Paracetamol (Acetaminophen): mechanisms of action
Summary
Paracetamol has a central analgesic effect that is mediated through activation of descending serotonergic pathways. Debate exists about its primary site of action, which may be inhibition of prostaglandin (PG) synthesis or through an active metabolite influencing cannabinoid receptors. Prostaglandin H2 synthetase (PGHS) is the enzyme responsible for metabolism of arachidonic acid to the unstable PGH2. The two major forms of this enzyme are the constitutive PGHS-1 and the inducible PGHS-2. PGHS comprises of two sites: a cyclooxygenase (COX) site and a peroxidase (POX) site. The conversion of arachidonic acid to PGG2 is dependent on a tyrosine-385 radical at the COX site. Formation of a ferryl protoporphyrin IX radical cation from the reducing agent Fe3+ at the POX site is essential for conversion of tyrosine-385 to its radical form. Paracetamol acts as a reducing cosubstrate on the POX site and lessens availability of the ferryl protoporphyrin IX radical cation. This effect can be reduced in the presence of hydroperoxide-generating lipoxygenase enzymes within the cell (peroxide tone) or by swamping the POX site with substrate such as PGG2. Peroxide tone and swamping explain lack of peripheral analgesic effect, platelet effect, and anti-inflammatory effect by paracetamol. Alternatively, paracetamol effects may be mediated by an active metabolite (p-aminophenol). p-Aminophenol is conjugated with arachidonic acid by fatty acid amide hydrolase to form AM404. AM404 exerts effect through cannabinoid receptors. It may also work through PGHS, particularly in areas of the brain with high concentrations of fatty acid amide hydrolase.
Introduction
Paracetamol (acetaminophen) remains the most popular analgesic/antipyretic used in children. The product became available in USA in 1955 and in UK the following year. Its ascension followed an association noted between Reye’s syndrome and aspirin (1). Despite the popularity of this medicine, the mechanism by which paracetamol achieves its effects on fever and pain is still debated.
It has been assumed that paracetamol probably acts through the cyclooxygenase (COX) pathway (Figure 1). This is the pathway through which the nonsteroidal anti-inflammatory drugs (NSAIDs) act. The NSAIDs inhibit production of prostaglandins (pro-inflammatory chemicals; PGE2, PGI2, PGF2α) and exert consequent effect (Table 1). They also influence thromboxane (TXA2). TXA2 is a vasoconstrictor, potent hypertensive agent, and facilitator of platelet aggregation. The ubiquitous nature of the PGs in the human body (Table 1) means that pediatric indications for NSAIDs range from pyrexia and analgesia to ductus arteriosis closure, prevention of vascular thrombosis and cystic fibrosis management.
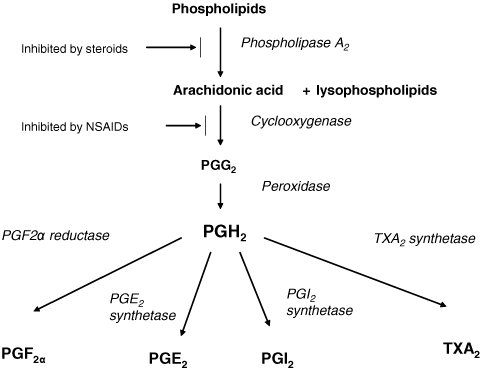
Schematic diagram of arachidonic acid metabolism.
| Prostaglandin type | Prostaglandin receptor | Function |
|---|---|---|
| PGI2 | IP | VasodilationInhibit platelet aggregationBronchodilation |
| PGE2 | EP1 | BronchoconstrictionGI tract smooth muscle contractionRegulation of blood pressure |
| EP2 | BronchodilationGI tract smooth muscle relaxationVasodilation | |
| EP3 | ↓ Gastric acid secretion↑ Gastric mucus secretionUterine contraction (when pregnant)GI tract smooth muscle contractionLipolysis inhibition↑ Autonomic neurotransmittersFever generationKidney reabsorption | |
| EP4 | Level and stability of COX-2 mRNANeonatal adaptation of circulatory system | |
| Not specified | HyperalgesiaPyrogenic | |
| PGF2α | FP | Uterine contractionBronchoconstrictionInduces parturitionModulates intraocular pressure |
- PG, prostaglandin; GI, gastrointestinal. Prostaglandin receptors are termed DP, EP, FP and IP that relate to the receptor that ligates the corresponding prostanoid e.g. EP (there are 9 known subtypes) is the receptor for PGE2.
Much investigation has centered on paracetamol’s inhibition of the COX enzyme because its analgesic and antipyretic effects are similar to those of aspirin, the archetype NSAID. However, paracetamol does not have significant anti-inflammatory activity nor does it inhibit production of the pro-clotting TXAs. Paracetamol does not appear to have a major effect peripherally; its action appears to be mostly central. It seems reasonable to assume that although there may be some effect on COX enzymes, this effect is different from that seen with the NSAIDs.
Alternative mechanisms of action proposed include inhibition of the l-arginine-nitric oxide (NO) pathway (2,3) mediated through substance P or N-methyl-d-aspartate (NMDA) (4), reinforcement of descending inhibitory serotonergic pain pathways (5), and active paracetamol metabolites that have effect on cannabinoid (CB) receptors (6,7). This current review attempts to link these disparate mechanisms, albeit loosely. Several recent reviews (8–12) cover this subject matter comprehensively.
A central serotonergic mechanism
A central mechanism of action for paracetamol has been proposed (13,14). Paracetamol concentrations in the cerebrospinal fluid mirror response to fever (15) and pain (16) to a greater extent than plasma concentrations. Paracetamol is effective in rat pain models after central administration (17). Animal data supports the contention that spinal 5-hydroxytryptamine type 3 (5-HT3) receptors are be involved in the antinociceptive effect of paracetamol (18,19) and that paracetamol interferes with serotonergic descending pain pathways. Support for these data in humans comes from the demonstration that co-administration of tropisetron or granisetron (5-HT3 receptor antagonists) with paracetamol completely blocked the analgesic effect of acetaminophen in volunteers (rapid metabolizers of tropisetron, n = 26) when assessed by pain induced from electrical stimulation of the median nerve. Volunteers given granisetron, a more specific antagonist, had greater pain (measured as area under the time-pain curve) than those given tropisetron (20). It is believed that paracetamol reinforces descending inhibitory pain pathways (5).
Data supporting the central effect of paracetamol through activation of descending serotonergic pathways do not refute arguments that its primary site of action may still be inhibition of PG synthesis, as for the NSAIDs (8). For example, the expression of a PGE2 receptor (EP3) by most of the serotonergic, noradrenergic, and adrenergic cell groups suggests that PGE2 modulates many physiologic processes. It may modulate nociceptive and autonomic processes by affecting the descending serotonergic pathway from the raphe magnus nucleus to the spinal cord (21). Serotonergic cell bodies in the raphe magnus nucleus provide dense projections to the dorsal horn of the spinal cord, and this descending pathway has been shown to mediate the antinociceptive action of morphine (22,23).
Prostaglandin H2 synthetase inhibition
Prostaglandin H2 synthetase is the enzyme responsible for metabolism of arachidonic acid to the unstable PGH2. The two major forms of this enzyme are the constitutive PGHS-1 and the inducible PGHS-2. These two enzymes are commonly referred to as COX-1 and COX-2 (24). However, the nomenclature PGHS is preferred because there are two active sites on this enzyme: a COX site and a POX site. The activity of the COX enzyme relies on its being in the oxidized form and it is suggested that paracetamol reduces the amount of the oxidized form by an action on the POX site (10). An alternative suggestion is that a PGHS variant (COX-3) exists in the central nervous system (CNS), and that this variant is exquisitely sensitive to paracetamol (25).
Paracetamol activity at the POX site
There is a two-step process for conversion of arachidonic acid to PGH2. First, arachidonic acid gains two molecules of O2 to form PGG2 (via COX) and then PGG2 is reduced to PGH2 by two electrons (via POX). These reactions occur at two different sites. POX occurs at an heme-containing active site at the protein surface while COX happens in a hydrophobic channel in the core of the enzyme (26). COX is dependent on POX, but POX can operate quite independently (27).
The conversion of arachidonic acid to PGG2 is dependent on the tyrosine-385 radical (Tyr385*) at the COX site (28) (Figure 2). However, generation of this radical from Tyr385 is reliant on generation of a ferryl protoporphyrin IX radical cation (Fe4+ = OPP*+) at the POX site. Paracetamol interferes with this process by acting as a reducing cosubstrate in a reaction that partially reduces Fe4+ = OPP*+ so that less Fe4+ = OPP*+ is available to be transferred to the COX site. Consequently, less Tyr385* is available to stimulate conversion of arachidonic acid to PGG2 (10,27).
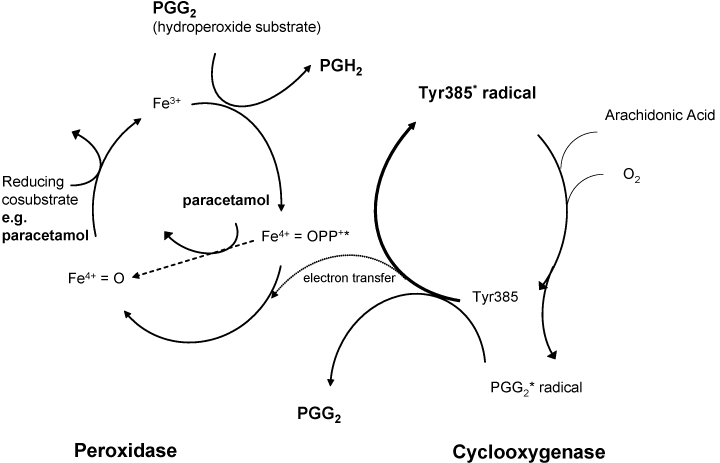
Prostaglandin H2 synthetase (PGHS) is the enzyme responsible for metabolism of arachidonic acid to the unstable PGH2. Formation of tyrosine-385 radical (Tyr385˙) at the cyclooxygenase site is dependent on the reduction of a ferryl protoporphyrin IX radical cation (Fe4+ = OPP*+) at the peroxidase site. Paracetamol is a reducing cosubstrate that partially reduces Fe4+ = OPP*+, decreasing the amount available for regeneration of Tyr385*. Figure adapted from Aronoff et al. (10).
Two factors contribute to paracetamol’s poor anti-thrombotic and anti-inflamatory effects. Paracetamol inhibitory potency against PGHS is antagonized by hydroperoxides such as PGG2. The increased generation of Fe4+ = OPP*+ guarantees available substrate for transportation to the COX site. Low levels of exogenous arachidonic acid are provided to brain endothelial cells where PGHS-2 is induced during fever. Consequently less PGG2 is generated. Paracetamol has effect in these cells. In contrast, there is explosive activation of phospholipase A2 biosynthesis in platelets when activated by thrombin. The burst in PGG2 formation inhibits any paracetamol effect. In addition, another lipid hydroperoxide (12-hydroperoxyicoatetraenoic acid) is also formed by platelet 12-lipoxygenase. This substance is also a good substrate for the reducing agent Fe3+, increasing Fe4+ = OPP*+ availability. Hydroperoxide-generating lipoxygenase enzymes are also extremely active in inflammatory leucocytes, negating paracetamol’s effect. This ‘peroxide tone’ of different cells may explain paracetamol’s differing effects in differing tissues (10).
Paracetamol activity at a variant PGHS (COX-3) site
Flower and Vane (29) demonstrated that paracetamol inhibits COX activity in brain homogenates more so than those from the spleen. This experiment supported the idea that variant COX enzymes exist and that paracetamol acts centrally. Such an enzyme (COX-3 or PGHS-1b) has been identified in the canine cerebral cortex (25). This enzyme, when expressed in dogs, shares a strong similarity to the other COX enzymes, produces pro-inflammatory chemicals, and is selectively inhibited by paracetamol (25). However, subsequent research has suggested that in humans and mice, the COX-3 encodes proteins with completely different amino acid sequences than PGHS-1 or PGHS-2 and without COX activity, so that it is improbable that COX-3 in these species plays a role in PG-mediated fever and pain (9,12).
Fever is associated with rapid induction of PGHS-2 and an increase in PGE2 production in the hypothalamus rather than the cerebral cortex. PGHS-1 or a variant of PGHS-1 (COX-3) seems to have little role here. Similarly, PGHS-2 is constitutively expressed in the CNS and rapidly up-regulated to reinforce pain perception. This would suggest an isoform variant of PGHS-2 rather than PGHS-1. Despite limitations with the belief that COX-3 may be site of paracetamol action, it has been suggested that there may be varied products from the two distinct COX proteins with overlapping contributions to prostanoid production throughout the body (30).
Cannabinoid receptor activity
Parents may administer paracetamol before bed time to relieve pain, reduce fever or simply to settle. The mechanism behind this settling or even the existence of paracetamol’s settling property has been doubted. Paracetamol results in only a modest improvement in activity and alertness in viral infections. Mood, comfort, appetite, and fluid intake were not improved compared with controls (31–33). Despite these doubts about paracetamol, subjective effects of euphoria, relaxation, and tranquillity are shared by aniline analgesics. These effects are similar to those of CBs.
Two research groups (6,7) have demonstrated an active metabolite of paracetamol (the fatty acid amide N-arachidonoylphenolamine; AM404); a compound that shares the ability of CBs to display analgesic activity and to lower body temperature (34,35). Paracetamol is mostly cleared by the liver through glucuronide and sulfate conjugation. However, it is de-acetylated in the mouse brain and spinal cord to p-aminophenol. This primary amine is then conjugated with arachidonic acid by fatty acid amide hydrolase to form AM404. AM404 does not work directly on CB receptors (36). It is a potent activator of the vanilloid subtype 1 receptor (TRPV1) (37), which is a ligand at CB1 receptors and an inhibitor of cellular anandamide uptake resulting in increased levels of endogenous CBs. Anandamide uptake would result in the activation of nociceptors. AM404 may also work through PGHS, particularly in areas of the brain with high concentrations of fatty acid amide hydrolase (mesencephalic trigeminal nucleus, primary sensory neurons), although this argument remains speculative.
Paracetamol’s mooted physical sites of action also appear similar to CB sites. The antinociceptive effect of paracetamol involves the activation of spinal serotonergic descending projections (18). CBs also produce their antinociceptive effect by descending spinal inhibition mediated mostly through CB1 receptors. CBs markedly lower body temperature (156) through the activation of CB1 receptors in the pre-optic area (38).
Nitric oxide
Depolarization of afferent neurones by peripheral harmful stimuli leads to activation of spinal NMDA receptors. Rodent studies suggest that these, in turn, promote the synthesis of NO; a neurotransmitter at a spinal level conveying nociceptive information (39,40) NSAIDs and paracetamol interfere with nociception associated with spinal NMDA receptor activation. This effect may involve an inhibitory action on spinal NO mechanisms (41). It seems counter-intuitive that NO-releasing NSAIDs or NO-releasing paracetamol (nitroparacetamol) should have enhanced potency and an improved safety profile (42,43). However, the small amounts of NO released by nitroparacetamol appear to have minimal effect on central pain mechanisms, but an improved peripheral anti-inflammatory effect (11).
The role that NO plays in nociception remains blurred. NO donors may induce either pronociception or antinociception. These opposing actions may be concentration-dependent with high doses of the NO donor intensifying ongoing pain (44). Positive modulation of the constitutive neuronal isoform of NO synthase occurs with activation of NMDA in the spinal cord (45). Of interest, however, are data confirming that NO modulates the biologic levels of arachidonate-derived cell signaling molecules by either enhancing or suppressing the activity of both PGH2 isoforms (PGHS-1 and PGHS-2) (46–48). The NO and eicosanoid biosynthetic pathways are linked in ways not yet fully disentangled.
Conclusions
Paracetamol antinociception is through interference with serotonergic descending pain pathways. This mechanism does not refute arguments that its primary site of action may still be inhibition of PG synthesis. An elegant model where paracetamol acts as a reducing cosubstrate on the POX site of the PGHS enzyme when combined with the ‘peroxide tone’ of different cells, explains paracetamol’s lack of platelet and anti-inflammatory effects. An active metabolite has been identified in mice. This metabolite (p-aminophenol) is then conjugated with arachidonic acid by fatty acid amide hydrolase to form AM404. AM404 exerts effect through CB receptors. It may also work through PGHS, particularly in areas of the brain with high concentrations of fatty acid amide hydrolase. Currently, the role and activity of this metabolic product have only been identified in mice and its role in humans unquantified. The theory that paracetamol is a prodrug and that an active metabolite may exert effect through CB receptors and the PGHS enzyme is certainly attractive. It is possible that paracetamol works at several stages within spinal pathways and future developments in this field are eagerly anticipated.
Conflict of interest
Brian Anderson has received honoraria for talks, consultancies, and support for travel costs to conferences from Neuren Pharmaceuticals, Bristol-Myer Squibb, Reckitt Benckiser, SmithKline Beecham, and McNiell Pharmaceuticals.




