Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems
Abstract
The cycles of the key nutrient elements nitrogen (N) and phosphorus (P) have been massively altered by anthropogenic activities. Thus, it is essential to understand how photosynthetic production across diverse ecosystems is, or is not, limited by N and P. Via a large-scale meta-analysis of experimental enrichments, we show that P limitation is equally strong across these major habitats and that N and P limitation are equivalent within both terrestrial and freshwater systems. Furthermore, simultaneous N and P enrichment produces strongly positive synergistic responses in all three environments. Thus, contrary to some prevailing paradigms, freshwater, marine and terrestrial ecosystems are surprisingly similar in terms of N and P limitation.
Introduction
Abundant data indicate that the growth and reproduction of photosynthetic biota (autotrophs hereafter) as well as large-scale ecosystem primary production are frequently limited by supplies of nitrogen (N) or phosphorus (P) in freshwater (Hecky & Kilham 1988; Elser et al. 1990), marine (Hecky & Kilham 1988; Vitousek & Howarth 1991) and terrestrial (Walker & Syers 1976; Vitousek & Howarth 1991) environments. Indeed, elevated inputs of these nutrients have been implicated worldwide in massive changes in biological diversity and ecosystem services (Smith et al. 1999), reflecting the fact that global cycles of N and P have been amplified by c.100% and c. 400%, respectively, by post-industrial human activities (Falkowski et al. 2000). Predicting and mitigating the effects of altered nutrient loading requires an understanding of if, where, and by how much these key nutrients limit production.
Past work has highlighted a diverse set of geochemical and ecological factors that can influence the identity and nature of N and P limitation in particular ecosystems (Vitousek & Howarth 1991). In terrestrial environments, soil age is key because P becomes increasingly sequestered because of mineralogical transformations over time scales of 103–105 years (Walker & Syers 1976; Vitousek 2004). Thus, tropical ecosystems that were not disturbed by glaciation are thought to be more frequently P-limited because of greater soil age. The regional fire regime can also have a major impact, as fire volatilizes ecosystem N pools while leaving P behind (Raison 1979; Hungate et al. 2003). In coastal marine systems, nitrogen has historically been considered to be the predominant limiting nutrient (Howarth 1988). However, sequestration of P in calcareous sediments is thought to drive P limitation in the tropics (Smith 1984), while constraints on planktonic N-fixation caused by insufficient light (Karl et al. 2001) or trace metal supply (Falkowski et al. 1998; Wu et al. 2000) are thought to influence the predominance of N or P limitation offshore. In freshwaters, redox-dependent P retention in sediments (Welch & Cooke 1995), the intensity of denitrification (Downing & McCauley 1992), watershed land use patterns (Downing & McCauley 1992; Carpenter et al. 1998) and internal food web structure (Elser et al. 1988) can all affect the absolute and relative supplies of N and P in particular lakes and streams.
This diversity of habitat-specific climatic, edaphic and ecological influences on N and P availability makes it difficult to obtain a broad picture of the relative importance of N and P limitation in the biosphere. Nevertheless, some existing paradigms identify N as the primary limiting nutrient in terrestrial (Vitousek & Howarth 1991) and marine (Howarth & Marino 2006) ecosystems and P as the main limiting nutrient in lakes (Schindler 1977). However, recent work has begun to question these generalizations, calling attention to an equivalence in N and P limitation in lakes (Elser et al. 1990) and streams (Francoeur 2001) and to frequent P limitation in the oceans (Downing et al. 1999b). As a result, the current state of knowledge has made it difficult for ecologists to make general recommendations about the need for joint nutrient controls in ameliorating eutrophication because existing paradigms may not provide accurate insight into the actual role of these nutrients in various ecosystems.
Here, we report the results of a meta-analysis that compiled and analysed results of field experiments evaluating the responses of primary producer biomass to manipulations of N and P availability. Our goal was to determine if patterns of autotroph nutrient limitation differ across systems, possibly because of differences in demand for N and P or in the major biogeochemical process controlling the supplies of N and P, or if they are broadly similar, as would be expected given the biochemical machinery shared by all autotrophs (Sterner & Elser 2002). Our dataset involves 653 freshwater, 243 marine and 173 terrestrial experiments and represents the largest study of its kind to date and the first to explicitly compare growth responses across aquatic and terrestrial realms. The experiments encompass diverse habitats across a broad range of latitudes within each of the three systems, including benthic and pelagic autotrophs in freshwater and marine environments and terrestrial habitats ranging from rainforest to desert to tundra. In light of existing paradigms about the primary limiting nutrient in different ecosystems, our results indicate a surprising uniformity in autotroph response to N and P enrichments. Specifically, the magnitude of producer response to P enrichment is similar in marine, freshwater and terrestrial ecosystems, combined N and P enrichment produces similarly strong synergistic effects in all habitats and N and P limitation appear to be of equal importance in terrestrial and freshwater ecosystems (although N limitation is stronger in marine systems).
Methods
Relevant studies were identified by searching titles and abstracts of publications returned from searches on ISI Web of Science using combinations of key words such as nitrogen, phosphorus, nutrient, enrichment, fertilization and bioassay. We also included studies summarized in previously published syntheses (DiTommaso & Aarssen 1989; Elser et al. 1990; Tanner et al. 1998; Downing et al. 1999b) and searched all subsequent papers citing those syntheses. For studies that included additional manipulations (such as grazer exclusion), we included only treatment combinations using the unmanipulated controls (grazers at natural densities). Studies including such secondary manipulations were a small subset of our data. Studies were included if they involved (minimally) independent manipulations of both N and P availability or (ideally) full factorial manipulations of N and P. (Some studies involved both N and P enrichment but did not apply, or report data from, both treatments in all individual experiments. Thus, the numbers of observations for +N and +P responses are not necessarily identical.) By including only studies that manipulated both N and P, we minimized potential biases induced by investigator focus on particular limiting nutrients thought to be most important in particular kinds of ecosystems. Furthermore, we analysed the data in two ways, one in which all data were included and another in which only data from fully factorial experiments were included. The overall patterns were the same for the two approaches. Thus, we present the results for the more inclusive data set in order to increase the scope of habitats and experimental approaches encompassed.
We included only studies that reported mean community-level biomass or production responses of autotrophs to nutrient enrichment. Single-species responses were eliminated unless drawn from a mono-dominant community in the judgment of the original authors or, if several species from a community were individually assayed for N and P response, an average across all species was taken for a given study. The preferred metric was biomass per unit area (terrestrial, wetland, benthic) or volume (pelagic). We also accepted proxy variables that are known to be correlated with standing biomass, such as chlorophyll concentration (most common in phytoplankton studies), ash-free dry mass, carbon mass, biovolume, percent cover and primary production. Many studies in forests and other systems dominated by woody plants and a small percent of marine benthos studies reported incremental rates (change in height or radius) rather than standing biomass. Inclusion of these studies did not qualitatively change the results of our analyses, and so we present results from the larger inclusive data set. Studies involving organism counts were excluded because of the orders-of-magnitude discrepancies in organism size among systems, and the expected inverse relation between organism size and abundance (Cohen et al. 1993; Cyr et al. 1997).
We defined a study as a temporally and spatially distinct experiment with internally consistent controls. Multiple studies could be reported from within one publication, for instance, if the same experimental treatments were performed in multiple streams with differing water quality or for water samples obtained from different stations along an oceanographic transect. When multiple measures were reported over time in a single experiment, we generally used the last temporal sample to avoid phases of transient dynamics in order to capture measures closer to when the system approached a potential equilibrium with the added nutrients. Exceptions were made to standardize duration within systems or to avoid excessively long incubations (mainly for bioassays with freshwater or marine phytoplankton). Data for multiple sampling dates in extended studies were averaged if phenological changes necessitated the use of mean values over all samples instead of the final value in order to be more ecologically relevant. In these cases, we used the most robust values by deferring to the working knowledge and intuition of the original authors.
We used the ln-transformed response ratio as our primary effect size metric RRX = ln (E/C), where E is the measured value of the response variable in enrichment treatment X (N or P or N + P) and C is its value in the unenriched control treatment. RR is one of the most frequently used effect metric in ecological meta-analysis (Hedges et al. 1999; Lajeunesse & Forbes 2003). Unlike Hedge’s d, the ln-response ratio does not require a measure of sample variability. Moreover, in comparisons across systems where response variables and experimental designs can differ considerably, the analysis of change relative to the control is more meaningful than standardized absolute differences between means.
For each study, we used a unique study identifier linked to the citation of the publication and obtained the following information wherever possible. We categorized the system as marine, terrestrial, or freshwater and the stratum within each system by assigning aquatic studies to either pelagic or benthic subcategories and the terrestrial to either aboveground or belowground. Some studies in wetlands and salt marshes were difficult to categorize. For these, we used the operational approach that studies addressing submersed or floating macrophytes, or microalgae growing on them, were classified as aquatic (marine or freshwater), whereas studies on above-water rooted plants were termed terrestrial. For studies involving submersed macrophytes, when nutrients were added to the sediments, only responses of the macrophytes were included. When nutrients were added to the overlying water, only responses of the epiphytes were included. Finally, we also created a standardized set for habitat subcategories consisting of: grassland/meadow; tundra; forest/shrubland; wetland; stream; lake pelagic; lake benthos; marine benthos (hard bottom), marine benthos (soft bottom); or marine pelagic. We also entered supporting data about incubation conditions and the local environment, including concentrations of available nutrients (nitrate, ammonium, soluble reactive phosphorus).
Original data can be obtained via the public data repository of the National Center for Ecological Analysis and Synthesis (http://knb.ecoinformatics.org/knb/metacat?action=read&qformat=nceas&docid=nceas.347). A map showing the global distribution of most of the study sites involved is given in Appendix S3. A summary listing all papers from which data were extracted is given in Appendix S3.
Results
Our data show that both N and P limitation are strong and widespread in the major habitats of the biosphere (Fig. 1, P < 0.001 for t-tests of RRX = 0 for all responses in all systems). Analysis of variance of the ln-response ratios (RRX, Table 1) indicates that there are no differences across the three ecosystem types in autotroph response to P enrichment (RRP; P = 0. 362). That is, the average strength of P limitation is similar in terrestrial, freshwater and marine ecosystems. In contrast to RRP, there are statistically significant cross-system differences in response to N enrichment (RRN; P < 0.0001) and to simultaneous N + P enrichment (RRNP; P < 0.0001), reflecting elevated RRN in marine systems and particularly high RRNP in freshwaters (Fig. 1).
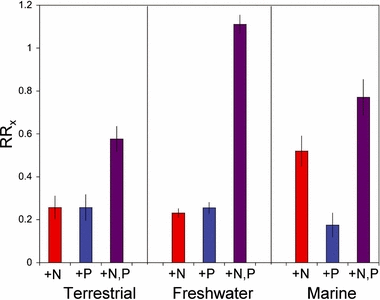
Responses of autotrophs to single enrichment of N (red) or P (blue) or to combined N + P enrichment (purple) in terrestrial, freshwater and marine ecosystems. Data are given as natural-log transformed response ratios (RRx) in which autotroph biomass or production in the enriched treatment is divided by its value in the control treatment and then ln-transformed (see Methods). Thus, a value of 0.5 indicates a value in the manipulated treatment that is c. 1.6 times its value in the control, while a value of 1.0 indicates a 2.7-fold increase. Sample sizes +N, +P and +N&P treatments were 112, 107 and 126 for terrestrial studies, 509, 506 and 618 for freshwater studies and 149, 141 and 197 for marine systems, respectively. Error bars indicate plus or minus one standard error.
| Parameter | d.f. | Sum of squares | F | P-value |
|---|---|---|---|---|
| RRN | 2, 767 | 9.758 | 14.77 | < 0.0001 |
| RRP | 2, 751 | 0.737 | 1.017 | 0.3621 |
| RRNP | 2, 938 | 39.53 | 17.61 | < 0.0001 |
Simultaneous additions of N and P produce higher responses than single nutrient additions across all systems (P < 0.0001; Table 2) but, across systems, overall responses to P or to N added separately are broadly equivalent (P = 0.222). N enrichment or P enrichment result in growth responses (Fig. 1) that are statistically indistinguishable in freshwater (P = 0.637) and terrestrial systems (P = 0.999) when systems are analysed separately (Table 2). N enrichment in marine environments produces significantly greater growth response than P enrichment (P = 0.002, Table 2), although, as noted above, average marine RRP is significantly greater than zero, indicating a positive response to P enrichment. In sum, these data lead to the overall conclusion that, in terms of the predominance of N vs. P limitation and synergistic effects of combined N + P enrichment, freshwater, marine and terrestrial systems are surprisingly similar.
| System | Factor | d.f. | Sum of squares | F | P-value |
|---|---|---|---|---|---|
| All systems | Nutrient treatment | 2 | 287.08 | 217.01 | < 0.0001 |
| C1: RRNP vs. (RRN and RRP) | 1 | 286.10 | 432.53 | < 0.0001 | |
| C2: RRN vs. RRP | 1 | 0.99 | 1.491 | 0.2222 | |
| Residuals | 2462 | 1628.5 | |||
| Terrestrial | Nutrient treatment | 2 | 8.121 | 10.549 | < 0.0001 |
| C1: RRNP vs. (RRN and RRP) | 1 | 8.121 | 21.097 | < 0.0001 | |
| C2. RRN vs. RRP | 1 | <0.001 | <0.0001 | 0.9998 | |
| Residuals | 342 | ||||
| Freshwater | Nutrient treatment | 2 | 288.65 | 232.582 | < 0.0001 |
| C1: RRNP vs. (RRN and RRP) | 1 | 288.52 | 464.942 | < 0.0001 | |
| C2: RRN vs. RRP | 1 | 0.14 | 0.22 | 0.6375 | |
| Residuals | 1630 | ||||
| Marine | Nutrient treatment | 2 | 29.11 | 16.181 | < 0.0001 |
| C1: RRNP vs. (RRN and RRP) | 1 | 20.48 | 22.765 | < 0.0001 | |
| C2: RRN vs. RRP | 1 | 8.63 | 9.5967 | 0.0021 | |
| Residuals | 484 |
- The effects of nutrient treatment are also analysed at two orthogonal contrasts: C1. RRNP vs. RRN and RRP and C2. RRN vs. RRP. Results are presented for the pooled data set across all systems and for each of the three systems analysed separately.
Substantial variation in nutrient enrichment response can be seen within systems (Fig. 1). This is not surprising, given the considerable heterogeneity in physical, chemical and biological characteristics associated with the diverse habitats we pooled into these broad categories. Consistent with this, there are highly significant subhabitat effects for each of the three ecosystem types (Fig. 2; P < 0.0001, Table 3), but these differences depend on the nutrient treatment (P < 0.0001). For example, RRNP is broadly similar across subhabitats within terrestrial environments but RRN is particularly high in wetlands while RRP is particularly high in forests. In freshwaters, lake phytoplankton and stream autotrophs (primarily attached algae) are equally responsive to N or P (as in the overall pattern) but lake benthic autotrophs (primarily attached algae) appear to be more strongly limited by P than N and synergistic responses are weak (Fig. 2). Finally, in the marine realm, benthic soft-bottom autotrophs (primarily seagrass and attached estuarine algae) show relatively weak responses to nutrients while coastal hard-bottom systems (rocky intertidal, temperate reef and coral reef macro- and microalgae) show substantial positive response to N and N + P but the strongest responses, especially to N or N + P enrichment, are for phytoplankton (Fig. 2).
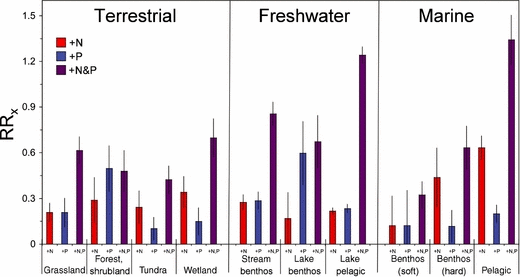
Relative responses (RRx) of autotrophs to single enrichment of N or P or to combined N + P enrichment in various subhabitats in terrestrial, freshwater and marine ecosystems. Data are expressed as in Figure 1.
| Factor | d.f. | Sum of squares | F | P-value |
|---|---|---|---|---|
| System | 2 | 11.23 | 9.092 | < 0.0001 |
| Nutrient treatment | 2 | 286.5 | 231.9 | < 0.0001 |
| Subhabitat (system) | 8 | 48.10 | 9.735 | < 0.0001 |
| Treatment × system | 4 | 30.88 | 12.50 | < 0.0001 |
| Treatment × subhabitat (system) | 14 | 35.66 | 4.124 | < 0.0001 |
| Residuals | 2434 | 1503 |
- The effects of nutrient treatment were also analysed at two orthogonal contrasts: (i) N (RRN) vs. P (RRP) addition (P = 0.91) and (ii) either N or P alone (RRN and RRP) vs. both N and P (RRNP) (P < 0.0001).
We considered whether autotroph response to enrichment varied across latitude, as it has been proposed that P limitation dominates in tropical terrestrial (because of effects of soil age) and marine (because of effects of sequestration in calcareous sediments) ecosystems while N limitation is predominant in temperate regions (Walker & Syers 1976; Smith 1984). In contrast, N has been said to be more limiting in tropical freshwaters with P more important in limiting production in temperate waters (Downing et al. 1999a). However, we found little evidence for strong latitudinal variation in autotroph nutrient limitation (see Supplementary Figure 1 in Appendix S1). We also evaluated some potential confounding factors that may have influenced the major patterns we report, such as differences among habitats in the range of ecosystem nutrient conditions encompassed and in the strength of nutrient enrichment applied in different ecosystem types. We found little potential for major effects. Details of these assessments are presented in Appendix S2 in the Supplementary Material.
It is possible that our results are influenced by major differences among studies and habitats in experimental duration relative to the size and generation time of dominant autotrophs in different systems. However, individual investigators likely choose their experimental durations to be appropriate for the approximate generation time of the biota in their study systems. Consistent with this, average experimental durations were c. 7 days for pelagic systems (freshwater and marine), c. 40 days for lake and stream benthos, c. 120 days for marine benthos (reflecting studies involving macroalgae and vascular plants), c. 450 days for wetlands, c. 960 for forest and shrubland, c. 1900 for grasslands and c. 2200 for tundra (see Appendix S4). Furthermore, correlations of response ratios with experimental duration (log-transformed) within ecosystem type (freshwater, marine, terrestrial) were generally weak and non-significant [P > 0.113, except for the correlation of RRP with log (duration) in freshwaters]. Considering such correlations by subhabitats (and thus more closely aligned with autotroph size and functional type), correlations were also generally weak (r2 < 0.25, except for tundra where only seven to eight observations were available) and associations were both positive and negative (Table S2 in Appendix S4). Only five of the twenty sets of correlations were significant at the P = 0.05 level. Details of these analyses can be seen in Appendix S4. Based on these results we suggest that response ratios can appropriately be compared across systems as ecologically meaningful indicators of overall autotroph response to nutrient enrichment.
Discussion
Our analyses clearly show that, despite differences in potentially important habitat-specific mechanisms of biogeochemical cycling and in the size, life history and phylogenetic affiliation of the autotrophs, broad similarities in nutrient limitation exist in marine, freshwater and terrestrial ecosystems. Significant synergistic effects of combined N and P enrichment are common to all ecosystems; there is no significant difference in RRP between freshwater, marine and terrestrial ecosystems; and N or P added singly have equally strong effects in both freshwater and terrestrial ecosystems. Thus, it appears that the N and P demands of fundamental core of biochemical machinery shared by all photoautotrophs (Sterner & Elser 2002) set the stage for growth limitation by N and P to similar degrees across the biosphere.
The similarity of N and P enrichment effects in freshwater and terrestrial ecosystems is in contrast with some existing views in which N is thought to dominate on land (Schlesinger 1997; Vitousek & Howarth 1991) and P in freshwater (Schindler 1977). While autotroph response to N is indeed stronger than response to P in marine ecosystems as suggested in existing paradigms (Vitousek & Howarth 1991; Howarth & Marino 2006), we note that average RRP is significantly different from zero, indicating that P-limitation is not unimportant in marine ecosystems. Most of the marine data included here are for seagrasses and attached macro- or microalgae growing in shallow coastal waters or estuaries (see Supplementary Figure S3 in Appendix S3). Thus, the apparent strength of marine N limitation may be overestimated if coastal and estuarine waters are broadly influenced by P-rich sources of pollution, as argued previously (Downing 1997; Downing et al. 1999b).
A clear pattern in our data was that strong positive synergistic effects of combined N and P enrichment are widespread. As the most likely mechanism for synergistic effects of joint N and P enrichment is that single enrichments quickly induce limitation by the alternative nutrient, the frequent and substantial synergistic effects observed suggest that N and P supplies are relatively closely balanced in most environments. Thus, our results indicate that, instead of focusing intense scrutiny on the supply and cycling of a particular nutrient under a system-specific presumption that it is limiting, community and ecosystem ecologists would benefit from a more balanced view of the impacts of multiple key nutrients, including N and P but also others (such as iron, silica, sulphur, or potassium).
Despite the surprising similarity across major ecosystem types in responses to N and P addition, our analysis did reveal some differences among particular subhabitats (Fig. 2). For instance, most fertilization experiments in forests were conducted in tropical latitudes, and this habitat type had a stronger response to added P than added N, suggesting support for the long-held belief that tropical ecosystems on old soils are predominantly P limited (Walker & Syers 1976). In contrast, while noting that only seven experiments contributed to the reported average values, tundra sites showed a greater response to added N than added P – potentially because these experiments tended to be in areas where frequent glaciation events resulted in younger soils with greater P supply. Despite these differing patterns among subhabitats, we found only a weak negative correlation fertilization response (RRP only) with latitude in terrestrial experiments (see Supplementary Figure 1 in Appendix S1) – perhaps signifying that latitude is a poor predictor of soil age or that geological parent material also plays a major role in addition to age. Subhabitat differences were also observed for the aquatic ecosystems, such as the apparent predominance of P-limitation in lake benthos relative to a pattern of balanced N and P limitation in stream and lake pelagic systems. Interpretation of this pattern is complicated by the somewhat limited sample size associated with the lake benthos (only 36 observations are involved in the RRN and RRP averages).
Our findings of widespread prevalence of both N and P limitation, of synergistic effects of N and P enrichment and of considerable variation within major ecosystem types in the strength of response to N or P enrichment have important implications for understanding and mitigating the effects of altered nutrient inputs on ecosystems. First, they call attention to the need for local assessments of ecological limiting factors in effectively addressing issues of eutrophication. Second, the dual importance of N and P limitation indicates that effects of alterations of a particular nutrient may be manifested not simply via quantitative changes in ecosystem production but also via qualitative shifts in the nature of nutrient limitation. This is likely to have subsequent impacts on competitive interactions among autotroph species (Grover 1997) and on stoichiometric processing of autotroph production by consumers (Sterner & Elser 2002). Finally, our results clearly show that enrichment by either N or P can increase autotroph production but that a simultaneous increase in both nutrients leads to dramatically higher levels of production in nearly all situations. Thus, ecosystem conservation and management efforts should take a balanced approach to N and P abatement throughout the biosphere.
Acknowledgements
This paper is a contribution from the Trophic Structure Comparisons working group at the National Center for Ecological Analysis and Synthesis, a centre funded by the National Science Foundation, the University of California and the State of California. We thank the staff of NCEAS for logistical support. We are grateful to many colleagues for providing digital versions and supplementary data for their published studies. P.M. Vitousek, R.W. Sterner and three anonymous referees provided useful comments on an early draft of the manuscript. This is Contribution Number 2383, Bodega Marine Laboratory, University of California at Davis. Correspondence and requests for materials should be sent to J.J.E. ([email protected]).




