Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers
Abstract
Chronic smoking is thought to cause changes in brain reward systems that result in overvaluation of cigarette-related stimuli and undervaluation of natural rewards. We tested the hypotheses that, in smokers, brain circuits involved in emotional processing: (i) would be more active during exposure to cigarette-related than neutral pictures; and (ii) would be less active to pleasant compared with cigarette-related pictures, suggesting a devaluation of intrinsically pleasant stimuli. We obtained whole-brain blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging data from 35 smokers during the presentation of pleasant (erotica and romance), unpleasant (mutilations and sad), neutral, and cigarette-related pictures. Whole-brain analyses showed significantly larger BOLD responses during presentation of cigarette-related pictures relative to neutral ones within the secondary visual areas, the cingulate gyrus, the frontal gyrus, the dorsal striatum, and the left insula. BOLD responses to erotic pictures exceeded responses to cigarette-related pictures in all clusters except the insula. Within the left insula we observed larger BOLD responses to cigarette-related pictures than to all other picture categories. By including intrinsically pleasant and unpleasant pictures in addition to neutral ones, we were able to conclude that the presentation of cigarette-related pictures activates brain areas supporting emotional processes, but we did not find evidence of overall reduced activation of the brain reward systems in the presence of intrinsically pleasant stimuli.
Introduction
Relapse is the most likely outcome of any smoking cessation attempt (Piasecki, 2006). Theoretical models attribute relapses to altered motivational processes. The affective motivational model (Baker et al., 2004) emphasizes the role of negative affect as the principal culprit in the maintenance of smoking addiction. The incentive-sensitization theory (Robinson & Berridge, 2003) highlights the power of drug-related cues to trigger compulsive drug seeking. In fact, cravings elicited by cigarette cues are often reported by smokers as the precipitating cause of relapse (Shiffman et al., 2007). Volkow et al. (Goldstein & Volkow, 2002; Koob & Volkow, 2010; Volkow et al., 2010) proposed that, in addition to the presence of drug cues, the reduction of the motivational value held by natural rewards (e.g. sex, food) could be another cause of relapse.
Studies testing the hypothesis that chronic drug use leads to the devaluation of natural rewards have provided mixed results. In animal models, chronic drug administration has been found to both reduce (Grigson & Twining, 2002) and increase (Fiorino & Phillips, 1999; Wyvell & Berridge, 2001) the incentive value attributed to natural rewards. In humans, Goldstein et al. (2010) showed that cocaine addicts attribute greater incentive value to cocaine cues than to natural rewards when ‘under the influence’. In contrast, Barr et al. (2008) found that, in nicotine-naive humans, nicotine administration results in response bias toward natural rewards.
Functional neuroimaging has the potential to link findings obtained by using self-report (Goldstein et al., 2010) and behavioral measures (Barr et al., 2008) to clarify the important question of how chronic drug use affects neural responses to rewarding stimuli. Two functional magnetic resonance imaging (fMRI) studies have found evidence of a link between addiction and decreased responses to natural rewards. Garavan et al. (2000) found that cocaine addicts had smaller blood oxygen level-dependent (BOLD) responses in reward-processing circuits when viewing an erotic video compared with a cocaine-related video. Heinz et al. (2007) found that blunted responses to intrinsically pleasant pictures in the ventral striatum were predictive of relapse in a sample of detoxified alcoholics. This raises the question of whether a similar phenomenon is evident in cigarette smokers.
Although prior neuroimaging studies of smokers reported increased activation at mesolimbic sites during cigarette cues compared with neutral cues (Due et al., 2002; David et al., 2005; McClernon et al., 2005; Franklin et al., 2007), none has directly compared neural activation with cigarette cues and other naturally rewarding stimuli. The novel aim of this study was to compare BOLD signal change when smokers viewed cigarette-related, pleasant, unpleasant and neutral pictures. We hypothesized that if cigarette cues are motivationally significant to smokers, their presence should heighten BOLD activation in regions activated by the presentation of pleasant pictures (e.g. erotic and romantic scenes). We also directly compared the magnitude of the BOLD signal change with cigarette-related and pleasant pictures to assess whether smoking sensitizes responses to cigarette cues at the expense of intrinsically pleasant stimuli, consistent with the fMRI results obtained in alcoholics and cocaine addicts (Garavan et al., 2000; Heinz et al., 2007).
Materials and methods
Participants
Smokers motivated to quit smoking and willing to participate in a clinical trial of smoking-cessation medications were recruited via local (Houston metropolitan area) radio, print and internet advertising. As part of this trial, participants were asked to complete two fMRI scanning sessions. The first session occurred before randomization to a medication group and while still smoking at their regular rate, while the second session occurred 24 h after quitting and while still receiving study medications. This article only presents data from the first session. The data from the post-quit fMRI session and its relationship to smoking cessation outcomes will be the focus of future articles, at the conclusion of the ongoing clinical trial.
Inclusion criteria for participation in the study were as follows – 18–65 years old, self-reported smoking of at least 10 cigarettes per day, baseline expired-air carbon monoxide (CO) > 10 ppm, not currently meeting diagnostic criteria for psychiatric or substance use disorders (other than smoking), being free of contraindications for the study medications (bupropion and varenicline), being fluent in English, having a working telephone, being right-handed (to reduce variability introduced by differences in brain lateralization), and self-reported European ancestry (to maintain a homogeneous sample for future genetic analyses). Of the 50 individuals who met the inclusion criteria, 40 completed the pre-quit fMRI session. Scanner-related equipment failure resulted in the loss of fMRI data for five participants, resulting in a total of 35 participants (14 female) for this analysis. Table 1 lists demographic and smoking-related characteristics for the group. All participants provided written informed consent, and the research was approved by the University of Texas MD Anderson Cancer Center Institutional Review Board. Participants were compensated $50 for completing each fMRI session.
| Variable | Total (N = 35) % (N) |
|---|---|
| Gender | |
| Female | 40.0 (14) |
| Mean (SD) | |
| Age (years) | 42.7 (11.3) |
| Years smoking | 23.4 (11.8) |
| Current smoking rate (cigarettes/day) | 21.8 (10.6) |
| Pre-scan expired CO (ppm) | 23.9 (12.5) |
| FTND score | 4.6 (2.3) |
- FTND, Fagerström test for nicotine dependence.
Stimuli
We based our picture-viewing task on a paradigm that has been used to reliably measure brain responses to emotional pictures (Bradley et al., 2003; Sabatinelli et al., 2007a). Participants viewed 64 pictures from the International Affective Picture System (IAPS; Lang et al., 2005), and from smoking-related picture collections previously used in our laboratory (Carter et al., 2006) and elsewhere (Gilbert & Rabinovich, 1999). Pictures were selected from four categories of affective valence, including pleasant (eight high-arousal erotic pictures and eight low-arousal romantic pictures), unpleasant (eight high-arousal mutilation pictures and eight low-arousal sad pictures), neutral (16 neutral people) and cigarette related (16 pictures of people smoking). The high-arousal and low-arousal subcategories were selected to have the same arousal levels (based on the IAPS normative ratings) in both the pleasant and unpleasant valence categories.1 The slides were displayed for 3 s each, separated by a 12-s intertrial interval, and delivered in a pseudorandom order with respect to category, with the restriction that no more than two pictures of the same category would be presented consecutively. Stimuli were presented with a Pentium 4 computer running e-prime software (Psychology Software Tools, Pittsburgh, PA, USA) and an MR-compatible stimulus-presentation system (IFIS-SA; Invivo, Orlando, FL, USA) such that they subtended a horizontal viewing angle of approximately 15 °.
Procedure
All laboratory sessions took place between 09:00 and 12:00 h. Participants were allowed to smoke ad libitum before the laboratory session to ensure that they would be in a non-deprived state. Upon arrival at the laboratory, the participant provided an expired-air CO sample to confirm that they had smoked, and completed smoking status and mood questionnaires. After completing the questionnaires, participants were escorted to the fMRI scanning suite, placed into the scanner, and instructed to simply look at the stimuli. The MR images were acquired using a GE Healthcare (Milwaukee, WI, USA) 3.0 T Signa HDxt (v 15.0) magnetic resonance scanner with TwinSpeed gradients in zoom mode (40 mT/m, 150 mT/m-s slew rate), and an eight-channel, high-resolution, transmit/receive brain volume coil (MRI Devices, Waukesha, WI, USA). After a short acclimation period, the BOLD signal was measured using a T2*-weighted, echo-planar imaging protocol, while the participants completed the picture-viewing task. The slice prescription consisted of 50 coronal slices (2.5 mm thick, 0.5 mm gap) that covered most of the brain, except for the most anterior and most posterior regions of the prefrontal and occipital cortices, respectively. The parameters for this pulse sequence were as follows – matrix size = 64 × 64; field-of-view = 160 × 160 mm; in-plane resolution = 2.5 × 2.5 mm; TR = 3000 ms; TE = 25.3 ms; flip angle = 90 °. The acquisition of each volume was time-locked to the slide presentation, such that, after discarding the first two volumes to allow T1 magnetization to reach steady-state, one volume was acquired while the slide was on and four volumes were acquired during the intertrial interval, for a total of 335 volumes for the entire picture-viewing task. After the picture-viewing task, a high-resolution (1 mm3 voxels), three-dimensional, T1-weighted anatomical image was obtained using a magnetization-prepared, fast spoiled gradient echo (IR-3DFSPGR) pulse sequence (TR = 6.432 ms, TE = 2.1 ms, inversion time = 400 ms, flip angle = 20 °). After obtaining the anatomical image, the participant was removed from the scanner and completed a post-experimental questionnaire before dismissal until the next experimental session.
Data reduction and analysis
The goal of this study was to determine whether BOLD responses to cigarette cues exceeded those to other categories of emotional stimuli. Primary analysis consisted of two steps. First, we identified regions of interest (ROIs) on the basis of a whole-brain analysis of the fMRI data that compared BOLD responses with cigarette and neutral cues, an approach followed in previous studies (McBride et al., 2006; Brody et al., 2007; McClernon et al., 2008, 2009; Janes et al., 2009). Second, we compared the BOLD responses across all stimulus categories within the ROIs identified in step 1 (Bradley et al., 2003). In addition, we conducted a secondary analysis comparing BOLD responses across all stimulus categories within two theoretically important ROIs that were not found to be significantly active in the whole-brain analysis from step 1. This analysis included the amygdala and the nucleus accumbens.
Step 1 – ROI identification
A whole-brain analysis was used to identify ROIs where BOLD responses to cigarette cues were greater than those to neutral cues. This analysis was conducted using BrainVoyager qx (version 2.2; Brain Innovation, Maastricht, the Netherlands; Goebel et al., 2006). First, the data for each participant were pre-processed, which consisted of slice-timing correction using cubic spline interpolation, motion correction using a six-parameter rigid-body transformation and trilinear interpolation, spatial smoothing with a three-dimensional Gaussian kernel (5-mm FWHM), and high-pass temporal filtering with a cutoff frequency of 0.02 Hz. The preprocessed functional images were aligned to the participants’ anatomical image, followed by transformation into standard Talairach–Tournoux space (3 mm isotropic voxels) through manual identification of reference points in the anatomical images.
The Talairach-transformed functional images for each subject were analysed using a fixed-effects general linear model. For each picture category (erotic, romantic, mutilation, sad, neutral and cigarette), a boxcar function representing the stimulus timing was convolved with a canonical hemodynamic response function to generate the predicted BOLD response for pictures from that category. The head motion parameters from preprocessing were added to the model as nuisance variables. Parameter estimates (β) were obtained for each of the picture categories for each voxel using least-squares regression. For group analysis, the individual subjects’β-values for each picture category were entered into a random-effects general linear model. The t-statistic from the cigarette vs. neutral contrast of the random-effects β-values was used to generate a statistical parametric map of the strength of cigarette cue reactivity. Clusters of voxels that exceeded a statistical threshold of t34 > 3.25 and cluster-size threshold of 125 mm3 were labeled as significantly active, which controlled the false discovery rate at P < 0.05. These clusters were defined as ROIs for the comparison of BOLD responses across all stimulus categories.
Step 2 – comparison of BOLD responses across all stimulus categories
Mean peristimulus time courses for each picture category were extracted from voxels within the ROIs identified from the whole-brain analysis and expressed as the percent change from the ROI’s entire time course. The volumes that started at 0, 3, 6, and 9 s after picture onset were then baseline-corrected by subtracting the value of the volume measured immediately before picture onset. To identify the post-stimulus time points that showed the largest differences across stimulus categories, we conducted a category × time within-subjects analysis of variance (anova) on the peristimulus time courses extracted from the largest ROI, the visual association cortex. The 6- and 9-s post-onset volumes showed the largest differences among picture categories (see Results). Thus, the data from these two time points were averaged for each of the ROIs identified using the whole-brain analysis and entered into anovas using picture category as a within-subjects factor.2 In the event of a significant F-test in these anovas, we used Tukey HSD post hoc tests to directly compare the difference in BOLD response among the stimulus categories. To evaluate arousal modulation of the BOLD response, we tested the significance of the quadratic trend in a polynomial contrast for the following picture categories: mutilation; sad; neutral; romantic; and erotic (coefficients of 2, −1, −2, −1, 2, respectively; Bradley et al., 2003). For all statistical tests, the significance threshold was set at P < 0.05. For effects involving repeated measures, significance was assessed using multivariate tests (Wilks λ and its approximate F-statistic).
Theory-based ROI analysis
Although the whole-brain analysis did not detect significantly greater activation for cigarette pictures than neutral pictures in the amygdala or the ventral striatum (see Results), we were interested in BOLD responses in these regions due to their theoretical significance for responses to emotional pictures (Sabatinelli et al., 2007a) and tobacco addiction (David et al., 2005; Franklin et al., 2007). Thus, we created ROIs for these structures using coordinates from a computerized version of the Talairach–Tournoux atlas (Lancaster et al., 1997, 2000). Extraction of peristimulus time courses from these regions and subsequent anovas for differences in the BOLD response among stimulus categories paralleled those described above.
Results
Participant characteristics
Table 1 shows demographic and smoking characteristics of the 35 participants included in the analyses.
Identification of ROIs from the whole-brain analysis
To identify ROIs for detailed comparisons of the BOLD response among cigarette cues and emotional stimuli, we examined the results of a whole-brain analysis of BOLD responses to cigarette vs. neutral cues. This analysis indicated that viewing cigarette related pictures, compared with neutral pictures, heightened BOLD activity in the visual association cortex in the occipital, posterior parietal and inferior temporal lobes, the dorsal striatum, the cingulate gyrus, the dorsolateral prefrontal cortex and the insula. Peak activation coordinates and statistics for the activated regions are listed in Table 2.
| Brain area | Hem | BA | Talairach coordinates (mm) | Cluster size (mm3) | t max | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Visual association cortex | |||||||
| Middle temporal gyrus | L | 37 | −47 | −64 | 4 | 6838 | 7.34 |
| R | 37 | 51 | −59 | 2 | 13671 | 8.58 | |
| Inferior parietal lobule | L | 40 | −40 | −44 | 43 | 13725 | 7.22 |
| R | 40 | 33 | −45 | 51 | 9870 | 8.14 | |
| Cuneus | L | 19 | −25 | −82 | 28 | 360 | 4.86 |
| R | 19 | 22 | −77 | 34 | 662 | 4.05 | |
| Precuneus | M | 7 | −4 | −61 | 33 | 1265 | 5.89 |
| Frontal lobe | |||||||
| Middle frontal gyrus | L | 46 | −45 | 30 | 18 | 448 | 4.18 |
| Dorsolateral prefrontal cortex | L | 9 | −48 | 5 | 25 | 1238 | 4.17 |
| Insula | L | 13 | −37 | −8 | 9 | 543 | 5.92 |
| Cingulate and subcortical | |||||||
| Anterior cingulate gyrus | L | 24 | 0 | 38 | 2 | 352 | 4.60 |
| Posterior cingulate gyrus | L | 31 | −2 | −27 | 31 | 1475 | 4.16 |
| Caudate head | R | 9 | 3 | 4 | 308 | 4.13 | |
- BA, Brodmann area; Hem, hemisphere; L, left; M, midline; R, right. Talairach coordinates are for the center of gravity of the cluster, and are expressed as follows, with the origin at the anterior commissure: x increases from left to right, y increases from posterior to anterior, z increases from inferior to superior. tmax = maximum t-statistic found within the cluster, with 34 degrees of freedom.
BOLD responses within each ROI
Visual association cortex
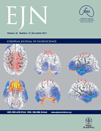
The whole-brain analysis revealed that responses to cigarette cues differed from neutral cues in several areas of the visual association cortex in the occipital, posterior parietal and temporal lobes (Fig. 1A). The clearest pattern of significant activation emerged bilaterally in the cuneus, inferior parietal lobule and middle temporal gyrus. Comparison of BOLD responses across all stimulus categories revealed a large increase in BOLD signal in response to erotic pictures, followed by all other emotional and cigarette-related pictures, and finally by neutral pictures (Fig. 1B), as shown by a statistically significant category × time interaction (F20,15 = 5.32, P < 0.001, λ = 0.12), which was the result of significant differences between stimulus categories during the 3-, 6- and 9-s post-stimulus onset volumes (F5,30 > 3.6, P < 0.05). The BOLD response peaked during the 6- and 9-s post-stimulus onset volumes, and the largest differences between picture categories were observed during this interval. Thus, subsequent analyses focused on the percent change in BOLD signal from baseline averaged across these two volumes. Figure 1C shows the BOLD responses in this time window averaged across the active clusters in the visual association cortex. The main effect of picture category was significant (F5,30 = 12.27, P < 0.001, λ = 0.33), and post hoc comparisons showed that responses to erotic pictures were significantly larger than those to all other picture categories (P < 0.001), and that responses to neutral pictures were significantly smaller than responses to all other picture categories (P < 0.05). The significant quadratic trend (F1,34 = 30.01, P < 0.0001) showed that in the visual cortex, BOLD activation increased as picture content became more emotionally arousing.
(A) Clusters of significantly higher functional activity during cigarette-related picture processing relative to neutral pictures in the visual association cortex. (B) Event-related time courses of the percent blood oxygenation level-dependent (BOLD) signal change from baseline averaged across cuneus, inferior parietal lobule and middle temporal gyrus in response to the different categories of pictures. (C) Percent signal change relative to the baseline from 6 to 12 s post-stimulus onset averaged across cuneus, inferior parietal lobule and middle temporal gyrus in response to the different categories of pictures. # Indicates that BOLD responses to neutral pictures were significantly smaller than those to all other picture categories (P < 0.05). ^ Indicates that BOLD responses to erotic pictures were significantly larger than those to all other picture categories (P < 0.001). CIG, cigarette-related pictures; ERO, erotic couples; MUT, mutilations; NEU, neutral people; ROM, romantic couples; SAD, sad contents.
Dorsal striatum
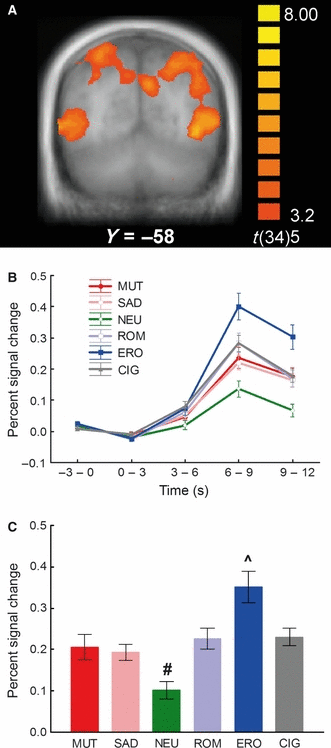
The whole-brain analysis found another area of significant cigarette > neutral cue reactivity in the head of the right caudate nucleus. Across all picture categories, the pattern of results in this cluster was similar to that seen in the visual association areas (Fig. 2A and B; stimulus category F5,30 = 4.67, P < 0.01, λ = 0.56), in which BOLD responses to erotic pictures were significantly greater than responses to all other picture categories (P < 0.05). Although the differences were not statistically significant (corrected P-values were between 0.11 and 0.39), BOLD responses to cigarette-related, romantic, sad and mutilation pictures appeared to be larger than responses to neutral pictures, similar to the result observed in the visual association areas. In fact, the significant quadratic trend (F1,34 = 17.16, P < 0.0005) confirmed the relationship between emotional arousal and BOLD activation.
(A) Cluster of significantly higher functional activity during cigarette-related picture processing relative to neutral pictures in the head of the right caudate nucleus (NC, Talairach coordinates: 9, 3, 4; yellow circle) and the left anterior cingulate gyrus (ACG, Talairach coordinates: 0, 38, 2; red circle). (B) Percent signal change relative to the baseline from 6 to 12 s post-stimulus onset in the head of the right caudate nucleus in response to the different categories of pictures. ^BOLD responses to erotic pictures were significantly larger than those to all other picture categories (P < 0.05). (C) Percent signal change relative to the baseline from 6 to 12 s post-stimulus onset averaged across the anterior and posterior cingulate gyri in response to the different categories of pictures. ACG, anterior cingulate gyrus; CIG, cigarette-related pictures; ERO, erotic couples; INS, insula; MUT, mutilations; NC, caudate nucleus; NEU, neutral people; ROM, romantic couples; SAD, sad contents.
Cingulate gyrus
Significantly active clusters from the whole-brain cigarette > neutral analysis were also evident in the left anterior (BA24) and posterior (BA31) cingulate gyrus (Table 2). Both clusters showed similar patterns of response, and Fig. 2C shows BOLD responses averaged across the two clusters. Examination of BOLD responses within this cluster to all picture categories found a significant main effect of picture category (F5,30 = 11.5, P < 0.001, λ = 0.34). Again, the largest BOLD increase occurred in response to erotic pictures, but the differences between these responses and the responses to romantic and cigarette pictures were not as large as those in the visual system and dorsal striatum. In fact, the difference between erotic and romantic or cigarette-related pictures did not reach significance (P < 0.10). Although the quadratic trend was significant (F1,34 = 16.69, P < 0.0005), an exploratory analysis using polynomial contrasts to compare the mean response to erotic, romantic and cigarette pictures with the mean response to neutral, sad and mutilation pictures found that there was more BOLD activation in response to erotic, romantic and cigarette pictures than to neutral, sad and mutilation pictures (F1,34 = 22.4, P < 0.001).
Prefrontal cortex
Two ROIs identified from the whole-brain cigarette > neutral analysis were found within the left dorsolateral prefrontal cortex, located primarily at the middle (BA46) and superior (BA9) frontal gyri (Table 2). In these regions, there was significant arousal modulation of the BOLD signal, with the largest responses to the highly arousing erotic and mutilation pictures, and the smallest responses to neutral pictures. This conclusion was supported by a main effect of picture category (F5,30 = 7.51, P < 0.001, λ = 0.44), and a significant quadratic trend of category (F1,34 = 16.48, P < 0.001). The response to cigarette-related pictures was similar to that seen in visual areas (i.e. smaller than erotic pictures but larger than neutral pictures), but these differences were not significant.
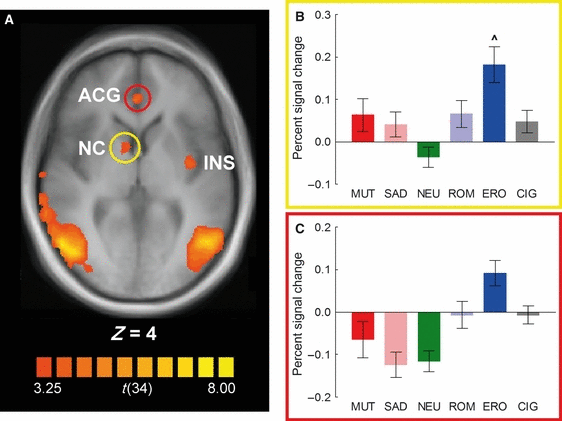
Insula
The whole-brain analysis also found a cluster of significant cigarette > neutral reactivity in the left insula (Fig. 3; main effect of picture category, F5,30 = 2.71, P < 0.05, λ = 0.69). In this region, comparison of BOLD responses across all picture categories revealed a pattern of results that was substantially different from that seen in the other regions, as cigarette-related pictures, rather than erotic, elicited the largest BOLD response. Although Tukey HSD post hoc tests found no significant differences between individual group means (P > 0.1), an exploratory polynomial contrast of BOLD responses to cigarette-related pictures vs. the mean of all other picture categories was statistically significant (F1,34 = 11.51, P < 0.01). This effect was primarily due to decreased BOLD responses relative to baseline in response to mutilation, neutral and pleasant pictures, and a small increase in BOLD responses to cigarette-related pictures.
(A) Cluster of significantly higher functional activity during cigarette-related picture processing relative to neutral pictures in the left insula (Talairach coordinates: −37, −8, 9). (B) Percent signal change relative to the baseline from 6 to 12 s post-stimulus onset in the left insula in response to the different categories of pictures. Unlike what is observed in all other areas of activation, cigarette-related pictures elicited a larger BOLD response than the other categories of pictures. ♦BOLD responses to cigarette-related pictures vs. the mean of all other picture categories P < 0.01. CIG, cigarette-related pictures; ERO, erotic couples; MUT, mutilations; NEU, neutral people; ROM, romantic couples; SAD, sad contents.
BOLD responses in theory-based ROIs
Amygdala
The amygdala ROI was defined as two 512-mm3 regions centered at ±20, −4, −11 mm in Talairach–Tournoux space (Fig. 4A, inset). The mean percent signal change from 6 to 12 s after picture onset over all voxels in these cubes was extracted and analysed for differences between picture categories. For non-cigarette-related pictures, the BOLD response varied as a function of the emotional arousal level of the picture (Fig. 4; main effect of picture category F5,30 = 3.87, P < 0.01, λ = 0.61), with the largest responses to the most highly arousing pictures (erotica and mutilation), and the smallest response to neutral pictures (quadratic trend of category –F1,34 = 5.82, P < 0.05). The BOLD response to cigarette-related pictures did not significantly differ from the BOLD response to neutral pictures.
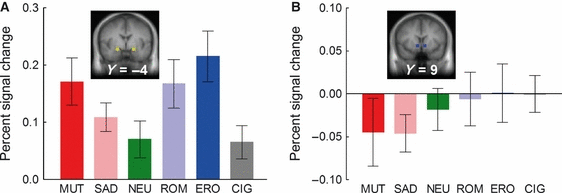
(A) Percent signal change relative to the baseline from 6 to 12 s after stimulus onset in the ROIs covering left and right amygdala (Talairach coordinates: ±20, −4, −11; see inset). (B) Percent signal change relative to the baseline from 6 to 12 s after stimulus onset in the two ROIs covering left and right ventral striatum (Talairach coordinates: ±9, 9, −4; see inset). CIG, cigarette-related pictures; ERO, erotic couples; MUT, mutilations; NEU, neutral people; ROM, romantic couples; SAD, sad contents.
Ventral striatum
The ventral striatal ROI was defined as two 512-mm3 regions centered at ±9, 9, −4 mm in Talairach–Tournoux space (Fig. 4B, inset). In this region, the BOLD response appeared to vary as a function of valence for non-smoking-related pictures (Fig. 4B), with decreased BOLD activity for mutilation and sad pictures, and no change from baseline for romantic and erotic pictures. The response to cigarette pictures resembled those to romantic and erotic pictures. However, the main effect of picture category was not statistically significant (F5,30 = 0.95, λ = 0.86), with an exploratory polynomial contrast between the mean response to sad and mutilation pictures, and the mean response to romantic, erotic and cigarette pictures only approaching significance (F1,34 = 3.31, P = 0.07).
Discussion
This study measured treatment-seeking smokers’ BOLD responses to cigarette cues and to non-drug-related pleasant, neutral and unpleasant cues. We tested the hypotheses that: (i) cigarette-related pictures, compared with neutral pictures, would produce significant BOLD activation in anatomical regions that are involved in the processing of emotional stimuli; and (ii) that the magnitude of BOLD responses to intrinsically pleasant stimuli would be reduced relative to the response to cigarette cues (Volkow et al., 2010). The first hypothesis was supported, but support for the second hypothesis was found only in the insula, where responses to cigarette cues were greater than responses to all other picture categories. In all other clusters of activation, the magnitude of the smokers’ BOLD responses to erotic stimuli exceeded the magnitude of their BOLD responses to cigarette cues. This finding has important implications for future studies of smoking cue reactivity and for theories of addiction.
Reactivity to cigarette and intrinsically pleasant cues
Through a learning process, chronic drug use is believed to result in the attribution of increased incentive salience to drug cues. Thus, major theories of addiction predict that brain regions that are involved in learning, memory and reward processing should be active in response to drug cues (Koob & LeMoal, 2001; Hyman, 2005; Volkow et al., 2010). Consistent with these models, we found evidence of significant cigarette cue reactivity in the visual association cortex, dorsal striatum, anterior cingulate cortex, prefrontal cortex and insula, and a trend toward significance in the nucleus accumbens. These areas have previously been implicated in reactivity to cigarette cues (Due et al., 2002; David et al., 2005; McClernon et al., 2005, 2008, 2009; Brody et al., 2007; Franklin et al., 2007; Janes et al., 2009, 2010a,b), alcohol cues (George et al., 2001; Heinz et al., 2004, 2007) and cocaine cues (Wexler et al., 2001; Kosten et al., 2006; Volkow et al., 2006). Specifically, we found that the magnitude of the BOLD response to cigarette cues closely resembled the magnitude of the response to mildly arousing, pleasant stimuli (i.e. the romantic pictures). This finding is consistent with self-report studies in which smokers reported cigarette cues as pleasant and mildly arousing (Orain-Pelissolo et al., 2004; Carter et al., 2006), studies that showed similar startle inhibition for pleasant and cigarette-related cues in smokers (Geier et al., 2000; Cinciripini et al., 2006), and studies that compared smokers’ brain reactivity with emotional and cigarette-related cues using event-related potentials (Versace et al., 2010, 2011). These results converge in supporting the hypothesis that chronic smoking leads to overvaluation of cigarette cues by the brain’s reward circuits.
In addition to an overvaluation of drug cues, chronic drug use is believed to lead to a devaluation of natural rewards and their associated cues (Koob & LeMoal, 2001; Volkow et al., 2010). For example, in the medial prefrontal cortex, cocaine addicts, compared with non-addicts, have been shown to have larger BOLD responses to cocaine videos and smaller responses to erotic videos (Garavan et al., 2000). The lack of alternative means of activating the brain’s reward circuitry may be one reason that makes long-term drug abstinence a goal difficult to achieve (Volkow & Li, 2005). Indeed, previous research has demonstrated that detoxified alcoholics with the largest BOLD responses to pleasant pictures in the ventral striatum were more likely to be abstinent at follow-up than those with smaller responses to pleasant pictures (Heinz et al., 2007), suggesting that responding to natural rewards may have a protective effect against relapse.
Building on previous neuroimaging studies that examined responses to drug cues in isolation, this study directly compared smokers’ brain responses to cigarette cues and naturally rewarding erotic stimuli using fMRI. Contrary to the results described above for cocaine users, we found that smokers had larger BOLD responses to erotic pictures than to cigarette-related pictures in nearly all ROIs, including the medial prefrontal cortex, where the effect was previously found in cocaine users (Garavan et al., 2000).
At first glance, this result may be interpreted as being inconsistent with the hypothesis that chronic drug use results in decreased sensitivity to naturally rewarding stimuli (Volkow et al., 2010), and with the data from cocaine users and animal models that support this hypothesis (Garavan et al., 2000; Grigson & Twining, 2002). However, there are several alternative explanations for the differences in response to erotic stimuli between the studies. Although cocaine and nicotine produce similar physiological effects throughout the brain, there are subtle differences between the actions of each drug, especially in the dorsal striatum and anterior cingulate cortex (Merlo Pich et al., 1997), which is where, in comparison to drug cues, the cocaine users were less reactive (Garavan et al., 2000) and the smokers more reactive to erotic stimuli. There are also important methodological differences between the studies. For example, the smokers in our study were entering a cessation program, but the cocaine users did not report serious plans to quit. Also, the erotic stimulus was presented to the cocaine users in the form of a single motion picture, whereas we used a set of several erotic photographs randomly interspersed within a series of stimuli from several categories. These differences might explain why smokers did not show the same suppression of BOLD responses to erotic stimuli as cocaine addicts did. Further research is necessary to determine whether this discrepancy is primarily due to the differences in the neuroadaptations following cocaine and nicotine addiction, or to procedural differences between smoking and cocaine cue reactivity studies.
Cigarette-specific cue reactivity in the insula
A notable exception to our finding of larger BOLD responses to erotica than cigarette-related pictures was found in the left insula, where responses to cigarette cues were larger than responses to all other stimulus categories. Several fMRI studies have found significant responses to cigarette cues in the insula (McClernon et al., 2005; McBride et al., 2006; Brody et al., 2007; Franklin et al., 2007; Janes et al., 2009, 2010a,b), but the lack of highly-arousing control stimuli in those studies made it difficult to determine whether the insula was selectively responsive to cigarette-related cues over other emotional stimuli. Our study extended the cue reactivity literature by suggesting that BOLD responses in the insula have some degree of selectivity to cigarette cues. Interestingly, animal models also provide support for selective processing of drug cues over natural rewards in the insula – inactivation of the insula disrupted nicotine self-administration and cue-induced reinstatement of nicotine-seeking, but had no effect on food self-administration or food-induced reinstatement of food-seeking (Forget et al., 2010). Thus, diminished cue reactivity may be one of the reasons why smokers who suffer damage to the insula region find it easy to quit smoking (Naqvi et al., 2007).
Although the insula is involved in the expression of drug-taking behavior in humans (Naqvi et al., 2007) and animal models (Contreras et al., 2007), and insular cue reactivity is associated with relapse (Janes et al., 2010a), the specific processes by which the insula influences this behavior are unknown. One possibility is that insular activity in response to cigarette cues mediates an interoceptive conditioned response that is subjectively correlated with expecting the ‘taste’ of a cigarette or the sensory qualities of smoking (Naqvi & Bechara, 2010). The insula has been implicated in interoceptive conditioning in general (Craig, 2002), and in taste perception specifically (Pritchard et al., 1999). In fact, the insula has been shown to be responsive to capsaicin-induced stimulation of the upper airway (Mazzone et al., 2007), a phenomenon similar to the effects of cigarettes on the upper airway (Behm & Rose, 1994).
Another possible role of the insula in cigarette cue reactivity may be in the subjective experience of cigarette ‘urge’ or ‘craving’, and cognitive efforts to resist that craving. The insula is interconnected with the anterior cingulate gyrus and medial prefrontal cortex (Mesulam & Mufson, 1982; Mufson & Mesulam, 1982), which are involved in reward-related decision-making (Liu et al., 2011). Data in support of this hypothesis come from a study that found a significant correlation between the amount of self-reported cigarette craving and insula activity to cigarette cues (Brody et al., 2007). However, other studies did not replicate this finding (McClernon et al., 2005; Franklin et al., 2007). Thus, further research is needed to clarify the relationship between craving and cue reactivity in the insula, as well as to address the other possible roles of the insula in smoking behavior, such as interoceptive conditioned responses.
Potential limitations and future directions
Because we drew our sample from a clinical trial of smokers seeking treatment for smoking cessation, a limitation of our study is the lack of a control group of non-smokers. The lack of a control group makes it difficult to determine whether the BOLD responses that we observed were due to chronic smoking, or whether they would have been evident in never smokers or light smokers as well. However, the possibility that the effects observed in this study were not due to chronic smoking is unlikely. In fact, our results are similar to those observed in other studies of smokers that also included non-smokers, as the cigarette cue reactivity that characterized smokers was not evident in the non-smokers (Due et al., 2002; David et al., 2005; Rubinstein et al., 2011). A second limitation is that our sample included only European-American, treatment-seeking smokers who met strict inclusion criteria for a clinical trial and therefore may not be representative of the population of smokers as a whole (e.g. ethnic minorities, smokers not attempting to quit). Future research must therefore extend sampling to more representative populations of smokers.
To evaluate BOLD responses to cigarette-related stimuli relative to other pleasant pictures differing in emotional arousal, we included both romantic (low arousing) and erotic (high arousing) pictures. Previous studies suggest that BOLD responses to erotic pictures are greater in males than in females (Lang et al., 1998; Hamann et al., 2004; Sabatinelli et al., 2004), which could bias the comparison between erotic and cigarette cues when averaging across a sample of males and females. However, we found no evidence of sex differences in BOLD responses to erotic stimuli in our analyses. The lack of sex differences in BOLD activation might be due to differences between the current sample (middle-aged, treatment-seeking smokers) and the samples used in previous research (undergraduate students). The lack of sex differences may also be the result of our identification of ROIs based on reactivity to cigarette cues rather than reactivity to erotic stimuli (Lang et al., 1998; Hamann et al., 2004; Sabatinelli et al., 2004). Also, we did not ask our participants about their sexual orientation, and because all erotic stimuli depicted heterosexual couples, we may have missed potential differences in the BOLD response to the erotic stimuli across participants of differing sexual orientation (Paul et al., 2008). However, large differences in the BOLD response as a function of sexual orientation are unlikely. Previous studies have found that, although men and women produce differences in self-reported affect across images of erotic couples, opposite sex nudes, and same sex nudes, their psychophysiological responses to these three categories of erotic picture are equal (Bradley et al., 2001).
Differences in the type of reinforcement provided by erotic and cigarette cues is also an issue that should be considered when interpreting the results of this study. Cigarette cues are not intrinsically reinforcing, but are instead thought to become motivationally significant through repeated pairing with the reinforcing effects of smoking (Koob & LeMoal, 2001; Robinson & Berridge, 2003; Hyman, 2005; Volkow et al., 2010). Erotica, however, appears to be intrinsically reinforcing (e.g. Hoffmann et al., 2004). This suggests that processes other than the drug-related content stimuli may have contributed to the differences in BOLD response between these two classes of picture. One of these additional factors may have been primary vs. secondary reinforcement processes, which have been shown to produce different patterns of BOLD response (Sescousse et al., 2010). Thus, an important direction for future research is to directly compare drug- vs. non-drug-related primary reinforcers and drug- vs. non-drug-related secondary reinforcers.
Previous studies have shown that the patterns of BOLD response to emotional pictures can be altered by the presence of active tasks during picture presentation (Hariri et al., 2000; Ochsner & Gross, 2005, 2008; Pessoa et al., 2005; Blair et al., 2007). Hence, to avoid confounds due to the presence of secondary tasks, we opted for a passive viewing paradigm. The lack of overt behavioral responses during picture presentation, however, does not allow us to be certain that participants maintained attentiveness throughout the whole paradigm. Our finding of significantly greater activation to highly arousing pictures than neutral pictures in the visual association areas and the amygdala replicates several previous fMRI studies of picture viewing (Lane et al., 1997; Bradley et al., 2003; Phan et al., 2004; Sabatinelli et al., 2007b), and suggests that the participants in our study were indeed paying attention to the pictures.
In our experiment we used a fixed, 12-s interstimulus interval. The selection of this interval was based on previous fMRI studies of emotional picture viewing (Lang et al., 1998; Bradley et al., 2003; Sabatinelli et al., 2004, 2005, 2007a,b). However, the use of a fixed interstimulus interval in event-related fMRI research is problematic because: (i) the participants may be able to predict when the stimuli are presented; and (ii) the amplitude of the BOLD signal may be decreased due to variability in the duration of the hemodynamic response that is not captured by a fixed interstimulus interval (e.g. Burock et al., 1998).
Despite these concerns, the current study provides a significant extension beyond existing smoking cue reactivity research. By including intrinsically pleasant and unpleasant pictures in addition to neutral ones, we were able to conclude that smokers, although more reactive to cigarette-related pictures than to neutral ones, were not more reactive to cigarette-related pictures than to intrinsically pleasant, erotic pictures. We also found evidence suggestive of a selective bias toward cigarette-related pictures, but not toward other emotional pictures, in the insula. It is therefore possible that smoking-cessation interventions that target the insula may be more effective than existing therapies at reducing cue-induced craving and relapse (Naqvi & Bechara, 2009). Thus, future research into the neurophysiological basis of the insula’s response to cigarette cues is warranted.
Footnotes
Acknowledgements
This work was supported in part by the National Institute on Drug Abuse through grant 1R01DA017073-S1 to Paul Cinciripini, by a faculty fellowship from The University of Texas MD Anderson Cancer Center Duncan Family Institute for Cancer Prevention and Risk Assessment to Francesco Versace, and by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant CA016672. The authors wish to thank David T. Evans, Jennifer Canul, Janeene Frerking and Christine Jeria for their help with data collection. Dr Cinciripini has served on the scientific advisory board of Pfizer Pharmaceuticals, has received grant support from Pfizer, and has conducted educational talks sponsored by Pfizer on smoking cessation for physicians. The other authors declare no conflict of interest. Clinical trial registration details: the parent project for this study is registered at http://www.clinicaltrials.gov as protocol NCT00507728.
Abbreviations
-
- BOLD
-
- blood oxygen level-dependent
-
- fMRI
-
- functional magnetic resonance imaging
-
- IAPS
-
- International Affective Picture System
-
- ROI
-
- region of interest