The role and regulation of programmed cell death in plant–pathogen interactions
Summary
It is commonly known that animal pathogens often target and suppress programmed cell death (pcd) pathway components to manipulate their hosts. In contrast, plant pathogens often trigger pcd. In cases in which plant pcd accompanies disease resistance, an event called the hypersensitive response, the plant surveillance system has learned to detect pathogen-secreted molecules in order to mount a defence response. In plants without genetic disease resistance, these secreted molecules serve as virulence factors that act through largely unknown mechanisms. Recent studies suggest that plant bacterial pathogens also secrete antiapoptotic proteins to promote their virulence. In contrast, a number of fungal pathogens secrete pcd-promoting molecules that are critical virulence factors. Here, we review recent progress in determining the role and regulation of plant pcd responses that accompany both resistance and susceptible interactions. We also review progress in discerning the mechanisms by which plant pcd occurs during these different interactions.
Introduction
Host cell death occurs during many, but not all, interactions between plants and the pathogens that infect them. This cell death can be associated with disease resistance or susceptibility, depending on the lifestyle of the pathogen. What is the role of host cell death during pathogenesis? Do all cells in an infection zone die by the same mechanism? Which other cellular processes are influenced when some host cells die during infection? As will be addressed in this review, the answers to these questions depend on the host–pathogen interaction being examined. However, what is clear is that cell death regulation is often intimately linked to a number of signalling pathways that influence other defence processes. Furthermore, cell death can serve to amplify other defence responses as well as to promote the aggressiveness and/or dissemination of some pathogens. As such, understanding cell death mechanisms of control and execution offers potential targets for modulating both host disease resistance and susceptibility. If cells die by different mechanisms depending on the host–pathogen combination, this information could be used selectively to target different pathways or, at the very least, could lead to the development of diagnostic tools that would help in the dissection of cell death and defence regulatory networks. Finally, if cells even within one host–pathogen interaction die by different mechanisms, it will be important to recognize that the function and regulation of these cell death events could be distinct.
Recent work suggests that pathogenesis-associated cell death in plants has common regulatory and mechanistic features with apoptosis in animals (for example, see Liang et al., 2003). This observation offers the possibility of understanding the basal eukaryotic cell death machinery and studying how its activation is linked to other cell biological and signalling events during pathogenesis. The purpose of this review is to analyse critically what is known about the role and mechanism of cell death in different host–pathogen interactions. The reader may also find several older reviews useful for insight into this topic (Greenberg, 1997; Morel and Dangl, 1997; Heath, 2000).
Cell death during disease resistance
Perhaps the most well-known cell death response in plants is the hypersensitive response (HR) associated with a phenomenon termed the resistance response (RR). The RR involves the co-ordinate activation of many defences that limit pathogen growth (Greenberg, 1997). For the purpose of this review, the HR is considered to be only the cell death component of the RR. An RR is triggered when the host has a dominant R gene that corresponds to a dominant avr gene in the pathogen. This gene-for-gene interaction results from either direct or indirect interaction between the R gene and avr gene products depending on the R–avr gene pair. Two cases of direct interactions have been described (Tang et al., 1996; Jia et al., 2000). Additionally, one interaction was shown to occur via formation of a complex probably involving an R protein, an Avr protein and an additional host protein(s) (Leister and Katagiri, 2000). Finally, an R–Avr interaction was recently found to result from the enzymatic activity of the Avr protein (Shao et al., 2003). Although RRs are often associated with the HR, in some cases, they can occur without or with very little cell death (for example, see Bendahmane et al., 1999).
Despite the observation that many RRs are accompanied by an HR, the signal transduction requirements for the RR and the HR can be different depending on the host–pathogen combination. Sometimes, the same gene can be required for the HR in response to one avirulent pathogen and can negatively regulate or be largely dispensable for the HR in response to different and/or related pathogens (see AtRBOHD/AtRBOHF, NDR1 and RAR1 examples in Table 1). Additionally, the major defence signal salicylic acid has both cell death-enhancing and cell death-suppressing roles depending on the avirulence factor or pathogen (see discussion in Vanacker et al., 2001). These different signalling requirements do not address whether the HRs eventually feed into a common cell death mechanism, although it is widely assumed that they do. Furthermore, the events that occur during different RRs can be quantitatively different. For example, the HR can occur with very different timing in response to highly related avirulent pathogens (Ritter and Dangl, 1996). Additionally, other defence-related events, such as the induction of cell wall papillae, which are thought to impede pathogen egress into plant cells, can occur with different timing and to different degrees depending on the R gene conditioning resistance (Freialdenhoven et al., 1994).
| HR effecta | Protein/functions | Comments/reference(s) |
|---|---|---|
| – | CPN1, possible phospholipid-binding protein and Ca2+-sensitive modulator of environmental responses | Affects Arabidopsis HR with P. syringae/avrRpt2. Loss of function mutant causes activation of basal defences and spontaneous cell death (Jambunathan et al., 2001) |
| –P | AtCYS1, cystatin with cysteine proteinase inhibitory activity | Ectopic expression in Arabidopsis suspension cells or tobacco plants reduces cell death in response to avirulent P. syringae (Belenghi et al., 2003) |
| –P | α-DOX1, oxygenates fatty acids and protects cells from oxidative stress | Arabidopsis with reduced α-DOX1 levels show an increased cell death with paraquat treatment and an increased HR with P. syringa e/avrRpm1. These plants also support higher growth of bacteria (Ponce de Leon et al., 2002) |
| –P | LeETR4, ethylene receptor | Tomato with antisense LeETR4 show a faster HR with X. campestris pv. vesicatoria (Ciardi et al., 2001) |
| – | FtsH, plastid protease presumed active during stress responses | Overexpression in tobacco enhances TMV resistance, reduced expression reduces resistance (Seo et al., 2000) |
| –P | HSR203J, similar to esterases/hydrolases | Tobacco with HSR203J antisense has an accelerated HR in response to avirulent P. syringae and P. parasitica. Transgenic plants are also affected in the transcription of defence-related genes (Tronchet et al., 2001) |
| –Pb | LSD1, Zn2+ finger protein. May interact with the related protein LOL1. May indirectly regulate superoxide levels | Loss of LSD1 in Arabidopsis leads to ectopic death and increased basal resistance (Jabs et al., 1996; Dietrich et al., 1997; Rusterucci et al., 2001; Aviv et al., 2002; Epple et al., 2003) |
| – | NAD7, mitochondria complex I subunit | Loss of NAD7 in tobacco enhances TMV resistance. Mutant has upregulated antioxidant enzymes (Dutilleul et al., 2003) |
| – | RDS, not yet cloned | Dominant suppressor of HR in oat–P. coronata interaction (Yu et al., 2001) |
| +P | EDS1, lipase-like protein | Important for HR induction of Arabidopsis by P. parasitica (Parker et al., 1996) |
| + | LOL1, Zn2+ finger protein, may interact with LSD1, possible cellular redox sensor | Affects HR of Arabidopsis in response to avirulent P. syringae; overexpression induces cell death (Epple et al., 2003) |
| + | MEK, mitogen-activated protein kinase (MAPK) cascade component | Gain of function allele in tobacco confers NbrbohB-dependent cell death (Yoshioka et al., 2003 and references therein). Other members of the MAPK cascade may also regulate the HR (Shirasu and Schulze-Lefert, 2003) |
| +P | AtMYB30, possible transcription factor | Important for HR induction in Arabidopsis and tobacco by various pathogens (Vailleau et al., 2002) |
| + | AmMYB308, possible transcription factor from antirrhinum. Negative regulator of phenolic acid metabolism when ectopically expressed in tobacco | Ectopic expression of AmMYB308 in tobacco leads to a faster HR in response to P. syringae, alterations in leaf morphology and precocious cell death (Tamagnone et al., 1998) |
| + | PTI, part of the PTO RR signal transduction cascade | Overexpression of PTI from tomato in tobacco enhances the HR induced by P. syringae/avrPto (Zhou et al., 1995) |
| + | RAR1, component of the ubiquitin proteolysis system, interacts with SGT1 | Important for barley Mla12-mediated HR but largely dispensable for Mlg-mediated HR during B. graminis infection (Freialdenhoven e t al., 1994). Functions with many R genes (Shirasu and Schulze-Lefert, 2003) |
| + | RIH, not yet cloned | Required for the HR as assayed indirectly by whole-cell autofluorescence of oat in response to P. coronata. Resistance appears to be unaffected by the rih mutation (Yu et al., 2001) |
| +E | NbrohA/NbrbohB (gp91phox homologues), components of the NADPH oxidase complex | Reduced expression leads to reduced HR of tobacco infected with P. infestans (Yoshioka et al., 2003) |
| + | SGT1, interacts with RAR1 | Important for the function of many R genes in a number of plant species (Shirasu and Schulze-Lefert, 2003) |
| +P | SID2/EDS16, isochorismate synthase, probable salicylic acid biosynthetic enzyme | Arabidopsis mutants are hypersusceptible to many pathogens. Important for the HR in response to P. syringae/avrRpt2 (Zhang et al., 2002) |
| –/+P | NDR1, possible membrane-associated protein, important for salicylic acid accumulation in response to P. syringae and UV-C, defective in H2O2 accumulation during the HR | HR-repressing in Arabidopsis in response to many P. syringae isolates carrying avr genes. HR-activating function in response to P. syringae/avrRpt2 (Century et al., 1995; Shapiro and Zhang, 2001) |
| –/+ | AtRBOHD/AtRBOHF, components of the NADPH oxidase complex involved in producing extracellular superoxide as part of the oxidative burst | The Arabidopsis double mutant has spontaneous cell death late in development. Proteins are HR-repressing only in response to a P. parasitica isolate that induces a weak RR response; they are HR-promoting in response to avirulent P. syringae (Torres et al., 2002) |
| –/+ | RIN4, membrane-localized protein that can interact with R proteins RPM1 and RPS2 as well as the Avr protein AvrB. rps2– mutants expressing avrRpt2 have no RIN4 protein | Reduced expression in Arabidopsis leads to decreased HR upon infection with P. syringae/avrRpm1 and constitutive activation of cell death (Mackey et al., 2002; Axtell and Staskawicz, 2003). Overexpression suppresses the HR induced by P. syringae/avrRpt2 (Mackey et al., 2003) |
- a . P indicates pathogen-inducible gene, E indicates elicitor-inducible gene.
- b . H. Lu and J. T. Greenberg, unpublished observations.
- Proteins are organized in alphabetical order within each HR-influencing class.
Cell death mechanisms during the HR
The view that the HR is an active process of the host and may be a form of programmed cell death (pcd) was supported by early observations that host cells must be metabolically active and, in some cases, the HR requires active host protein synthesis for its induction by fungi (Nozue et al., 1977 and references therein; Heath, 2000) and bacteria (Keen et al., 1981). Subsequent studies have shown that the HR is subject to genetic control, with factors important for its positive and negative regulation being identified (Table 1). The morphology of cells undergoing the HR at late stages suggest that it is a form of pcd with some apoptotic features. In particular, apoptotic-like bodies with avirulent Pseudomonas syringae infections were observed (Levine et al., 1996). We know of only a few studies using ultrastructural analysis of morphological events that occur during a time course of the HR. Bestwick et al. (1995) found early changes in mitochondrial morphology (swelling and cristae disorganization) in avirulent P. syringae-infected lettuce, similar to what occurs in animal cells undergoing apoptosis (Wakabayashi and Karbowski, 2001). Later stages of the infection were accompanied by membrane dysfunction (loss of ability to be plasmolysed) and progressive vacuolization of the cytoplasm. Membrane damage was proposed to be the critical event for cell death. Apoptosis-related chromatin condensation and endonucleolytic cleavage were not reported. However, there was a gap in the time course in which these events may have occurred. Thus, the HR in this system has a subset of apoptotic features and may also be similar to a pcd process called oncosis that involves vacuolar disruption (Jones, 2000).
Further studies looking at a range of host–pathogen interactions using detailed time courses could establish whether apoptosis-related events such as mitochondrial swelling, chromatin condensation and endonucleolytic cleavage occur before general organelle dysfunction. This would be expected if the HR occurs by an apoptotic-like mechanism. To begin to address this question, in collaboration with Shigeyuki Mayama at Kobe University, we have analysed the interaction between oat and the fungal pathogen Puccinia coronata as well as the interaction between Arabidopsis and avirulent P. syringae. Strikingly, we often observe that cells adjacent to the first cells that die have the apoptotic features of chromatin condensation and endonucleolytic cleavage in both interactions (Fig. 1). In these cells, we found no evidence for oncosis. We suggest that cells in an infection zone may die by multiple mechanisms, a possibility that requires further investigation. It is also possible that there are differences in the mechanisms of pcd used in different host–pathogen interactions.
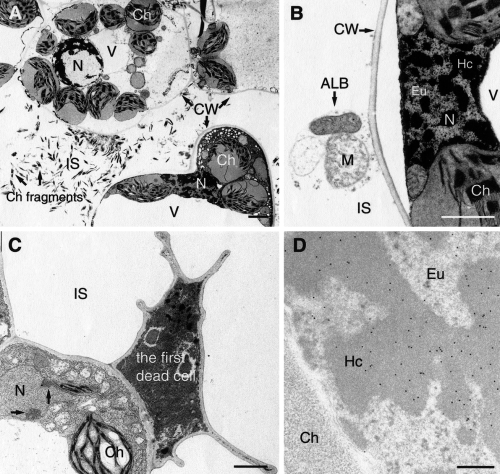
Ultrastructural features of cells undergoing the HR. A, B and D. Primary leaves of oat cv. Shokan 1 were inoculated with the HR-inducing incompatible race 226 of P. coronata f. sp. avenae for 24 h. The cells adjacent to the first HR collapsed cell were observed. The corpses of the initial HR cells are fragmented, as evidenced by the chloroplast fragments (A) and membrane-bound body (representing an apoptotic-like body) containing swollen mitochondria (B) in the intercellular spaces. Cells adjacent to the fragmented initial collapsed cells had the typical apoptotic features of chromatin condensation and intact tonoplasts (A and B). C. 21-day-old Arabidopsis (ecotype Col-0) leaves were infiltrated with avirulent P. syringae carrying avrRpm1 at 5 × 106 cfu ml−1 for 21 h. Note the initiation of chromatin condensation (arrows) in the cell adjacent to the first dead cell. D. Abundant gold label detecting endonucleolytic cleavage DNA using an Apoptag plus fluorescein in situ apoptosis detection kit (Intergen) can be seen in the condensed heterochromatin portion. ALB, apoptotic-like body; Ch, chloroplast; CW, cell wall; Eu, euchromatin; Hc, heterochromatin; IS, intercellular space; M, mitochondrion; N, nucleus; V, vacuole. Bar in (A), (B) and (C) = 1 µm; in (D) = 200 nm.
Evidence for endonucleolytic cleavage of DNA during the HR of cowpea was found during infections with cowpea rust fungus (Heath, 2000). Additionally, the involvement of proteases in the RR and/or HR has been shown in a number of host–pathogen interactions (Heath, 2000; Grey, 2002). In animals, apoptosis often involves proteases called caspases (Green and Reed, 1998). Although clear homologues of caspases have not been found in the complete genome of the model plant Arabidopsis, caspase-like activities in plants have been documented biochemically or inferred from inhibitor studies (for example, see Elbaz et al., 2002; Lincoln et al., 2002; del Pozo and Lam, 2003). Lam et al. (2001) have suggested that, as in animals, mitochondria could have a role in controlling the HR. Evidence for a role of plastids in virus-induced HR is indicated by the change in the amount of cell death during infection in plants when levels of the plastid protease FtsH are increased or decreased (Seo et al., 2000).
Signalling during the HR
A strategy for studying the signalling requirements and mechanism of cell death during the HR is to use simplified experimental systems. In particular, pathogen-derived molecules, called elicitors, that induce HR-like cell death reactions have been used either by applying them directly to plant cells or by expressing the genes for these elicitors directly in plant cells. In some cases, the elicitors have been shown to be Avr products. Some researchers have also used pathogen infections of plant cell cultures. Such systems are potentially very powerful for identifying HR signalling components, for determining the relationship between cell death and other defence-related events and for studying the involvement of organelle changes in cell death.
Using such approaches, an oxidative burst (Heath, 2000), ion channels (Atkinson et al., 1996; Heath, 2000; Wendehenne et al., 2002), NO (Delledonne et al., 2001) and the interaction between some of these different signals (Delledonne et al., 2001) were implicated in HR control. The exact role of these molecules is still the subject of intense investigations by many researchers. Using protoplasts of Arabidopsis, we recently found that the HR in response to specific Avr proteins could be recapitulated. Using this system and careful flow cytometric and microscopic analysis, we have established that the HR involves a mitochondrial permeability transition (N. Yao and J. T. Greenberg, unpublished observations). In animal cells, such transitions are often important components of the apoptotic mechanism because of the release of the cell death-inducing cytochrome c (Green and Reed, 1998). Whether cytochrome c release is necessary for HR induction has not yet been examined. However, Hansen (2000) found that Agrobacteria-infected maize suspension cells exhibited an apoptotic-like response that was inhibited in transgenic maize cells producing antiapoptotic proteins from animal viruses. Cell death was accompanied by cytochrome c release to the cytoplasm. A careful analysis of the timing of cytochrome c release relative to other apoptosis-related events needs to be established to determine whether such release could be causal to cell death.
Use of simplified systems in which one or two proteins from pathogens are expressed in host cells has also revealed that the extracellular bacterial pathogen P. syringae injects into plants antiapoptotic proteins in addition to the known proapoptotic Avr activities (Abramovitch et al., 2003; Espinosa et al., 2003). Experiments by Abramovitch et al. (2003) in particular suggest that the antiapoptotic activity acts at a different place in the cell death pathway from the proapoptotic activities. Indeed, the antiapoptotic activity was active in the heterologous system of yeast Saccharomyces cerevisiae (Abramovitch et al., 2003). It is intriguing that P. syringae secretes antiapoptotic activities. Doke (1983) showed that the fungal pathogen Phytophthora infestans also possesses both cell death-promoting and cell death-inhibitory molecules. We speculate that, in some infections, the cell death pathway may be only partially or transiently activated. In support of this view, we have observed that host cells two or three cells distal to the primary fungus-infected cells undergoing an HR have some apoptotic features early after infection. However, similarly positioned cells late in the infection appeared to recover, showing thicker cell walls and fewer chloroplasts, but lacking apoptotic features (N. Yao, unpublished observations). The role of such partially apoptotic and/or recovered cells remains to be elucidated. Interestingly, some viral pathogens of animals also use both proapoptotic and antiapoptotic proteins to manipulate their hosts at different times in the infection (Munger et al., 2003).
It is possible that cells in an infection zone undergoing an RR may not all die from the same signals. In particular, the HR has been suggested to require the correct relative levels of both NO and H2O2 to be induced in the host (Delledonne et al., 2001). However, NO was found to be generated first at cell surfaces in avirulent P. syringae-infected Arabidopsis at a time too late to be causal to the first cell death events during the HR. The pattern of NO generation suggests that it has a role in cell–cell signalling and the spreading of cell death as an infection proceeds (Zhang et al., 2003). Furthermore, inhibition of NO synthesis or action only attenuates the HR, in support of this role. Interestingly, an Arabidopsis AtbohF/AtbohD double mutant in NADPH oxidase complex components, thought to generate an oxidative burst during the HR, lacks detectable H2O2 accumulation during an RR (Torres et al., 2002). This double mutant still activated some early cell death. Torres et al. (2002) suggested that the first cell deaths occur independently of H2O2 and subsequent death requires H2O2 generation. Thus, initial cell deaths during the HR may be both NO and H2O2 independent. One caveat to this conclusion is that the studies on NO and H2O2 production relative to cell death relied largely on whole-tissue analyses. It is possible that highly localized subcellular generation of these signals was missed using these techniques. For example, when cell death in oat is induced by the toxin victorin, H2O2 accumulation is mainly found in localized mitochondrial pores (Yao et al., 2002a).
Is the HR important for pathogen resistance?
A number of attempts have been made to address the role of cell death in disease resistance. Many experiments have involved using pharmacology and genetics combined with an analysis of the timing of pathogen arrest relative to cell death. Interpretations of these experiments can be difficult as a number of genes and signal molecules affect more than just cell death (Table 1). What has emerged is that the importance of cell death in resistance depends on the host–pathogen interaction. This is because, as mentioned above, not all RR are the same because of differences in the strength of signalling and the downstream defences that are activated. Certainly, in some cases, pathogen arrest can occur in the absence of cell death (see below) and, conversely, cell death may not be sufficient for pathogen arrest when other defences are compromised (Century et al., 1995).
A seminal paper from Baulcombe and colleagues examined Rx-mediated resistance against the PVX virus (Bendahmane et al., 1999). They found that resistance to this virus occurs without the HR. Intriguingly, the Rx protein resembles many other R proteins that are known to trigger an HR. Ectopic expression of the viral Avr elicitor and the Rx protein can induce an Rx-dependent HR. This has led to the suggestion that, in some host–pathogen interactions, a phenomenon called ‘extreme resistance’ without cell death occurs because sufficient R protein signalling can arrest the pathogen. A quantitatively greater interaction would then lead to the HR, which Baulcombe and colleagues consider as a mechanism to reinforce the front-line ‘extreme resistance’ defence reaction in some host–pathogen interactions. A prediction of their idea is that some RR's produce defences that are extremely rapid and robust, arresting the pathogen before cell death. Work from Schulze-Lefert and colleagues has shown that resistance against the obligate pathogen powdery mildew (Blumeria graminis f. sp. hordei) fits well with this hypothesis (Freialdenhoven et al., 1994; Peterhänsel et al., 1997). In the case of Mlg-mediated resistance, rapid and robust activation of cell wall alterations restricts pathogen growth before cell death. In contrast, Mla12-mediated resistance is very closely correlated with the HR. Similarly, increasing the HR strength against an isolate of the obligate pathogen Peronospora parasitica, which normally triggers a weak RR, strongly enhances disease resistance (Torres et al., 2002). In general, it is likely that cell death may have a larger contribution to defence with obligate pathogens that require living cells in order to replicate.
To evaluate rigorously the contribution of the HR to the RR, the ideal experiment would involve the selective inhibition of the HR. To do this, one needs to know the components of the cell death machinery and the selectivity of the reagent used to inhibit the machinery. As mentioned above, in plants, there is some evidence that the HR involves the activation of caspase-like activities. If in plants these caspase-like activities are truly specifically involved in activating pcd, then they provide an ideal target to disrupt in order to test the involvement of the HR in resistance. A challenge for future studies is to establish rigorously whether these plant caspase-like activities are solely involved in cell death control.
To test the possible involvement of plant caspases in resistance, del Pozo and Lam (2003) placed the caspase-inhibitory protein p35 from baculovirus in tobacco that normally shows resistance to TMV because of the presence of the N resistance gene. They infected the transgenic plants with TMV and found that the lesions were the same size and number as in infected wild-type plants, but their appearance was ‘less dehydrated’. This suggests that the process of pcd was partially interrupted. Strikingly, in p35-expressing plants, TMV was not restricted to local lesions as in non-transgenic N plants, but rather spread systemically throughout the plant. It will be important to examine the ultrastructure of the p35-expressing TMV-infected plants to see exactly how the HR is altered. However, these results argue strongly for a role for the HR in limiting TMV replication. Importantly, a catalytically inactive form of p35 was ineffective in altering the host–virus interaction. A similar approach was attempted using plants with altered expression of the plant-derived Bax inhibitor-1 (BI-1) protein (Huckelhoven et al., 2003). Overexpression of BI-1 attenuates cell death caused by Magnaporthe grisiae-derived elicitors (Matsumura et al., 2003). However, BI-1 manipulation also strongly affects other defences in addition to cell death (Huckelhoven et al., 2003), making it possible that the cell death machinery is not the only target of BI-1. The identification of the basal cell death machinery in plants will hopefully lead to additional rigorous experiments to test the role of the HR in resistance.
Other experiments to alter the HR, mainly using genetics and transgenic approaches to cause its attenuation or acceleration, may also be informative for examining the role of the HR in resistance. However, the interpretation of the experiments must be done cautiously, especially when pleiotropic effects of manipulations are known. For example, the RR can be uncoupled from the HR to most avirulent P. syringae in the dnd1 and hlm1 mutants of Arabidopsis (Yu et al., 1998; Balague et al., 2003). However, these mutants have constitutively active defences, making the interpretation of the lack of an HR difficult. Many studies have found that conditions that caused attenuated or increased disease resistance also caused similar changes in HR strength at least with some pathogens (Freialdenhoven et al., 1994; Seo et al., 2000; Jambunathan et al., 2001; Tronchet et al., 2001; Torres et al., 2002; Vailleau et al., 2002; Epple et al., 2003; Yoshioka et al., 2003). Exceptions to this pattern have also been described for some host–pathogen combinations (for example, see Century et al., 1995; Yu et al., 1998; Rate and Greenberg, 2001; Ponce de Leon et al., 2002; Torres et al., 2002). In most of these cases, however, the alterations were documented to be pleiotropic, affecting other defence-related processes or causing some spontaneous cell death.
Other functions for the HR
In addition to playing a role in limiting pathogen growth directly, the HR may have additional contributions to defence. Careful work by Kauffmann and colleagues using the fungal glycoprotein elicitin from Phytophthora megasperma to elicit HR-like cell death has shown a strong association between cell death and the subsequent activation of specific defences in neighbouring tissue (Costet et al., 1999). Such systemic signalling is important for protecting plants from future infections. Similarly, plants showing an HR after infection with avirulent P. syringae exhibit stronger systemic resistance to subsequent infection with virulent P. syringae than if the initial response lacks an HR (Shapiro and Zhang, 2001).
Other forms of disease resistance-associated cell death
Induction of systemic signalling resulting in acquired resistance is often accompanied by the rapid induction of localized cell death by pathogens that normally would not induce rapid cell death (reviewed by Greenberg, 1997). For example, plants with acquired resistance show very rapid HR-like cell death upon virulent P. syringae infection. Signalling for this rapid cell death appears not to be associated with H2O2 accumulation, unlike the HR (Wolfe et al., 2000). Overexpression of the Arabidopsis protein AtMyb30 in plants can also cause otherwise slow cell death-inducing pathogens to induce an HR-like response as well as inducing the HR-associated accumulation of oxylipins (Vailleau et al., 2002). Finally, loss of function mlo and edr1 mutants have increased resistance to Blumeria graminis f. sp. hordei and Erysiphe cichoracearum respectively (Peterhänsel et al., 1997; Frye and Innes, 1998). In the case of edr1 mutants, E. cichoracearum triggers increased host cell death around the fungal hyphae (Frye and Innes, 1998). In the case of mlo, the mutant plants develop some spontaneous cell death, but disease resistance upon infection is thought not to require cell death (Peterhänsel et al., 1997). Whether cell death described in these various cases is mechanistically and/or functionally similar is not known.
Cell death during successful pathogen infections (susceptible host–pathogen interactions)
The occurrence of cell death during susceptible host–pathogen interactions in which pathogens can replicate well in their hosts is common. A recent study of oat infected with a broad variety of pathogens (fungi, viruses and bacteria) established that many virulent pathogens induce cell death with apoptotic features (Yao et al., 2002b). Apoptotic-like events occurred with widely different timing depending on the infectious agent and occurred in the directly infected cells and/or the neighbouring cells. Although morphological criteria argue that cells may die by the same mechanism, a more rigorous test would be to use an inhibitor of the apoptotic cell death machinery and examine whether disease symptoms and pathogen growth are altered. This approach has the same caveats as mentioned for the HR studies, in that the specificity of the reagents used must be established.
Tomato producing the anticaspase baculovirus protein p35 showed reduced cell death with a well-characterized mycotoxin that can promote apoptotic cell death on its own (Lincoln et al., 2002). These plants also showed reduced symptoms with a number of pathogens. Recall that tobacco carrying the same protein had less dehydrated cell death and enhanced spread of a virus to which the plants had genetic resistance due to the N resistance gene (del Pozo and Lam, 2003). The alteration of both susceptible and resistance responses by the same anticaspase protein argues that a cell death pathway target is common to the HR and susceptible host responses. In support of apoptotic-like cell death being important for symptoms caused by virulent pathogens, Dickman et al. (2001) found that a number of antiapoptotic proteins from animals, when ectopically expressed in plants, can protect them from symptoms caused by fungal pathogens. However, a recent retraction of some of these data (Dickman et al., 2003) suggests that caution should be used in forming any firm conclusions based on this study. It must be emphasized that these experiments assume that there is a unique plant target for p35 and each of the other antiapoptotic proteins from non-plant organisms, and that this target's only function is to control cell death. A challenge for future experiments is to test these assumptions rigorously.
That pcd and other disease symptoms result from host-encoded functions is supported by a number of findings. Arabidopsis mutants that form disease-like lesions spontaneously have been documented by us and others (Greenberg et al., 2000; Pilloff et al., 2002). One of these mutants, acd5, shows increased disease symptoms and modestly increased growth of P. syringae when infected before spontaneous lesion formation. Interestingly, ACD5 encodes a ceramide kinase (CERK) that is induced during virulent P. syringae infections (Liang et al., 2003). Ceramides are known to be bioactive lipids that activate apoptosis in animals (Hannun and Obeid, 2002). We have shown that ceramide is sufficient to induce apoptotic-like cell death, while its phosphorylated derivative (the product of the CERK reaction) can partially block pcd in Arabidopsis protoplasts. Finally, acd5 mutant plants accumulate increased amounts of the ACD5 CERK substrate. Together, these findings suggest that cell death activated by ceramide is important for P. syringae virulence.
Ceramides are part of the sphingolipid family of bioactive lipids (Hannun and Obeid, 2002). This sphingolipid pathway may be commonly targeted by plant pathogens. Indeed, some fungal pathogens secrete cerebrosides (which are derived from sphingolipids) that induce HR-like cell death in rice (Koga et al., 1998). Additionally, a number of fungal pathogens secrete related mycotoxins (for example, AAL and fumonisin) that cause pcd and disrupt sphingolipid metabolism (Abbas et al., 1994).
Multiple signals control susceptible cell death
Like the HR, disease symptoms caused by at least some pathogens may be a mixture of events, each of which is under the control of different signals. In tomato–Xanthomonas campestris interactions, disease symptoms are in part attributable to the known host signal molecules ethylene and salicylic acid (O’Donnell et al., 2001). However, plants blocked for these pathways show a normal initial cell death response but then fail to develop subsequent cell death and chlorophyll loss (a late pathogenesis symptom). Ethylene-insensitive, ethylene-deficient and salicylic acid-deficient tomato plants were described as pathogen tolerant because they did not have altered replication of the pathogen even though symptoms were attenuated. A similar finding was made with ethylene-insensitive Arabidopsis mutants infected with bacterial pathogens (Bent et al., 1992). We have also found that attenuation of chlorophyll catabolism in Arabidopsis during P. syringae infection reduced overall cell death without reducing pathogen growth (Mach et al., 2001).
A few additional examples of mutants and natural variants of Arabidopsis that display tolerance to bacterial pathogens have been described (reviewed by Greenberg, 1997). In most cases, the molecular basis of tolerance is not known. The Arabidopsis COI gene that confers sensitivity to the hormone jasmonic acid is implicated in the control of lesion formation during P. syringae infection (Kloek et al., 2001). COI1 is thought to be involved in targeting proteins for degradation through the ubiquitin pathway (Xie et al., 1998). Because genes involved in tolerance to bacterial pathogens seem largely to involve hormone signalling, it is likely that disease symptom development is not the only process altered in the tolerant plants. We note that the initial cell death events, which usually still occur even in tolerant plants during infection, appear to be important for contributing to pathogen replication (see below).
Contribution of host cell death to virulent pathogen replication
In some host–pathogen interactions, pcd has a clear role in promoting pathogen growth. This is especially true for pathogens that secrete toxins to kill host cells rapidly, presumably to gain nutrition. We have already mentioned that the AAL toxin and related molecules induce pcd. Alternaria alternata f. sp. lycopersici lacking the AAL toxin has severely reduced growth on susceptible plants (Akamatsu et al., 1997). Likewise, the pathogenicity of Cochliobolus victoriae was correlated with the secretion of the victorin toxins (Wolpert et al., 2002). Exogenous application of victorin to susceptible oat plants causes an apoptotic-like response, including mitochondrial alterations (Curtis and Wolpert, 2002). Interestingly, pathogens that secrete AAL or victorin have a very narrow host range, only infecting specific hosts. For this reason, AAL and victorin are considered to be host-specific toxins (Wolpert et al., 2002).
In addition to the very clear cases outlined above, evidence has also emerged to support pcd as an important event promoting the growth of other pathogens. Tomato carrying the anticaspase p35 protein show both attenuated symptoms and growth of normally virulent P. syringae (Lincoln et al., 2002). In this case, the primary disease lesions were smaller in the transgenic p35-expressing plants than in the controls, and the replication of P. syringae was reduced. These transgenic tomato showed reduced symptoms with other pathogens, but their replication was not measured. Importantly, basal defences in the transgenic tomato appeared to be unaltered by the presence of p35. The involvement of pcd in promoting P. syringae virulence can also be inferred by the phenotype of Arabidopsis fbr mutants (Stone et al., 2000). These mutants were selected based on their resistance to the pcd-inducing toxin fumonisin. When infected with virulent P. syringae, the fbr mutants showed reduced disease symptoms and pathogen replication. Basal defences in fbr mutants did not appear to be altered.
Other roles for susceptible cell death
For some bacterial pathogens, cell death lesions associated with water-soaked tissue may be formed to facilitate both release of the bacteria on to leaf surfaces and subsequent transmission of the bacteria. Some bacterial proteins that are injected into plant cells during infection seem to play a role specifically in inducing water soaking (Yang et al., 1994) and/or causing lesion formation (Badel et al., 2003). We have found a tight correlation between severe disease symptoms and bacterial release on to leaf surfaces using the P. syringae–Arabidopsis model system (Guttman and Greenberg, 2001). We envisage such release to be an important step in the dissemination of bacteria, especially in cases where bacteria do not cause a systemic infection.
Concluding remarks
Many questions about the role, regulation and mechanism of pcd during host–plant interactions remain unanswered. Are there multiple mechanisms of cell death execution and regulation in infection zones and in different plant species? In which host–pathogen interactions is cell death truly important for disease resistance or susceptibility? We find it intriguing that ectopic expression in plants of the anticaspase p35 protein affects cell death in both the HR and susceptible host–pathogen interactions. This raises the possibility that there is a basal cell death machinery engaged during the different responses. It is possible that, although some pcd steps are common between resistant and susceptible responses, some aspects of pcd are different. Further analysis of pcd mechanisms in different conditions will be necessary to resolve this question. For future evaluation of the role of cell death in plant pathogenesis, it will be important to try to inhibit the cell death machinery selectively and simultaneously to monitor other defence- and pathogenesis-related events. Using this approach, it should be possible to determine whether cell death can be uncoupled from other responses and, if so, what its contribution to resistance or susceptibility is.
Acknowledgements
We thank members of the Greenberg laboratory for helpful discussions and comments on the manuscript. We acknowledge the help and support of Shigeyuki Mayama at Kobe University in whose laboratory the oat infection study shown in Fig. 1 was performed. Our research is funded by grants to J.T.G. from the NSF and NIH.




