-1β. These require transcriptional gene induction to acquire their function in immune defence and inflammation. In contrast to the latter group of cytokines, IL-18 bioactivity appears to be regulated primarily on the post-translational level. Specifically, activation of the inflammasome (Martinon and Tschopp, 2004), an intracellular multiprotein complex, mediates conversion of biological inactive pro-IL-18 into fully active mature IL-18 (mat-IL-18) by action of the cystein protease caspase-1. Processing of IL-18 is tightly coupled to its secretion. Both processes, IL-18 processing and secretion, are supposed to be very similar to those controlling IL-1β release (Tsutsui et al., 2000; Dinarello and Fantuzzi, 2003; Mühl and Pfeilschifter, 2004; Wewers, 2004). The best stimulus known to mediate ample processing and secretion of IL-18 is extracellular ATP that acts on the purine P2X7 receptor thereby inducing a cellular K+ efflux (Perregaux et al., 2000; Mehta et al., 2001; Mühl et al., 2003; Kahlenberg and Dubyak, 2004; Martinon and Tschopp, 2004; Rampe et al., 2004; Sluyter et al., 2004). This activity is connected with inflammasome and caspase-1 activation as well as IL-18 secretion (Andrei et al., 2004). Due to its constitutive expression, processing and release of mat-IL-18 from appropriately stimulated monocytes is a rather rapid process and detectable within minutes rather than hours (Fig. 2D, inset). This rapid kinetic of secretion together with its biological functions suggests that IL-18 is located at a particular proximal position within the proinflammatory cytokine cascade.
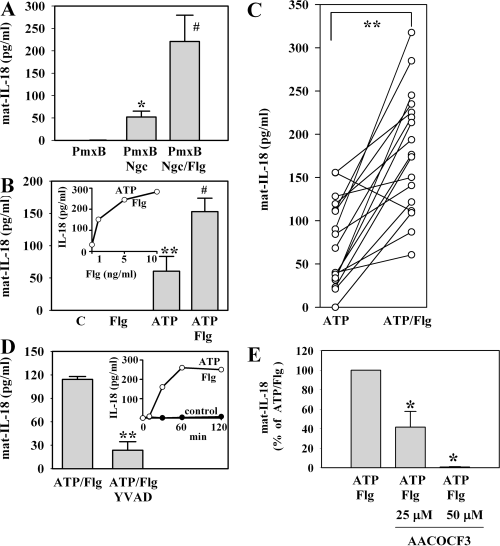
Flagellin augments secretion of mat-IL-18 from nigericin (Ngc)- or ATP-stimulated PBMC. A. PBMC were kept as control or were stimulated with Ngc (20 µM) for 45 min. Where indicated, cells were pretreated with Flg (5 ng ml−1) for 3 h. All other cells were kept in control medium during this pre-incubation period. After the adjacent 45 min stimulation with or without Ngc, mat-IL-18 release was determined by ELISA. In these experiments, Flg was pretreated with PmxB (final concentration during stimulation: 5 µg ml−1). Accordingly, an identical PmxB pretreatment was included in all other experimental conditions. Data are expressed as means ± SEM (n = 3); *P < 0.05 compared with PmxB control; ♯P < 0.05 compared with Ngc/PmxB alone. B. PBMC were kept as control or were stimulated with ATP (5 mM) for 2 h. Where indicated, unstimulated and ATP-treated cells had underwent a 3 h pre-incubation period with Flg (5 ng ml−1). All other cells were kept in control medium during this pre-incubation period. After the adjacent 2 h incubation with or without ATP, mat-IL-18 release was determined by ELISA. Data are expressed as means ± SEM (n = 6); **P < 0.01 compared with unstimulated control; ♯P < 0.05 compared with ATP alone. Inset: dose–response curve of Flg enhancing ATP-induced release of mat-IL-18. PBMC from one donor were pre-incubated with the indicated concentrations of Flg for 3 h. Flg-pretreated or untreated cells were then stimulated with ATP (5 mM) for 2 h. Thereafter, mat-IL-18 release was determined by ELISA. C. According to the protocol specified in (B), 17 experiments (ATP versus ATP plus Flg) with PBMC obtained from eight different donors were performed during this study. These are summarized in this figure. **P < 0.01 compared with ATP alone. D. After a 3 h pre-incubation with Flg (5 ng ml−1), PBMC were exposed to ATP (5 mM) for 2 h. Where indicated, the 3 h pre-incubation period with Flg included a 1 h pre-incubation with YVAD (10 µM). All other cells were kept in control medium during this pre-incubation period. After the adjacent 2 h stimulation with ATP (total incubation time: 5 h), mat-IL-18 release was determined by ELISA. Data are expressed as means ± SEM (n = 3); **P < 0.01 compared with ATP/Flg. Inset: kinetic of ATP/Flg-induced release of mat-IL-18 from PBMC. PBMC from one donor were kept as unstimulated control or were pre-incubated with Flg at 5 ng ml−1 for 3 h. Flg-pretreated cells were then stimulated with ATP (5 mM) for the indicated time periods. Thereafter, mat-IL-18 release was determined by ELISA. E. PBMC were pre-incubated for 3 h with Flg (5 ng ml−1) in the presence or absence of the indicated concentrations of AACOCF3. Cells were then stimulated with ATP (5 mM) for 2 h. Thereafter, mat-IL-18 release was determined by ELISA. Data are expressed as (% of ATP/Flg alone) ± SEM (n = 3); *P < 0.05 compared with ATP/Flg alone.
The toll-like receptor (TLR) system provides an inherent cellular recognition device that is of paramount importance for the development and function of innate as well as adaptive immunity (Takeda and Akira, 2005). Among the at least 10 different human TLRs, TLR5 is able to specifically detect bacterial flagellin (Flg), the major protein component of the flagella from Gram-negative and Gram-positive motile bacteria (Hayashi et al., 2001; Szabo, 2003; Ramos et al., 2004). TLR5 is expressed on epithelial cells, immature dendritic cells, natural killer cells, T cells and monocytes (Muzio et al., 2000; Hornung et al., 2002; Szabo, 2003). Accordingly, expression of TLR5 is readily detectable in human PBMC (Smith et al., 2003). Hallmarks of TLR activation, including that of TLR5, are stimulation of the IL-1 receptor-associated kinase/nuclear factor-κB (NF-κB) pathway and of mitogen-activated protein (MAP) kinases, with subsequent induction of proinflammatory cytokines like TNFα and IL-8 (Takeda and Akira, 2005). Although Flg was shown to increase production of mat-IL-18 in murine macrophages (Mariathasan et al., 2004), data on consequences of TLR5 activation for Th1 versus Th2 decisions in the murine system are not uniform (Eaves-Pyles et al., 2001; McSorley et al., 2002; Didierlaurent et al., 2004). In this context it is noteworthy that at least in mice IL-18 appears to be able to upregulate also Th2 responses under certain conditions (Yoshimoto et al., 2000). Therefore, effects of Flg on the IL-18/IL-12/IFNγ pathway in human PBMC were investigated in the present study.
Results
Recombinant Flg mediates a proinflammatory response in PBMC
In an initial set of experiments we sought to characterize the recombinant Flg in use. Flg mediated a robust proinflammatory response in PBMC as detected by significant induction of IL-8 (Fig. 1A). In fact, Flg was as potent as the TLR4 ligand lipopolysaccharide (LPS) in inducing release of IL-8 from these cells. The concentration of Flg used (5 ng ml−1) is concordant with a previous study on induction of TNFα by adherent PBMC. In that report, a half-maximal TNFα release was noted at 0.75 ng ml−1 of recombinant Flg (McDermott et al., 2000). Analogous inducibility of TNFα was observed by Flg in the present study (data not shown). To exclude that an LPS contamination of the Flg preparation is responsible for its biological activity, Flg was pretreated with PmxB for 1 h (final concentration during the stimulation: 2 µg ml−1). PmxB pretreatment efficiently suppressed LPS-induced IL-8 (Fig. 1A) and TNFα (data not shown). In contrast, PmxB was unable to affect production of both cytokines mediated by Flg. Therefore, a relevant LPS contamination in the Flg preparation used in the present study can be ruled out. Furthermore, Flg induction of IL-8 release from PBMC was potently suppressed (> 92% inhibition of IL-18 release) by pretreatment of Flg with trypsin further excluding a relevant contamination of recombinant Flg with ‘non-protein’ TLR ligands such as the TLR2 activator lipoteichonic acid (data not shown). The monocyte-enriched adherent cell fraction of PBMC was used to detect IκBα degradation as a read-out for NF-κB activation. In accord with the concept of TLR5 coupling to NF-κB, we observed a marked downregulation of cellular IκBα content under the influence of Flg (Fig. 1B).
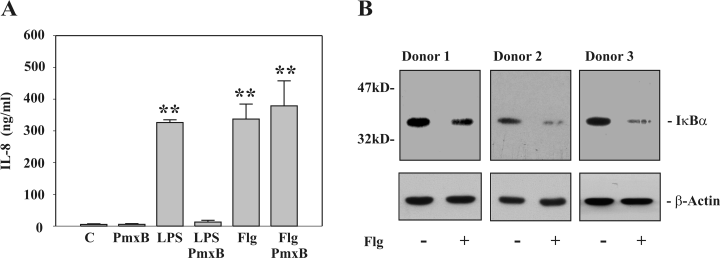
Immunostimulatory effects of recombinant flagellin on PBMC. A. PBMC were either kept as unstimulated control or stimulated with LPS (10 ng ml−1) or Flg (5 ng ml−1). Where indicated, cells were incubated as PmxB-pretreated (final concentration: 2 µg ml−1) control or were stimulated with PmxB-pretreated LPS (10 ng ml−1) or Flg (5 ng ml−1). After 18 h, IL-8 release was determined by ELISA. Data are expressed as means ± SEM (n = 3); **P < 0.01 compared with unstimulated control. B. IκBα expression was evaluated by immunoblot analysis. Adherent PBMC from three different donors were kept as unstimulated control or stimulated with Flg at 5 ng ml−1. After 1 h, cell lysates were analysed for IκBα expression by immunoblot analysis. In addition, β-actin on these blots was determined as a control for equal loading.
Flagellin enhances secretion of mat-IL-18 from monocytic THP-1 cells and PBMC activated by nigericin or by the ATP/P2X7 pathway
The microbial toxin nigericin is an ionophore that triggers potassium efflux from human monocytes. This process is associated with caspase-1 activation, with processing and release of IL-1β and IL-18, and with induction of cell death (Cheneval et al., 1998; Hentze et al., 2003). Initially, this nigericin model of caspase-1 activation and IL-18 processing/secretion was used herein to investigate immunoregulatory pathways that potentially modulate secretion of mat-IL-18 by the human monocytic cell line THP-1.
Here we report that a 3 h pre-incubation period with Flg (1 ng ml−1) significantly upregulated secretion of mat-IL-18 in response to nigericin (20 µM, 1 h) (135.9 ± 27.6 pg ml−1 versus 260.2 ± 86.6 pg ml−1 for nigericin versus nigericin plus Flg respectively; n = 8, P < 0.01). In contrast, mat-IL-18 was not detectable in culture supernatants from unstimulated THP-1 cells or from cells stimulated with Flg alone. Ethanol, the solvent for nigericin (final concentration for nigericin at 20 µM: 0.05%), did not activate release of mat-IL-18 from THP-1 cells (data not shown). In accord with the pivotal role of caspase-1 for processing of pro-IL-18 we observed that addition of Z-YVAD-FMK (20 µM), a specific inhibitor of the inflammatory caspases-1/-4, potently suppressed appearance of mat-IL-18 in supernatants of THP-1 cells exposed to nigericin or nigericin plus Flg respectively (99.1 ± 0.9% inhibition for nigericin plus Z-YVAD-FMK, 93.3 ± 1.3% inhibition for nigericin/Flg plus Z-YVAD-FMK; n = 3, P < 0.01). In addition, immunoblot analysis revealed that Flg did not upregulate cellular protein levels of pro-IL-18 (data not shown).
In accord with the data on THP-1 cells we observed significant upregulation of mat-IL-18 release by PBMC exposed to nigericin after pre-incubation with Flg. These experiments were performed using Flg pretreated with PmxB (final concentration during stimulation: 5 µg ml−1). Accordingly, all other incubations in this experimental set-up were identically performed in the presence of PmxB (Fig. 2A). In the next step we sought to investigate secretion of mat-IL-18 in response to P2X7 receptor activation by ATP. Here we omitted pretreatment with PmxB because it has been shown that PmxB modulates P2X7 receptor function (Ferrari et al., 2004). As shown in Fig. 2B, incubation with ATP alone was sufficient to trigger secretion of mat-IL-18 from PBMC. In accord with the data on stimulation of cells by nigericin, we observed that Flg significantly augmented ATP-induced mat-IL-18 secretion. Altogether, during the current study 17 experiments were performed with PBMC obtained from eight different donors. Flg failed to upregulate secretion of mat-IL-18 only in one case out of these 17 experiments (Fig. 2C). In contrast, Flg was incapable of initiating release of mat-IL-18 as a single stimulus (Fig. 2B). Potent enhancement of mat-IL-18 secretion was also observed in human whole blood cultures (WBC) (Table 1). Co-incubation with Z-YVAD-FMK efficiently suppressed release of mat-IL-18 from PBMC exposed to ATP/Flg (Fig. 2D). In addition, time-course analysis revealed that mat-IL-18 secretion is indeed rapid and actually completed within 1 h of stimulation (Fig. 2D, inset). Augmentation of mat-IL-18 secretion by the TLR5 ligand Flg resembles actions of the TLR4 ligand LPS in this regard. In fact, a 3 h pre-incubation with LPS (5 ng ml−1) significantly enhanced mat-IL-18 release by PBMC exposed to ATP (5 mM) for 2 h [60.6 ± 22.8 pg ml−1 for ATP alone versus 326.3 ± 22.6 pg ml−1 for ATP plus LPS (n = 6; P < 0.01)]. In keeping with previous data on IL-1β (Andrei et al., 2004), secretion of IL-18 was suppressed by phospholipase A2 inhibition using the arachidonyl derivative AACOCF3 (Fig. 2E).
| Control | Flg | ATP | ATP/Flg | |
|---|---|---|---|---|
| Donor 1 | 54.4 | 59.3 | 96.4 | 170.6 |
| Donor 2 | 71.1 | 76.6 | 108.8 | 371.0 |
| Donor 3 | 49.4 | 74.1 | 168.2 | 306.7 |
- Flagellin enhances release of mat-IL-18 from human WBC. WBC from three different donors were kept as unstimulated control or stimulated with ATP for 2 h (2 mM). Where indicated, WBC were pre-incubated for 3 h with Flg at 10 ng ml−1. After the adjacent 2 h incubation with or without ATP, plasma levels of mat-IL-18 were determined by ELISA. Basal levels of mat-IL-18 in these plasma samples obtained from the three healthy donors were 58.5 ± 6.8 pg ml−1 and concurred with previous data (Perregaux et al., 2000).
Effects of Flg on production of IFNγ, IL-10 and IL-4 by PBMC
To further characterize immunomodulatory properties of Flg, we sought to investigate the impact of Flg on the production of IFNγ, IL-10, and IL-4 by PBMC (Fig. 3). Whereas Flg (AB), IL-18 (B), and the combination IL-18 plus Flg (B) did not induce significant amounts of IFNγ during an 18 h incubation period, a modest but significant induction was evident by IL-12 as a single stimulus (A). A strong synergism was observed by the combination IL-12 plus Flg (A). Similar data were obtained after 36 h of incubation (data not shown). This synergism was not due to induction of mat-IL-18 by the combination Flg/IL-12 as release of mat-IL-18 was not detectable in these experiments (data not shown). This observation concurs with the notion that proinflammatory activation of PBMC, e.g. by LPS, is not sufficient to trigger efficient maturation and release of IL-18 (Puren et al., 1999). PBMC activated by IL-12/Flg mediated IFNγ-dependent inducible nitric oxide synthase (iNOS) expression in adjacent co-cultured DLD-1 colon carcinoma cells. Thus, IFNγ released under these conditions is biological active. Activation of PBMC by IL-12/IL-18 served as positive control for induction of iNOS in adjacent cells (Fig. 3D). In contrast, IL-12/Flg (Fig. 3C) or IL-12/IL-18 (not shown) were unable to induce iNOS in DLD-1 cells in the absence of PBMC. Flg (5 ng ml−1) did not augment production of IFNγ by PBMC stimulated with the combination IL-12 (5 ng ml−1) plus IL-18 (50 ng ml−1) (60.6 ± 18.4 ng ml−1 versus 62.6 ± 21.1 ng ml−1 for IL-12/IL-18 plus Flg versus IL-12/IL-18 alone; incubation: 18 h; n = 4). It became also apparent that exposure of PBMC to Flg, alike LPS, not only induces proinflammatory cytokines but also results in production of immunomodulatory IL-10 (Fig. 3E), a known deactivator of both, Th1 and Th2 cytokine responses (Mocellin et al., 2004). Induction, however, was not as pronounced as that seen under the influence of LPS (Fig. 3E). In contrast, Flg was not capable of mediating production of the Th2 cytokine IL-4 neither as a single stimulus nor in combination with the ionophore A23187 (Fig. 3F). In these same experiments, Flg potently enhanced release of IL-8 indicating efficient activation of PBMC under these conditions (data not shown).
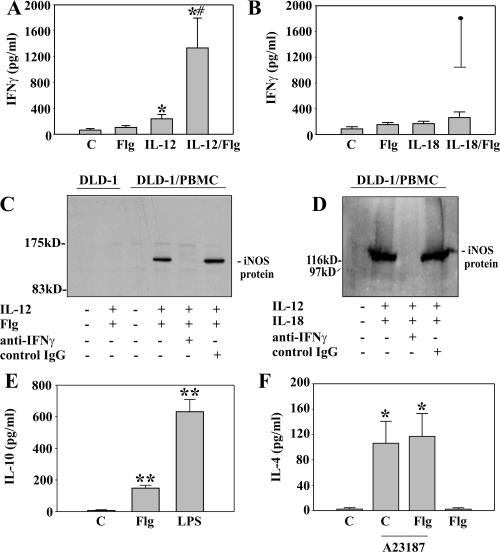
Effects of Flg on production of IFNγ, IL-10 and IL-4 by PBMC. A. PBMC were kept as unstimulated control or stimulated with Flg (5 ng ml−1), IL-12 (5 ng ml−1) and Flg (5 ng ml−1) plus IL-12 (5 ng ml−1). After 18 h, IFNγ release was determined by ELISA. Data are expressed as means ± SEM (n = 7); *P < 0.05 compared with unstimulated control. ♯P < 0.05 compared with IL-12 alone. B. PBMC were kept as unstimulated control or stimulated with Flg (5 ng ml−1), IL-18 (50 ng ml−1) and Flg (5 ng ml−1) plus IL-18 (50 ng ml−1). After 18 h, IFNγ release was determined by ELISA. Data are expressed as means ± SEM (n = 4). In this same set of experiments, IFNγ was induced by the combination Flg plus IL-12 (indicated by the filled circle). C and D. Transwell co-cultures of PBMC and DLD-1 cells were kept as unstimulated controls or stimulated with IL-12 (10 ng ml−1)/Flg (5 ng ml−1) (C, 48 h incubation) or with IL-12 (10 ng ml−1)/IL-18 (20 ng ml−1) (D, 45 h incubation) in the presence or absence of anti-IFNγ antibody (20 µg ml−1) or control IgG (20 µg ml−1). Thereafter, iNOS expression in DLD-1 cells was evaluated by immunoblot analysis. E. PBMC were either kept as unstimulated control or stimulated with LPS (2.5 ng ml−1) or Flg (2.5 ng ml−1). After 18 h, IL-10 release was determined by ELISA. Data are expressed as means ± SEM (n = 5); **P < 0.01 compared with unstimulated control. F. PBMC were either kept as unstimulated control or stimulated with Flg (5 ng ml−1), A23187 (1 µM) and the combination A23187 (1 µM) plus Flg (5 ng ml−1). After 16 h, IL-4 release was determined by ELISA. Data are expressed as means ± SEM (n = 4); *P < 0.05 compared with unstimulated control.
Flagellin synergizes with IFNγ for induction of IP-10 but has no effect on IFNγ-induced IL-18-binding protein
After having observed that Flg is able to promote production of IL-18 and IFNγ, we investigated whether Flg can as well enhance activation of monocytic cells and PBMC by IFNγ. As IP-10 is key to the development of Th1-like immune responses and because this chemokine is a prototype IFNγ-inducible gene (Gangur et al., 1998; Dufour et al., 2002), we chose to evaluated this parameter with the intention to further illustrate the immunoregulatory potential of Flg. In fact we observed a stunning synergism between IFNγ and Flg for IP-10 release by THP-1 cells. Both mediators alone induced significant IP-10 production (Fig. 4A, inset). However, the combination of both resulted in a further up to 50-fold increase of IP-10 release (Fig. 4A). IP-10 production by IFNγ-stimulated PBMC was as well significantly enhanced by Flg (Fig. 4B). However, upregulation by Flg was not as pronounced as that seen in THP-1 cells. This is likely due to the fact that induction of IP-10 by IFNγ alone was much more efficient in PBMC as opposed to THP-1 cells (Fig. 4A and B). Another prototype IFNγ-regulated gene is the IL-18 opponent IL-18-binding protein (IL-18BP) (Novick et al., 1999; Paulukat et al., 2001; Hurgin et al., 2002; Mühl and Pfeilschifter, 2003). IL-18BP was neither in THP-1 cells (Fig. 4C, inset) nor in PBMC (Fig. 4D) inducible by Flg as a single stimulus. Even more, Flg was unexpectedly not capable of enhancing IL-18BP expression in combination with IFNγ in either cell type (Fig. 4C and D).
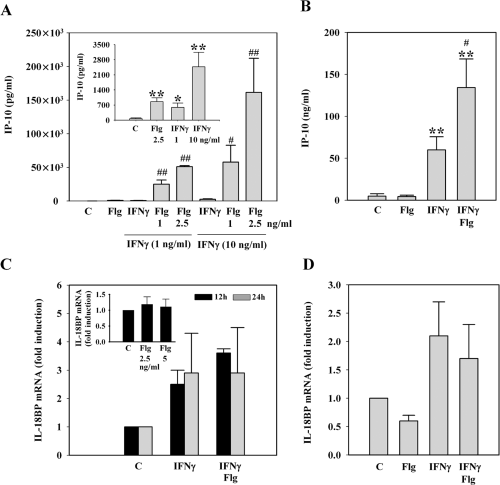
Flagellin differentially regulates expression of IP-10 and IL-18BP. A. Flg synergizes with IFNγ for release of IP-10 by THP-1 cells. Cells were kept as control, incubated with Flg at 2.5 ng ml−1 or were stimulated with IFNγ (1 ng ml−1 or 10 ng ml−1 as indicated) alone or in combination with the indicated concentrations of Flg. After 16 h, IP-10 release was determined by ELISA. Data are expressed as means ± SD (n = 3); ♯P < 0.05 compared with IFNγ alone; ♯♯P < 0.01 compared with IFNγ alone. Inset: IP-10 release from THP-1 cells stimulated with Flg and IFNγ alone is almost invisible with the scale used in (A). Therefore, these same data are shown using a suitable scale. Data are expressed as means ± SD (n = 3); *P < 0.05 compared with control; **P < 0.01 compared with control. B. Flg synergizes with IFNγ for release of IP-10 by PBMC. PBMC were kept as control or were stimulated with Flg (5 ng ml−1), IFNγ (0.5 ng ml−1) and Flg (5 ng ml−1) plus IFNγ (0.5 ng ml−1). After 16 h, IP-10 release was determined by ELISA. Data are expressed as means ± SEM (n = 7); **P < 0.01 compared with control; ♯P < 0.05 compared with IFNγ alone (data were analysed by paired t-test). C. Flg does not enhance expression of IL-18BP by IFNγ-activated THP-1 cells. THP-1 cells were kept as control or stimulated with IFNγ (10 ng ml−1) or IFNγ (10 ng ml−1) plus Flg (2.5 ng ml−1). After the indicated incubation periods, cells were harvested and mRNA expression of IL-18BP was evaluated by quantitative real-time PCR. Data are expressed as fold induction compared with unstimulated control ± SD (n = 3). IL-18BP expression was normalized to that of GAPDH. Inset: Flg does not affect IL-18BP expression in THP-1 cells. Cells were kept as control or stimulated with the indicated concentrations of Flg. After 12 h, cells were harvested and mRNA expression of IL-18BP was evaluated by quantitative real-time PCR. Data are expressed as fold induction compared with unstimulated control ± SD (n = 4). IL-18BP expression was normalized to that of GAPDH. D. Flg does not affect expression of IL-18BP by PBMC. PBMC were kept as control or stimulated with Flg (5 ng ml−1), IFNγ (20 ng ml−1) or IFNγ (20 ng ml−1) plus Flg (5 ng ml−1). After 12 h, cells were harvested and mRNA expression of IL-18BP was evaluated by quantitative real-time PCR. Data are expressed as fold induction compared with unstimulated control ± SD (n = 4). IL-18BP expression was normalized to that of GAPDH.
Discussion
In the present study effects of Flg on Th1-like cytokine responses were investigated in the mixed mononuclear cell population of human PBMC. We demonstrate that activation of PBMC by the TLR5 ligand Flg not only results in a proinflammatory cytokine response that is characterized by activation of NF-κB and production of IL-8 and TNFα but is clearly associated with a Th1-like cytokine response. Plasma levels of Flg in septic patients are in the range of 2–20 ng ml−1. In contrast, levels in healthy volunteers are below 0.1 ng ml−1 (Liaudet et al., 2003). Concentrations of Flg used herein (1–10 ng ml−1) are thus precisely in the pathophysiological relevant range. Using the nigericin model of caspase-1 activation, a significant augmentation of mat-IL-18 secretion under the influence of Flg became apparent. At this point it should be emphasized that the enzyme-linked immunosorbent assay (ELISA) used is specific for mat-IL-18. Therefore, IL-18 detected in these supernatants has been processed before by activated caspase-1 in intact cells. Passive release of pro-IL-18 due to cell death is thus not detected in these assays. To further elaborate on this activity of Flg, experiments were performed using PBMC activated through the P2X7 receptor. In agreement with constitutive expression of IL-18 and with previous studies on human whole blood (Perregaux et al., 2000) and microglial cells (Rampe et al., 2004) we observed that P2X7 activation by ATP is sufficient to trigger secretion of mat-IL-18. Three-hour pre-incubation with Flg significantly enhanced mat-IL-18 release from PBMC and whole blood. As expected, secretion of mat-IL-18 in response to ATP/Flg was detectable within minutes after onset of stimulation by ATP and suppressed by inhibition of inflammatory caspases.
To further clarify the role of Flg in regulating Th1-like responses its regulatory potential concerning cytokine-induced IFNγ was investigated. Flg synergized with IL-12 for induction of biological active IFNγ in PBMC. This activity of Flg was not via IL-18 because the latter was not detectable in these culture supernatants. This agrees with the assumption that P2X7 activation is a prerequisite for induction of mat-IL-18 secretion. Whereas IL-12 mediates a variety of its effects through signal transducer and activator of transcription-4, Flg activates NF-κB. Both transcription factors certainly have the potential to synergistically mediate production of IFNγ (Tsutsui et al., 2000). Recently it has been demonstrated that Flg has the capability to enhance IFNγ production by purified human NK cells. However, high concentrations of Flg (≥ 1000-fold higher compared with the present PBMC study) were used to achieve this function (Chalifour et al., 2004). As TLR5 is expressed by human T cells (Hornung et al., 2002), these appear to be the most likely candidates responsible for IFNγ production by PBMC under the influence of IL-12/Flg. In addition to a direct effect of Flg on T cells or NK cells, Flg-stimulated monocytes may provide a co-stimulus for efficient IFNγ production mediated by IL-12. Neither Flg or IL-18 as single stimuli nor both in combination were capable of mediating IFNγ release from PBMC. This observation concurs with the concept of widely overlapping signal transduction pathways mobilized by TLR ligands and members of the IL-1 family of cytokines (Tsutsui et al., 2000; Dinarello and Fantuzzi, 2003; Mühl and Pfeilschifter, 2004; Takeda and Akira, 2005). Co-culture experiments revealed that IL-12/Flg-activated PBMC mediate IFNγ-dependent iNOS in adjacent cells, indicating that the net outcome of this stimulatory condition is in fact proinflammatory and in accord with Th1-like conditions.
We also investigated whether Flg can augment expression of IFNγ-inducible genes. For that purpose two cytokines with divergent functions in Th1/Th2 decisions were evaluated. Here we identified a potent synergism between Flg and IFNγ in THP-1 cells or PBMC with regard to production of IP-10. This CXC-chemokine is a potent amplifier of Th1 responses by attracting activated Th1 cells to sites of inflammation and immunoactivation and by enhancing production of IFNγ (Gangur et al., 1998; Dufour et al., 2002). We and others have previously reported on similar synergisms involving IFNγ and IL-1β or TNFα. These observations are based on the potential of NF-κB to augment expression of IP-10 (Majumder et al., 1998; Hellmuth et al., 2004). The second IFNγ-inducible gene that we chose to investigate is IL-18BP. IL-18BP, a soluble decoy receptor for IL-18 that operates as its functional antagonist, is constitutively expressed but also upregulated by IFNγ (Novick et al., 1999; Paulukat et al., 2001; Hurgin et al., 2002; Mühl and Pfeilschifter, 2003). Notably, analysis of IL-18BP revealed a different picture. In accord with previous observations (Corbaz et al., 2002), we observed a fairly modest upregulation of IL-18BP by IFNγ in THP-1 cells or PBMC. Neither Flg alone nor Flg in combination with IFNγ affected IL-18BP expression. Taken together, Flg differentially regulates IP-10 and IL-18BP and thus appears to exhibit an immunomodulatory profile that favours inflammation and the development of Th1-like cytokine responses. This assumption is furthermore supported by the present observation that release of IL-4 by PBMC exposed to A23187 was not affected by Flg.
Data presented herein relate TLR5 ligation by Flg to protective or pathological functions of IL-18 in host defence or inflammation respectively. Upregulation of IL-18 has been associated with a broad array of acute and chronic inflammatory conditions, among them sepsis and Crohn's disease (Tsutsui et al., 2000; Dinarello and Fantuzzi, 2003; Mühl and Pfeilschifter, 2004). Targeting the TLR4 ligand LPS by antibody therapy repeatedly failed to improve survival in sepsis clinical trials (Riedemann et al., 2003) implying that other factors including Flg may contribute to this syndrome. The present data support the concept that neutralization of Flg might be an additional strategy to control the cytokine storm in sepsis induced by motile bacteria. This may particularly apply to sepsis-associated pulmonary inflammation which appears to be highly sensitive towards Flg (Liaudet et al., 2003; Szabo, 2003). Inflammation in Crohn's disease is associated with upregulation of local (Monteleone et al., 1999) and systemic (Kanai et al., 2000) IL-18. Protection by IL-18BP in experimental colitis (ten Hove et al., 2001) suggests a role for IL-18 in this disorder. In fact Crohn's disease is considered a Th1-like condition (Rogler, 2004) which is highlighted by clinical success of IL-12 neutralization in patients (Mannon et al., 2004). In accord with this concept it has been demonstrated that lack of IP-10 ameliorates colitis in IL-10–/– mice (Singh et al., 2003). The function of TLRs in colonic inflammation is complex. Although it has been shown that TLR4 via IL-12 can augment experimental colitis (Kobayashi et al., 2003), recent data imply that physiological TLR activation by commensal bacteria mediates protective functions (Rakoff-Nahoum et al., 2004), likely by upregulation of protective factors, e.g. heat-shock proteins and transforming growth factor-β1. In addition, physiological TLR signalling may restrain bacterial invasion into the colonic mucosa by inducing shelter molecules such as defensins (Vora et al., 2004). However, once the intestinal barrier has collapsed, TLR signalling is obviously detrimental. Notably, Flg is an efficient proinflammatory signal for colon epithelial cells (Szabo, 2003; Ramos et al., 2004). The relevance of Flg for the pathogenesis of this disorder is furthermore highlighted by the fact that antibodies against Flg are highly abundant in sera of Crohn patients (Sitaraman et al., 2004). Flg may thus be of pivotal importance for innate as well as adaptive mechanisms that trigger chronic inflammation in Crohn's disease.
Signal transduction and thus cellular functions of TLRs widely overlap. In fact, upregulation of IL-18 secretion has been observed as well with the TLR4 ligand LPS (Mehta et al., 2001; Hentze et al., 2003). Nevertheless, dependent on the pathophysiological context, distinct TLRs certainly mediate specific functions in host defence. The relevance of the Flg/TLR5 pathway for immune defence has been highlighted by the observation that humans lacking a functional TLR5 are prone to pneumonia induced by Gram-negative Legionella pneumophila (Hawn et al., 2003). Notably, L. pneumophila infections are controlled by IFNγ and IL-18 contributes to production of IFNγ in murine models of this infection (Brieland et al., 2000; Deng et al., 2001). Therefore, despite the apparent redundancy of the TLR system, specific TLR5 functions can be of pathophysiological relevance. Interestingly, L. pneumophila is not detected by TLR4 on murine macrophages which may be due to the fact that LPS from these bacteria has a markedly reduced immunostimulatory potential (Hawn et al., 2003). In addition, mobile Gram-positive bacteria such as Listeria monocytogenes are detected by TLR5 (Hayashi et al., 2001). Actually, human dendritic cells infected with L. monocytogenes release IL-18 (Kolb-Maurer et al., 2003) which is of pivotal importance for the control of these bacteria in the respective murine infection model (Neighbors et al., 2001). These observations suggest that Flg-induced amplification of mat-IL-18 release as detected in the present study may contribute to protection against certain bacterial infections in humans.
In summary, we present evidence that pathophysiologically relevant concentrations of Flg potently enhance release of mat-IL-18 and prototype Th1-like cytokines such as IFNγ and IP-10 from human PBMC. These observations indicate that under certain conditions TLR5 may significantly contribute to immune defence against infections and to the pathogenesis of acute and chronic inflammation associated with infection by motile bacteria.
Experimental procedures
Materials
Interferon-γ and IL-18 were from Peprotech (Frankfurt, Germany). Nigericin and LPS (Escherichia coli Serotype 0127:B8) were from Sigma (Deisenhofen, Germany). IL-12 was from R&D systems (Wiesbaden, Germany). Recombinant Flg (Salmonella muenchen) and Polymyxin B (PmxB) were from Calbiochem-Novabiochem GmbH (Bad Soden, Germany). Z-YVAD-FMK was from Bachem AG (Weil am Rhein, Germany). AACOCF3 and A23187 were from Alexis (Grünberg, Germany). Trypsin was from Gibco-BRL (Eggenstein, Germany).
Isolation and cultivation of PBMC, co-cultures of PBMC with DLD-1 colon carcinoma cells and cultivation of THP-1 cells
The study protocol and consent documents were approved by the ‘Ethik Kommission’ of the Klinikum der Johann Wolfgang Goethe-Universität. Informed consent was obtained from volunteers. Healthy donors abstained from using drugs during 2 weeks before the study. PBMC were freshly isolated using Histopaque®-1077 (Sigma). PBMC were resuspended in RPMI 1640 supplemented with 10 mM Hepes, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin and 10% heat-inactivated FCS (Gibco-BRL) and seeded at 3 × 106 cells ml−1 in round-bottom polypropylene tubes (Greiner, Frickenhausen, Germany). In some experiments adherent PBMC, which constitute a monocyte-enriched fraction, were prepared. For that, PBMC (6 × 106 cells per 2 ml in the aforementioned medium) were seeded in six-well polystyrene plates (Greiner). After 2 h, non-adherent cells were removed, adherent cells was washed twice with culture medium, and 2 ml of the aforementioned supplemented culture medium were added per well. For transwell co-cultures (in the aforementioned) medium PBMC (7.5 × 106 cells per insert) were seeded into Transwell-Clear inserts (0.4 µm pore size, Costar, Bodenheim, Germany). Inserts were placed onto confluent human DLD-1 colon carcinoma cells (Centre for Applied Microbiology and Research, Salisbury, UK), grown in six-well polystyrene-plates (Costar). Monocytic THP-1 cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Cells were maintained in the aforementioned supplemented RPMI 1640 culture medium using polystyrene flasks (Greiner, Frickenhausen, Germany). For experiments, 5 ml or 1 ml of cell suspensions were seeded into 10 cm or 24-well polystyrene plates (Greiner) at 106 cells ml−1 using supplemented RPMI 1640 cell culture medium. All incubations of either PBMC or THP-1 cells were performed at 37°C and 5% CO2.
Human whole blood cultures
Heparinized blood was mixed with an equal volume of culture medium (RPMI 1640 supplemented with 25 mM Hepes, 100 U ml−1 penicillin, 100 µg ml−1 streptomycin) and 1 ml aliquots were transferred into round-bottom polypropylene tubes (Greiner). The sealed tubes were incubated upright at 37°C and 5% CO2 for the indicated time periods.
Detection of cytokines in cell-free culture supernatants by ELISA
Levels of IL-18 in culture supernatants obtained from PBMC or THP-1 cultures were determined by ELISA according to the manufacturer's instructions. This ELISA specifically detects mat-IL-18 (MBL/Biosource, Solingen, Germany). Levels of IL-10, IL-4 (Biosource), IL-8, interferon-inducible protein-10 (IP-10, CXCL-10), IFNγ and TNFα (Pharmingen, Heidelberg, Germany) were determined by ELISA according to the manufacturers’ instructions.
Detection of human IL-18, IκBα and iNOS by immunoblot analysis
For detection of intracellular proteins, cells were treated with lysisbuffer [100 mM NaCl, 20 mM TrisCl, pH 7.8, 0.1% NP-40, supplemented with protease inhibitor cocktail (Roche Molecular Biochemicals) and DTT, Na3VO4, PMSF and NaF each 1 mM]. Fifty micrograms of total protein lane−1 were used. Antibodies: IL-18 (15% SDS-PAGE), murine monoclonal antibody (Immunotools, Friesoythe, Germany); inhibitor (I)κBα (10% SDS-PAGE), rabbit polyclonal antibody (Santa Cruz Biotechnology, Heidelberg, Germany); β-actin (10% SDS-PAGE), murine monoclonal antibody (Sigma); iNOS (7.5% SDS-PAGE), murine monoclonal antibody (Transduction Laboratories Hamburg, Germany).
Analysis of IL-18BP by real-time quantitative polymerase chain reaction (PCR) analysis
Real-time polymerase chain reaction (PCR) analysis of IL-18BP and glyceraldehyde-phosphate dehydrogenase (GAPDH) was performed as recently described (Möller et al., 2003). Changes in fluorescence are caused by the Taq-polymerase degrading the probe that contains a fluorescent dye (FAM for IL-18BPa, VIC for GAPDH) and a quencher (TAMRA). Primers and probe for IL-18BPa were designed using Primer Express (Applied Biosystems) according to the published sequence (XM 035063.1): forward 5′-acc tcc cag gcc gac tg-3′; reverse 5′-cct tgc aca gct gcg tac c-3′; probe 5′-cac cag ccg gga acg tgg ga-3′. The possibility of amplification of contaminating genomic DNA was eliminated by selecting an amplicon which crosses an exon/intron boundary. For GAPDH pre-developed assay reagents were used (Applied Biosystems). Specificity of PCR products was tested by classic PCR using the aforementioned primers. One microgram of total RNA was transcribed using random hexameric primers and Moloney virus reverse transcriptase (Applied Biosystems) according to the manufacturer's instructions. Real-time PCR was performed on the AbiPrism 7700 Sequence Detector (Applied Biosystems) as follows: one initial step at 50°C for 2 min and 95°C for 2 min was followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Detection of the dequenched probe, calculation of threshold cycles (Ct values) and further analysis of these data were performed by the Sequence Detector software. mRNA expression was quantified by use of cloned cDNA standards for IL-18BPa and GAPDH. All results for IL-18BPa expression were normalized to that of GAPDH.
Statistics
Data are shown as mean ± SD (experiments using THP-1 cells), or mean ± SEM (experiments using PBMC), and are presented as pg ml−1, ng ml−1, % of ATP/Flg alone, or as fold induction compared with unstimulated control. Unless otherwise stated, raw data were analysed by unpaired Student's t-test on raw data using Sigma Plot (Jandel Scientific).
Acknowledgements
The authors gratefully acknowledge the skilful technical assistance of Silke Kusch. This work was supported by a grant of the Deutsche Forschungsgemeinschaft DFG MU 1284/3-2 (to H.M.).