Novel antagonistic interactions associated with plant polyploidization influence trait selection and habitat preference
Abstract
Ecology Letters (2010) 13: 330–337
Polyploidization is an important mechanism for sympatric speciation in plants. Still, we know little about whether plant polyploidization leads to insect host shifts, and if novel interactions influence habitat and trait selection in plants. We investigated herbivory by the flower bud gall-forming midge Dasineura cardaminis on tetraploids and octoploids of the herb Cardamine pratensis. Gall midges attacked only octoploid plant populations, and a transplantation experiment confirmed this preference. Attack rates were higher in populations that were shaded, highly connected or occurred along stream margins. Within populations, late-flowering individuals with many flowers were most attacked. Galling reduced seed production and significantly influenced phenotypic selection on flower number. Our results suggest that an increase in ploidy may lead to insect host shifts and that plant ploidy explains insect host use. In newly formed plant polyploids, novel interactions may alter habitat preferences and trait selection, and influence the further evolution of cytotypes.
Introduction
The factors generating, maintaining and constraining interactions between plant hosts and insect herbivores are fundamental to understand patterns of diversity, population dynamics and trait evolution in many ecosystems. The mechanisms by which new plant–insect associations arise, e.g. the relative importance of evolution vs. ecological fitting, has been the focus of many recent studies (Agosta 2006; Agosta & Klemens 2008). One potentially important source of changes in plant–insect associations is plant polyploidization. Polyploidization constitutes an instantaneous and dramatic shift of the genome and has long been recognized as an important mechanism in plant speciation and trait evolution (Stebbins 1971; Levin 1983). Plant polyploidization may be of two different types; in allopolyploidization traits of multiple plant lineages are combined while in autopolyploidization genomes from the same lineage is duplicated. Both allo- and autopolyploids often differ from their diploid progenitors in traits such as number and size of flowers, flowering phenology and overall plant size (reviewed in Stebbins 1971; Ramsey & Schemske 2002), and such trait changes may lead to new or altered interactions with mutualist and antagonist insects, directly or via altered plant habitat preferences (Segraves & Thompson 1999; Arvanitis et al. 2007, 2008; Thompson 2009). Changes in interaction patterns may, in turn, result in differential selection on plant ploidy levels and play an important role in the differentiation of polyploids and their ancestors (Bretagnolle & Lumaret 1995; De Kovel & De Jong 2000).
Plant polyploidization is also potentially an important mechanism for insect host range expansion, either because insects present on the diploid progenitor are able to use also the polyploid cytotype or because the polyploid constitutes a novel resource that can be used by insects that were not present on the diploid. The polyploid may encounter novel insect herbivores if ploidy-related changes act on the spatial and temporal distribution of plants, or on phenotypic plant traits (Stebbins 1971; Ramsey & Schemske 2002). Host shifts would not require evolutionary changes in the herbivore if the polyploid plant represents the same resource as the previously used host, or if the herbivore possesses traits that allow it to use the polyploid as a novel resource (Agosta 2006; Agosta & Klemens 2008). Alternatively, plant polyploidy may induce a barrier to a herbivore present on the lower ploidy level, allowing the newly formed polyploid to escape the interaction (Burdon & Marshall 1981; Reinert et al. 1986). Current differences in intensity of herbivory between polyploids and their ancestors may, however, not only depend on changes directly associated with the polyploidization event but also on subsequent selection.
Synthetically produced polyploids do not always show the pronounced differentiation in traits found in established polyploids, suggesting that not only instantaneous changes at polyploid formation but also subsequent selection plays an important role in the differentiation of polyploids and their ancestors (Bretagnolle & Lumaret 1995; De Kovel & De Jong 2000; Thompson & Merg 2008). One potentially important source of differential selection on plant ploidy levels is interactions with mutualistic and antagonistic animals. We know that insect herbivores and pollinators differ between plant populations in different physical environments and respond to variation in the same phenotypic plant traits that are changed by polyploidization events (Kolb et al. 2007). Still, only a handful of studies have investigated how plant polyploidization is related to interactions with insects. These studies suggest that the intensity of interactions differs between plant populations of different ploidy levels (Segraves & Thompson 1999; Arvanitis et al. 2007; but see Halverson et al. 2008). However, we are still not aware of any case where an insect visitor is restricted to the higher ploidy level, which would suggest that polyploidization has resulted in a host shift and a novel interaction. Within populations, floral visitors have been shown to discriminate between ploidy levels (Thompson et al. 2004; Kennedy et al. 2006; Arvanitis et al. 2008), but we still know very little about whether insect-mediated trait selection differs between ploidy levels (but see Nuismer & Ridenhour 2008).
In this study, we asked if plant polyploidization can be associated with host shifts in insect herbivores and if such novel interactions influence habitat preference and trait selection in plants. We used the study system consisting of the perennial herb Cardamine pratensis L. (Brassicaceae) and the bud gall-forming midge Dasineura cardaminis Winnertz (Cedomyiidae). In the study area, Cardamine pratensis is represented by tetraploid and octoploid cytotypes. Cytotypes differ in several phenotypic traits as well as in habitat preferences (Lövkvist 1956; Arvanitis et al. 2007). Evidence from morphological and molecular data suggests that octoploids have arisen via autopolyploidy from tetraploids (Khatri 1989; Franzke & Hurka 2000). We studied the relationship between gall abundance and habitat and plant characteristics in 196 C. pratensis populations and performed a transplantation experiment. In three populations, we studied how the number of galls and plant seed production was correlated to number of flowers, flower shoot size, flowering time and canopy cover in 339 octoploid individuals. We asked: (1) Can an interaction with an antagonist be restricted to a higher ploidy level, suggesting a host shift associated with polyploidization? (2) Can differences in interactions with the gall midge between ploidy types influence habitat preferences and trait selection in the plant?
Methods
Study system
Cardamine pratensis L. (Brassicaceae) exists as a diploid and several polyploid cytotypes. Only polyploid cytotypes are known from the study area where they grow in pastures, meadows, damp woods and ditches in lowland areas (Lövkvist 1956). The only known tetraploid cytotype in Northern Europe has 4n = 30 while the octoploid cytotypes have 8n = 56 or 64 (Lövkvist 1956). In the field, it is not possible to distinguish between the two octoploid cytotypes whereas tetraploids and octoploids differ in several phenotypic traits (Lövkvist 1956; Arvanitis et al. 2007). Tetraploids have normally a single 15–50 cm high flower shoot, 5–79 (median = 18.1) flowers, with 8–11 mm long petals, 20–40 mm long siliques and sessile cauline leaves with distinct veins. Octoploids have up to 65 cm high flower shoots, 4–39 flowers (median = 10.5) with 12–19 mm long petals, 30–55 mm long siliques and petiolated cauline leaves. Individuals of both ploidy levels flower with white to purple flowers assembled in the top of the flower shoot from mid May to the end of June. Differences in ploidy levels have previously been shown to affect intensity of seed predation by the butterfly Anthocharis cardamines (Arvanitis et al. 2007, 2008).
The gall midge Dasineura cardaminis Winnertz (Cedomyiidae), has been reported to cause flower bud galls on C. pratensis and C. amara (Coulianos & Holmåsen 1991). Larvae of D. cardaminis induce a chickpea shaped gall in flower buds of C. pratensis. The gall is c. 1 cm in diameter and consists of abnormally grown stamens, pistil, petals and sepals (Connold 1909). Each gall contains several orange larvae that fall to the ground for pupation in the soil in late June (Connold 1909; L. Arvanitis pers. obs.). In spite of extensive observations, we have not observed any aborted galls in this system.
Gall midges, plant ploidy levels, and habitat and population characteristics
A population survey was carried out between 21 May and 6 July 2004 in the Ludgo Parish, southern Sweden (17°06′ E, 58°56′ N). Because previous studies have documented morphological differences between ploidy types, we assessed the ploidy level of populations in two steps. First, we used shoot and flower morphology of a large number of individuals in each population for a preliminary assessment and categorized 88 populations as tetraploids and 108 as octoploids (Lövkvist 1956). Eight of the 108 populations categorized as octoploids also contained very small numbers (1–3) of tetraploid individuals (overall proportion 0.7%, n = 2051). Second, we used flow cytometry to confirm the preliminary ploidy level assessment. We examined the DNA content of 1–9 individuals in each of 117 populations (a total of 345 plants). We collected leaf cuttings and grew plants from them in greenhouse and common garden. We chopped small pieces of fresh leaf (c. 0.5 cm2) of the target plant and mixed with leaf tissue of an internal standard (Lycopersicon esculentum cv. Stupické polní tyčkové rané or Glycine max cv. Polanka), with known nuclear DNA content (Mandakova & Münzbergova 2006). Flow cytometry measurements were performed with a Partec PA II machine (Partec GmbH, Münster, Germany). Mean nuclear DNA content of 41 populations assigned to tetraploids was 1.60 ± 0.02 pg (mean ± SD). The variation in mean nuclear DNA content among 75 populations assigned to octoploids (mean ± SD = 3.66 ± 0.13 pg) was in concordance with two somatic numbers, 56 and 64, commonly found in Scandinavia at the octoploid level (Lövkvist 1956). Flow cytometry estimation of nuclear DNA content confirmed the classification of ploidy level based on morphology in all except one population. This population, preliminary assigned to octoploid, had a mean nuclear DNA content of 5.26 ± 0.04 pg (mean ± SD) and was excluded from the subsequent analyses.
To examine whether differences in gall midge attacks among populations were related to population and habitat characteristics we investigated several parameters. We estimated the area with C. pratensis in all populations. Plant population size was estimated as the total number of flowers and calculated as the product of the number of flower shoots in the population and the mean number of flowers per shoot. The number of flowers per shoot (the sum of buds, open flowers and siliques) was counted on up to 30 flower shoots. Population isolation was measured both in terms of distance to nearest plant population occupied by D. cardaminis and in terms of connectivity, i.e. weighted distances to all plant populations occupied by the gall midge. Population edge-to-edge distances were used for both connectivity measures. We used plant population size as the weighting factor and assumed a negative exponential relationship between distance and connectivity (Hanski 1994). Because we had no independent estimate of effect of distance on connectivity (α) available for D. cardaminis, we used the best-fitting value (α = 1.0). All areas and distances were measured using the software package Arc View 3.2. We categorized vegetation as dry, fresh, and moist meadow, moist wood, marsh or stream margin, and canopy openness as open (shaded < 30% of the day), partly shaded (30–60%) or shaded (> 60%). Flowering state was recorded as the mean proportion of flowers that had produced siliques. To correct for differences in record day between populations, we used the residuals from the regression of this measure on record day as the estimate of flowering state.
To separate direct effects of differences in ploidy level from effects via differences in habitat preferences, we also performed a transplantation experiment. In September 2005, we planted 100 pairs of tetraploids and octoploids in close proximity to a naturally occurring population of octoploid C. pratensis in the same area. Before planting, the ground vegetation was cut and removed. Individuals within pairs were spaced 20 cm apart, with c. 1 m between pairs. The plant material used was produced vegetatively from leaflets collected from sites within 50 km from the experimental site and cultivated in plastic pots in a common garden. Mean and standard deviation of nuclear DNA content of the three octoploid populations used in the transplantation experiment (and for the selection study, see below) were the same as the overall mean of the 75 octoploid populations examined for the population survey (n = 12, mean ± SD = 3.66 ± 0.14 pg). In 2006, presence of galls was recorded every second day between 30 May and 19 June in all flowering individuals (81 tetraploids and 86 octoploids).
Gall midges and plant trait selection
To examine how oviposition by D. cardaminis is related to individual plant traits and fitness, all flowering plants in three populations of octoploid C. pratensis (17°04′ E, 58°57′ N, 17°04′ E, 58°58′ N and 17°26′ E, 59°06′ N) were followed through 2005. Individuals were marked and recorded with 7-day intervals (7–8, 14–15 and 21–22 June). At each visit the numbers of galls, buds, flowers and siliques were counted. The sum of buds (including galls), flowers and siliques of an individual plant was used as a measure of total number of flowers. Flowering time of individual plants was classified into four categories according to the state of flowers and fruits at the three recordings; flowering plants with siliques at the first recording, plants without siliques but with open flowers at the first recording, plants that started to flower between the first and second recording, and plants that started to flower between the second and third recording. Height and basal diameter were measured in all shoots during flowering. We used flower shoot volume as a measure of size and calculated it as the volume of a cylinder: (basal diameter)2 · π/4 · height. Seeds from each individual in two plant populations were collected just before dispersal and counted. Because shading conditions may influence both the gall midge preference, and fitness (Rossi & Stiling 1998), we estimated the canopy cover above each individual plant by digital photographs in the middle of June. The camera was placed at a height of 50 cm next to each plant and orientated perpendicular to the ground. Canopy cover was analysed using the open source software ImageJ (http://rsb.info.nih.gov/ij/).
Data analysis
To study the relationship between galling intensity, and habitat and population characteristics in C. pratensis populations, we used generalized linear models with Poisson error distribution and a log-link function. The response variable was the number of galls per plant population, and the predictor variables considered were population size in terms of the total number of flowers, population area, distance to nearest occupied population, connectivity, shading, vegetation type and mean phenological state. Because tetraploid populations were never galled (see Results), population level analyses were carried out only for octoploid populations. To avoid problems associated with collinearity, models with either total flower number or population area, and either connectivity or distance to nearest occupied population, were fitted separately and the residual deviances were compared. Mean phenological state was clearly not significant (P = 0.93) and was not included in the presented models. At the level of plant individuals, we examined the relationships between the number of galls per plant individual and population identity, flower shoot size, the number of flowers and flowering time, again using Poisson regression. For individual models, we included an interaction term for the plant trait variables, i.e. flower number and flowering time. Because of collinearity, models with flower number and flower shoot size were fitted separately, and the residual deviance was compared. All analyses were carried out in r 2.9.1 (R Development Core Team 2009). Overdispersion was taken into account when computing standard errors of parameter estimates and related statistics by using the quasipoisson ‘family’ in the ‘glm’ function in r.
We used Selective Source Analysis (SSA; Ridenhour 2005) to examine the effects of gall midges on plant trait selection. Ridenhour (2005) showed that the indirect effect of interacting species on trait selection can be quantified by partitioning total fitness into direct effects of traits of the focal species and indirect effects via interactions with other organisms. Nuismer & Ridenhour (2008) applied this method to examine plant–parasite interactions. In our analyses, the fitness measure, seed number, was relativized and all traits were standardized before analysis. The number of flowers and galls (+1 as it contained zero values) were transformed to their natural logarithms before standardization. There were no differences in fitness or in the relationship between traits and fitness between populations, and no effects of the interaction between flowering time and flower number on fitness. Thus populations were pooled and the interaction term was dropped from the model for the final analyses. Canopy cover was clearly not significant (P = 0.94) and was not included in the presented models. Including interactions or canopy cover did not affect the SSA results. Lastly, because flower number and flower shoot size were closely correlated and because flower number was more strongly correlated with gall midge attacks (see Results), flower shoot size was not included in these analyses. For the SSA, we thus fitted a multiple regression model of relative fitness on flowering time, flower number and number of galls (main and interaction effects). The partial regression coefficients related to the plant traits and the variance–covariance matrix for traits and the number of galls were used to partition the selection differential for flowering time and flower number into direct effects of trait variance, indirect effects due to covariance with other traits, and effects resulting from the interactions with the gall midge (each of the regression coefficients pertaining to the gall midge interaction times the covariance of the plant trait with number of galls) (Lande & Arnold 1983; Ridenhour 2005; Nuismer & Ridenhour 2008). Using the package ‘boot’ in r 2.9.1, we calculated 20 000 bootstrapped values for each partition of the selection differentials and calculated (bias corrected) confidence intervals to statistically test the effects of the gall midges (cf. Nuismer & Ridenhour 2008). The SSA results were very similar to those from a model of trait selection using path analysis (Scheiner et al. 2000) in the ‘sem’ function in r (not shown).
Results
Gall midges and plant ploidy level
Galls were found exclusively in plant populations of the higher ploidy level. None of 88 C. pratensis populations dominated by tetraploids were galled by D. cardaminis, while galls were found in 42 of 107 octoploid populations (39%). Four of the octoploid populations with galls also contained flowering tetraploids, but none of these tetraploids were galled. In the transplantation experiment 21% of octoploids had single or multiple galls while only 2% of tetraploids had single galls (Fisher’s exact test: P = 0.0002).
Gall midges and plant population characteristics
Habitat and population characteristics significantly influenced gall midge attacks in octoploid C. pratensis. The number of galls was highest in populations with intermediate shading and lowest in populations in open habitats (Table 1; Fig 1). Larger populations, populations in stream margins and populations more connected to other populations also contained more galls. The estimate of population size was not significantly different from 1.0 (P = 0.23) making the presented model similar to one where population size was included as an offset (due to the log-link function in the Poison regression). This means that the probability of an individual flower being galled was independent of population size and that the effects of the other variables may be interpreted as acting on number of galls per flower.
| Source of variation | d.f. | Estimate | χ2-value | P-value |
|---|---|---|---|---|
| Population size | 1 | 0.869 | 100.36 | <0.001 |
| Shading | 2 | 36.90 | <0.001 | |
| Vegetation type | 5 | 20.97 | <0.001 | |
| Connectivity | 1 | 0.352 | 5.45 | 0.020 |
- The null deviance was 3398.4 (106 d.f.) and 81.4% of the deviance was explained by the model. A model where area replaced population size had a much higher residual deviance than the presented model, and a model where the distance to the nearest neighbouring population replaced connectivity a slightly higher deviance. Chi-square is the likelihood-ratio χ2 of type II tests.
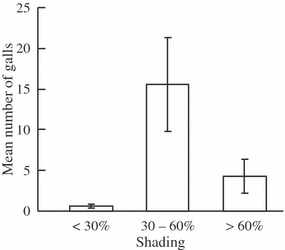
Mean number of Dasineura cardaminis galls in octoploid Cardamine pratensis populations for three categories of shading (n < 30% = 36, n30–60% = 46 and n > 60% = 25). Bars are ± SE.
Gall midges and plant trait–fitness relationships
Gall midges significantly reduced fitness and changed trait selection in plants. Gall midges attacked 42% of octoploid plants and the number of galls decreased with increasing canopy cover. There was a significant effect of the interaction between number of flowers and flowering time; late-flowering shoots with many flowers had the highest number of galls (Table 2). The number of seeds per plant decreased with an increasing number of galls and flower shoots with three or more galls never produced seed (Fig. 2). As a result of their preferences and negative effects on fitness, gall midges significantly influenced trait selection in plants. Partitioning of the selection differentials using SSA showed that there were both a significant positive direct effect of number of flowers on fitness and a significant negative effect via gall midge attacks (Table 3). For flowering time, there was significant direct selection for earlier flowering while effects via gall midge attacks were not significant.
| Source | d.f. | Estimate | χ2-value | P-value |
|---|---|---|---|---|
| No. flowers | 1 | −0.164 | 64.35 | <0.001 |
| Population | 2 | 70.78 | <0.001 | |
| Canopy cover | 1 | −0.013 | 9.25 | 0.002 |
| Flowering time | 1 | −1.033 | 4.88 | 0.027 |
| No. flowers × flowering time | 1 | 0.558 | 16.81 | <0.001 |
- The null deviance was 754.7 (338 d.f.) and 32.3% of the deviance was explained by the model. A model where flower shoot size replaced number of flowers had a higher residual deviance. Chi-square is the likelihood-ratio χ2 of type II tests.
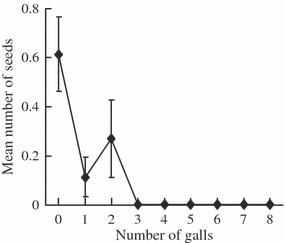
Relationship between the number of Dasineura cardaminis galls and the number of seeds in 208 octoploid Cardamine pratensis individuals. Plants with three or more galls never produced seeds (n = 46). Sample size for plants with 0, 1 and 2 galls were 105, 27 and 30 respectively. Bars are ± SE.
| Trait | Selective source | β-estimate | 95% Confidence interval |
|---|---|---|---|
| No. flowers | No. flowers | 0.549 | 0.189, 1.271 |
| Galling | −0.204 | −0.400, −0.026 | |
| Total | 0.345 | ||
| Flowering time | Flowering time | −0.452 | −0.838, −0.188 |
| Galling | −0.018 | −0.168, 0.132 | |
| Total | −0.470 |
- Total effects (excluding indirect effects via the other trait) were partitioned into direct effects and effects via the gall midge Dasineura cardaminis. Estimates are selection gradients (β).
Discussion
Our results suggest that the increase in ploidy in C. pratensis is associated with a novel interaction with the antagonist D. cardaminis, and that this interaction significantly reduces fitness and changes trait selection in the plant. Gall midge attacks were restricted to the higher ploidy level. The attack rate in octoploid populations increased with connectivity and shading, and was higher in stream margins. Dasineura cardaminis females preferred late-flowering individuals with many flowers and this preference altered selection on flower number in octoploids.
To the best of our knowledge, our results are the first to show that a specialized insect herbivore uses only the higher ploidy level of an autopolyploid plant species. Gall midge attacks were restricted to the higher ploidy level in natural populations and octoploids were much more often galled in the transplantation experiment, although also a few tetraploids were attacked. This preference for the higher ploidy level is particularly interesting because it occurred in an autopolyploid species. It suggests that effects of plant polyploidization on insect host-plant ranges may not only occur via the combination of traits from different lineages, but that also genome duplication per se changes plant traits and distribution, thereby bringing plants in contact with novel herbivores. This issue merits further attention both in case studies and in phylogenetic studies of the relationship between host shifts and polyploidization events (cf. Thompson 2009). If the newly formed plant polyploids are instantly attacked by insects from another host plant than the lower ploidy level, or suffer a greater reduction in fitness from attack by an insect already present at the lower ploidy level, then this would imply that the polyploid is at a disadvantage from the start compared with its ancestors. This disadvantage should decrease the likelihood of polyploid establishment and hence our ability to detect novel antagonisms in polyploids. If the newly formed polyploid is structurally and chemically similar to the previously used host plant and occurs in the same habitat, a host shift from a different species does not necessarily involve behavioural and physiological changes in the herbivore (Bush 1969; Thomas et al. 1987; Becerra 1997), and hence could be achieved by ecological fitting (Janzen 1980, 1985; Agosta 2006). In our study area, the other known host plant of the gall midge, C. amara, flowers during the same period and occurs in moist shady habitats, similar to those where intensity of galling was highest in octoploid C. pratensis, making a shift from C. amara likely. In a similar manner, some populations of the moth Greya politella have shifted from the ancestral host Lithophragma parviflorum to the related polyploid Heuchera grossulariifolia (Brown et al. 1997; Janz & Thompson 2002). Unlike D. cardaminis, the moth uses both ploidy levels but shows a preference for tetraploid over diploid H. grossulariifolia (Thompson et al. 1997; Nuismer & Thompson 2001). This preference has been suggested to be a consequence of a higher degree of overlap in flowering time between L. parviflorum and tetraploid H. grossulariifolia than between L. parviflorum and diploid H. grossulariifolia (Janz & Thompson 2002). In C. pratensis, the use of only the higher ploidy level by D. cardaminis appears not to be the result of different habitat preferences of the two ploidy levels exclusively. We did not find galls in tetraploid C. pratensis populations in the habitat preferred by D. cardaminis or in tetraploid individuals at sites with galled octoploids. Moreover, in the experiment where both ploidy types were transplanted into a common environment, tetraploids were only galled very rarely while octoploids were frequently galled. We observed no ovipositing failures or gall abortions in spite of almost daily observations of a large number of plants during several seasons (L. Arvanitis pers. obs.). This suggests that observed differences in attack rates may be largely due to differences in female midge preferences, and that tetraploid C. pratensis is not considered a suitable host plant, while octoploid C. pratensis has either evolved into a suitable host or became suitable immediately following the polyploidization event. The acquired suitability allowed a host shift, or host expansion, most probably from C. amara. One possible explanation of why the higher ploidy level is preferred is that flower shoots and individual buds and flowers of octoploid C. pratensis are larger than those of tetraploids, offering more resources for larval development (Lövkvist 1956; Arvanitis et al. 2008). A preference for larger plants or plant parts among gallmakers has been documented in other systems (Cornelissen et al. 2008). An additional possibility is that differences in flowering phenology between ploidy levels influence the gall midge preference for the higher ploidy level. In the transplantation experiment, octoploids flowered earlier than tetraploids (L. Arvanitis pers. obs.).
The intensity of gall midge attack in octoploids was related to habitat characteristics, suggesting that the interaction has the potential to alter the habitat preference of the octoploids. As in several other plant herbivore systems (e.g. Östergård & Ehrlén 2005; Arvanitis et al. 2007), we found that intensity of insect attacks increased with increasing connectivity. This is consistent with the findings that gall midges are weak fliers that usually remain near the emergence site (Gagné 1989) and means that the strength of the interaction between plants and gall midges is determined largely by local factors. Gall midge attacks were less frequent in octoploid C. pratensis populations in open habitats than in populations in more shaded environments. Shading is likely to increase the humidity of the habitat which, in turn, may improve adult gall midge activity and survival of first instar larvae (Skuhrava et al. 1984; Gagné 1989). A positive effect of humidity on intensity of gall midge attack is also supported by that attacks were most frequent in stream margin habitats. In contrast, shaded C. pratensis populations are exposed to a lower risk of pre-dispersal seed predation by butterfly A. cardamines than populations growing in sunny habitats (Arvanitis et al. 2007). This means that the net relationship between shading and overall damage by antagonists will depend on the relative abundance of gall midges and butterflies.
The novel interaction with gall midges that octoploid C. pratensis individuals experienced resulted in reduced seed production and altered trait selection. Gall midges preferred late-flowering individuals with many flowers. Ovipositing midge females may prefer individuals with a higher flower number because they provide more oviposition sites and a larger overall abundance of resources. The preference for later flowering individuals may be due to that these better matched gall midge occurrence in the study year. The emergence of adult gall midges lasts only about a week and the developmental stage of buds determine their quality as oviposition sites (Gagné 1989; Yukawa 2000). As a result of their negative effect on plant fitness, gall midge preferences significantly influenced trait selection in octoploid plants. In our system, insect antagonists reduced selection for a higher number of flowers. In H. grossulariifolia the seed-parasitic moth G. politella favoured increased floral display size and earlier flowering in the polyploid population but later flowering in the diploid population (Nuismer & Ridenhour 2008). Taken together, such results suggest that polyploidization in plants may lead to altered selection and trait evolution via changes in interactions with antagonistic and mutualistic insects. Given that observed phenotypic selection gradients to some extent reflect additive genetic variation, we would expect gall midges to decrease selection for higher flower number in the higher ploidy level. If flower production in the absence of midges is associated with a cost in terms of a lower future reproduction and there is an optimal flower number, then gall midges will shift this optimum downwards. For our study system, it is also true that the presence of other selective agents, such as A. cardamines may considerably modify trait selection in octoploids (Arvanitis et al. 2008).
Our results suggest that plant polyploidization events lead to novel interactions with insects and that these result in shifts both in selection gradients and in relative performance among environments. Because the novel interaction in our study system involved an autopolyploid, it suggests that plant genome duplication per se can constitute a mechanism for insect host range expansion. In plants, the further diversification of polyploids from their ancestors may be influenced not only by changes in inherited interactions with antagonistic and mutualistic animals but also by novel interactions that did not occur in their diploid ancestor.
Acknowledgements
We thank Ove Eriksson and John N. Thompson for comments on the manuscript, and H. Sirén, K. Hamza, J. Karlsson and T. Luotola for field assistance. This study was financially supported by the Swedish Research Council (VR to JE).




