Corticomotor representation to a human forearm muscle changes following cervical spinal cord injury
Abstract
Functional imaging studies, using blood oxygen level-dependent signals, have demonstrated cortical reorganization of forearm muscle maps towards the denervated leg area following spinal cord injury (SCI). The extent of cortical reorganization was predicted by spinal atrophy. We therefore expected to see a similar shift in the motor output of corticospinal projections of the forearm towards more denervated lower body parts in volunteers with cervical injury. Therefore, we used magnetic resonance imaging-navigated transcranial magnetic stimulation (TMS) to non-invasively measure changes in cortical map reorganization of a forearm muscle in the primary motor cortex (M1) following human SCI. We recruited volunteers with chronic cervical injuries resulting in bilateral upper and lower motor impairment and severe cervical atrophy and healthy control participants. All participants underwent a T1-weighted anatomical scan prior to the TMS experiment. The motor thresholds of the extensor digitorum communis muscle (EDC) were defined, and its cortical muscle representation was mapped. The centre of gravity (CoG), the cortical silent period (CSP) and active motor thresholds (AMTs) were measured. Regression analysis was used to investigate relationships between trauma-related anatomical changes and TMS parameters. SCI participants had increased AMTs (P = 0.01) and increased CSP duration (P = 0.01). The CoG of the EDC motor-evoked potential map was located more posteriorly towards the anatomical hand representation of M1 in SCI participants than in controls (P = 0.03). Crucially, cord atrophy was negatively associated with AMT and CSP duration (r2 ≥ 0.26, P < 0.05). In conclusion, greater spinal cord atrophy predicts changes at the cortical level that lead to reduced excitability and increased inhibition. Therefore, cortical forearm motor representations may reorganize towards the intrinsic hand motor representation to maximize output to muscles of the impaired forearm following SCI.
Introduction
Traumatic spinal cord injury (SCI) usually leads to loss of motor and sensory function below the site of injury (Dietz & Curt, 2006), owing to degenerative processes affecting ascending and descending tracts (Jurkiewicz et al., 2006; Wrigley et al., 2009; Freund et al., 2011). Recent functional imaging studies in humans have demonstrated changes in the usual somatotopic pattern of task-related brain activation within the primary motor cortex (M1) (Freund et al., 2011; Lundell et al., 2011). In a patient cohort with low cervical traumatic injury, we found that movement of the impaired hand was associated with increased blood oxygen level-dependent (BOLD) responses, not only in the expected hand area of the motor cortex, but also in the expected location of the motor output-deprived leg area. The extent of this activation was related to the degree of atrophy in the spinal cord above the lesion, and was larger in patients with greater disability (Freund et al., 2011).
However, whether this kind of cortical reorganization translates into functional gain is unclear; indeed, even its pathophysiological basis is incompletely understood. This is partly because the interpretation of functional magnetic resonance imaging (fMRI) studies is limited to the nature of the BOLD signal. The BOLD signal represents indirect neuronal activation that is mediated by both motor and sensory inputs. To gain insights into the nature of cortical reorganization, it is therefore crucial to study the contributions of the motor system and the sensory system independently.
One non-invasive approach to studying physiological changes in corticomotor projections is by using transcranial magnetic stimulation (TMS). Previous mapping studies with conventional scalp reference points (e.g. elastic cap) suggested that the motor cortical representation of upper limb muscles innervated by roots rostral to the lesion had expanded towards areas that normally innervated the now-paralysed muscles below the lesion (Levy et al., 1990; Topka et al., 1991; Streletz et al., 1995). Such results were interpreted as indicating an increase in excitability of corticospinal (CS) projections to non-paralysed muscles above the lesion, and were consistent with reports of reduced latency of motor-evoked potentials (MEPs) (Topka et al., 1991), and reduced excitability of inhibitory circuits as tested in paired pulse TMS studies by Saturno et al. (2008). However, this was not seen in all studies. For example, Lotze et al. (2006) and Laubis-Herrmann et al. (2000) found reduced excitability of CS neurons to some muscles and no changes in others; Lotze et al. (2006) also reported an increase rather than a decrease in excitability of cortical inhibitory circuits, again suggestive of reduced CS excitability.
Scalp mapping with TMS is limited by the variation in brain anatomy with reference to scalp topography in individual subjects. However, it is now possible to integrate individual three-dimensional magnetic resonance imaging (MRI) with TMS such that output maps can be projected directly onto the anatomical surface of each subject’s brain. This allows more precise comparison of cortical muscle maps from different individuals (Julkunen et al., 2009).
Given our previous results, in which we had noted spread of brain activation into the ‘paralysed’ M1 leg area during a handgrip task (Freund et al., 2011), we hypothesized that the CS output map of the forelimb representation would shift towards the denervated cortical leg representation. Therefore, we used three-dimensional mapping to investigate the physiological properties of the CS system in the same SCI cohort.
In addition, we compared the cross-sectional spinal cord area (SCA) at cervical level C2 in all participants (Freund et al., 2011), and compared this with the motor threshold (a measure of CS excitability), cortical silent period (CSP) duration (a measure of excitability in cortical inhibitory circuits) and position of the centre of gravity (CoG) of representational maps of the extensor digitalis communis muscle (EDC) in cervical SCI participants and a group of healthy individuals. Regression analysis was used to determine associations between spinal changes (i.e. SCA) and upper limb function and TMS-derived physiological measures.
Materials and methods
Participants
We examined nine male participants with a cervical SCI, all of whom fulfilled the following inclusion criteria: (i) bilateral upper and lower limb impairment; (ii) no head or brain lesion associated with the trauma leading to the injury; (iii) no seizure, and no medical or mental illness; and (iv) no contraindications for MRI.
All SCI participants were right-handed prior to injury and remained so thereafter, except for one. For this participant, we mapped the right hemisphere during contraction of the left EDC. On the day of the experiment, no medication (i.e. baclofen) was taken.
Fourteen age-matched and gender-matched right-handed healthy participants [mean age, 39.3 years; standard deviation (SD), 15.8 years] without any history of neurological or psychiatric illness were recruited.
All participants were assessed on the nine-hole peg test (9HPT) bilaterally (Goodkin et al., 1988). Furthermore, the maximum grip strength and the action research arm test (ARAT) (Lyle, 1981) were assessed with the dominant hand in SCI subjects. To obtain the grip strength, we asked all participants to perform a dynamic isometric handgrip with a handgrip device that consisted of two force transducers (Honeywell FSG15N1A; Honeywell, Morristown, NJ, USA) situated between two moulded plastic bars spanning a width of 6 cm. Compression of the two bars then resulted in the generation of a differential voltage signal (mV) that was linearly proportional to the exerted handgrip force. The signal was then fed into a signal conditioner (CED 1902; Cambridge Electronic Design, Cambridge, UK), digitized (CED 1401; Cambridge Electronic Design), and stored on a PC. The averages of two trials for each hand of the 9HPT and of the grip strength of the dominant hand were calculated. Three SCI participants were scored with the maximum time allowed for the 9HPT (300 s), as they were unable to perform the 9HPT with their non-dominant hand within this time window (Hoogervorst et al., 2002). The Mann-Whitney test was used to assess differences in motor performance between the groups, and a P-value of < 0.05 was considered to be significant.
The study was approved by the Joint Ethics Committee of the UCL Institute of Neurology and the National Hospital for Neurology and Neurosurgery (ref. 08/0243). All participants gave informed, written consent before the study.
MRI protocol
Image acquisition
Prior to the TMS experiment, participants were scanned on a 1.5-T whole body Magnetom Sonata MRI scanner (Siemens Medical Systems, Erlangen, Germany) operated with a radiofrequency body transmit coil and an eight-channel receive head coil.
T1-weighted anatomical scan
We used a T1-weighted three-dimensional MDEFT sequence (Deichmann et al., 2004) that is optimized for high-resolution brain and cervical cord imaging in order to study morphometric changes in the brain and spinal cord on the same anatomical scan (Freund et al., 2010). The following imaging parameters were used: 176 sagittal partitions; 256 × 256 image matrix; field of view, 256 × 256 mm2; isotropic 1-mm3 resolution; repetition time, 12.14 ms; echo time, 3.56 ms; inversion time, 530 ms; bandwidth, 106 Hz/pixel; α = 23°; total acquisition time, 13 min 43 s.
Cord atrophy
In a previous study (Freund et al., 2011) with the same study cohort, we measured the SCA on the optimized T1-weighted anatomical reference scans with a semi-automated segmentation method (Losseff et al., 1996). Here, we present only the data in the context of the newly performed regression analysis that sought to explain interindividual changes in SCA in relation to independent electrophysiological measures of cortical reorganization.
MEPs
MEPs were recorded from the right EDC. In one SCI subject, the left hand was used as his hand dominance changed after the injury. Electromyographic (EMG) recordings were obtained with silver/silver chloride-plated surface electrodes (9 mm in diameter), with the active electrodes positioned over the muscle belly and the reference electrode over the interphalangeal joint. Raw signals were amplified and filtered (Digitimer, Welwyn Garden City, UK) with a time constant of 3 ms and a low-pass filter of 3 kHz. Data were acquired at a sampling rate of 5 kHz via a CED 1401 interface (Cambridge Electronic Design,) and stored on a PC for offline analysis (Signal 2.0; Cambridge Electronic Design).
TMS
A Magstim 200 stimulator (Magstim, Dyfed, UK) was used to deliver transcranial magnetic stimuli via a figure-of-eight coil (outer wing diameter, 70 mm).
Corticomotor thresholds
To determine the active motor threshold (AMT), sites near the estimated centre of the corticomotor projection of the EDC were explored as a percentage of maximum stimulator output during 10% of their maximum muscle contraction that evoked an MEP of approximately 1 mV in amplitude (Rothwell, 1997). At the site of maximum MEP, the stimulus was then increased in 5% steps from a level below threshold until at least five MEPs could be measured from 10 consecutive stimuli.
Navigated TMS mapping procedure
We mapped, at 110% AMT, the cortical muscle representation of the EDC in each participant with the Brainsight Frameless Navigation system (Rogue Research, Montreal, QC, Canada), in order to monitor the position of the TMS coil throughout the experiment and to assess topographical changes in cortical excitability and inhibition. Therefore, we adapted a protocol that was initially developed for the assessment of training-induced changes in CS output (Kleim et al., 2007). In brief, we imported the anatomical scans into the neuronavigation system, and superimposed a target grid (1 × 1 cm) on the hemisphere to be stimulated (Fig. 1A) (Kleim et al., 2007). The grid was centred at the point where the central sulcus meets the longitudinal fissure at the dorsal aspect of the brain. The grid extended 7 cm anterior, 2 cm posterior and 8 cm lateral to this point, with a dimension of 8 × 9 cm (72 sites). We then used the stereotaxic neuronavigation system to position the TMS coil to each grid point, and applied 10 TMS pulses at each position, in pseudorandom order. Following each block of 10 trials, participants were given a short break of approximately 30 s, during which the TMS coil was positioned over the next grid point. During the experiment, participants were asked to maintain 10% of their maximum muscle contraction for a duration of 10 trials. A stable background activation level was achieved by online visual feedback of their individual current EMG activity. Throughout the experiment, the TMS coil was orientated at a 45° angle to the midline, with the handle of the coil pointing posteriorly at a scalp site (Orth et al., 2003). From these data, the peak-to-peak amplitude and CSP were measured.
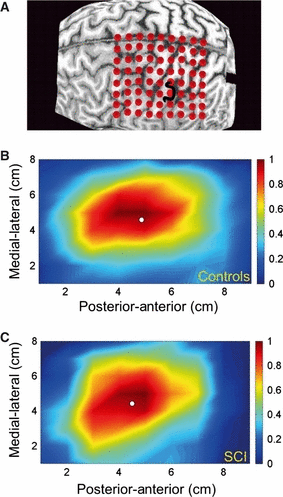
(A) Grid (8 × 9 cm) superimposed over the reconstructed cortical surface. (B and C) Pseudo-color map of EDC-MEP amplitude as a function of stimulated positions. Red indicates high and blue indicates low amplitudes of motor evoked potentials (MEPs). The colour bar gives the normalized MEP amplitudes corresponding to a colour in the map. The circle indicates the position of the CoG.
CoG
All analyses were conducted with Matlab 7.0 (The Mathworks, Natick, MA, USA). First, background EMG activity of the EDC (during 10% background contraction of the EDC) from each stimulated site was rectified and averaged for the 100 ms preceding the TMS pulse. The peak-to-peak MEP amplitudes between 15 and 35 ms after delivery of TMS were measured at each positive site, and the average of 10 stimulation pulses per scalp site was calculated and normalized by the average background EMG activity of that site. The resulting matrix, including all sites, was then normalized to the muscle-specific maximum mean peak-to-peak MEP amplitude and rescaled between 0 and 1 in order to allow comparison between participants. Magnetic stimulation induces current spread that enlarges the motor cortical map, so defining the map boundaries is difficult (Uy et al., 2002). To minimize this problem, normalized values below 0.25 of the mean peak-to-peak response were discarded. We then generated a pseudo-colour map of MEP amplitudes as a function of stimulated position (Fig. 1B and C).
From the rescaled normalized maps, the CoG specifying an amplitude-weighted indication of the map position (Wassermann et al., 1992) was calculated.
To adjust for high intersubject and between-group anatomical variability, we referenced each CoG to an individual anatomical landmark, namely the anatomical ‘hand knob’ as identified from the MRI scan (Yousry et al., 1997; Dechent & Frahm, 2003). To identify the most posterior point of the hand knob, we used the superimposed grid as a coordinate system (Fig. 1A). We then calculated the Euclidian distance (ED) between this anatomical landmark (i.e. hand knob) and the CoG. The ED between two points in a plane is calculated with the Pythagorean theorem, and provides an absolute value independently of direction. Thus, a two-dimensional displacement vector is created that can better describe the overall change in location than the use of a mean displacement, expressed as the difference between two consecutive x-coordinates or y-coordinates.
CSP
We additionally quantified the mean and peak (hot spot) CSP duration on the same dataset as that on which the MEPs were measured to detect changes in the inhibitory cortical processes. CSP duration was measured from the time-point of TMS stimulus artefact to the resumption of sustained EMG activity. The duration, measured in milliseconds of the CSPs, was then fed into the Matlab scripts to create a CSP map, from which the corresponding CoG of the CSP–MEP map was derived.
Statistical analysis of TMS physiology
We used the Stata 9.2 software package (http://www.stata.com) for all analyses. Results associated with P < 0.05 are reported. For group comparison, we applied the non-parametric Mann–Whitney test. Two multiple linear regression models were designed to explore associations between physiological measures (response variables) and (i) spinal changes (e.g. SCA = predictors) and (ii) clinical measures of upper limb function. For the first regression model, we included age, height, weight and scalp–cortex distance as covariates of no interest. Specifically, we included scalp–cortex distance because it has been shown to directly influence the magnitude of cortical stimulation in TMS (Stokes et al., 2007). For the second regression model, we included age and scalp–cortex distance as confounds. In order to determine whether these associations were different in SCI participants and controls, subject type indicator and type × predictor interaction terms were added; however, there was no evidence that associations were different in SCI participants and controls, and therefore the combined group was used in these analyses.
Results
Clinical results
Nine SCI participants (mean age, 46.7 ± 11.3 years) had a chronic traumatic lesion between cervical levels C5 and C8. The mean period following SCI was 145.9 months (±59.8 months; range, 57–288 months). Seven of the nine SCI participants had incomplete lesions of the cervical cord and two had complete lesions of the cervical cord, based on the American Spinal Cord Injury Association (ASIA) Impairment classification. As a result of trauma, all SCI participants had severe upper and lower limb impairment, as confirmed by reduced ASIA motor scores of upper and lower limbs and impairment on the ARAT (mean score of 38.72 of a maximum of 57). SCI participants were impaired bilaterally on the 9HPT [dominant hand, SCI, mean = 98.77 s (SD, 84.45) vs. dominant hand, controls, mean = 16.37 s (SD, 1.69), Mann–Whitney U = 117, number of controls (nc) = 14, number of participants (np) = 9, P < 0.001, two-tailed; non-dominant hand, SCI, mean = 141.71 s (SD, 121.44) vs. non-dominant hand, controls, mean = 17.72 s (SD, 2.12), Mann–Whitney U = 117, nc = 14, np = 9, P < 0.001, two-tailed]. The grip strength of the dominant hand was also lower than normal [SCI, mean = 0.16 mV (SD, 0.15) vs. controls, mean = 0.61 mV (SD, 0.19), Mann–Whitney U = 112, nc = 14, np = 9, P < 0.001, two-tailed]. More details on SCI participants’ characteristics are summarized in Table 1 and also in a previous report (Freund et al., 2011).
| Participant | Age (years) | Aetiology of the injury | Time since injury (years) | Level of motor impairment/ASIA* | Upper limb motor score | Lower limb motor score | Dominant hand 9HPT | Non-dominant hand 9HPT | Grip strength | ARAT |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 43 | Fracture | 14 | C6/D | 25 | 30.5 | 68 | 54.35 | 0.22 | 36 |
| 2 | 29 | Fracture | 9 | C6/B | 19 | 0 | 52.6 | 59.2 | 0.05 | 42 |
| 3 | 44 | Fracture | 7 | C7/C | 14 | 19 | 56.75 | 118.4 | 0.25 | 57 |
| 4 | 35 | Fracture | 14 | C5/A | 18.5 | 0 | 190.5 | 300 | 0.02 | 26 |
| 5 | 60 | Spinal stenosis | 12 | C6/C | 46.5 | 19 | 26.25 | 24.55 | 0.47 | 57 |
| 6 | 61 | Fracture | 19 | C6/A | 34 | 0 | 68.3 | 76.5 | 0.05 | 26.5 |
| 7 | 40 | Disc prolapse | 19 | C5/C | 20.5 | 18.5 | 283 | 300 | 0.01 | 26 |
| 8 | 53 | Fracture | 7 | C8/D | 43.3 | 48 | 38.55 | 42.45 | 0.25 | 53 |
| 9 | 56 | Fracture | 15 | C5/D | 23.5 | 34.5 | 105 | 300 | 0.11 | 25 |
| Mean | 27.14 | 18.33 | 98.77 | 141.72 | 0.16 | 38.72 | ||||
| SD | 11.50 | 16.94 | 84.45 | 121.44 | 0.15 | 13.93 |
- *ASIA impairment scale: A, no sensory or motor function is preserved; B, sensory function is preserved below the level of the injury, but there is no motor function; C, motor function is preserved below the neurological level, and more than half of the key muscles below the neurological level have a muscle grade of < 3; D, motor function is preserved below the neurological level, and at least half of the key muscles below the neurological level have a muscle grade of ≥ 3.
TMS results
The background level of EMG activity of the EDC (approximately 10% of the maximum EDC muscle contraction) did not differ significantly between SCI participants and controls (SCI, median = 0.11 mV [interquartile range (IQR), 0.04] vs. controls, median = 0.13 mV (IQR, 0.04); Mann–Whitney U = 49, nc = 14, np = 9, P > 0.05, two-tailed) (Fig. 2A–C). The AMT in the EDC was higher in SCI participants [SCI, median = 52% (IQR, 25.5) vs. controls, median = 46% (IQR, 12.5); Mann–Whitney U = 22, nc = 14, np = 9, P = 0.01, two-tailed; Table 2]. The duration of the CSP, calculated as the mean over all stimulation sites, was longer in SCI participants [SCI, median = 76 ms (IQR, 20) vs. controls, median = 47 ms (IQR, 20); Mann–Whitney U = 23, nc = 14, np = 9, P = 0.012, two-tailed], and the CSP duration measured at the motor cortical hot spot was also longer [SCI, median = 130 ms (IQR, 60) vs. controls, median = 96 ms (IQR, 30); Mann–Whitney U = 26, nc = 14, np = 9, P = 0.02, two-tailed; Table 2].
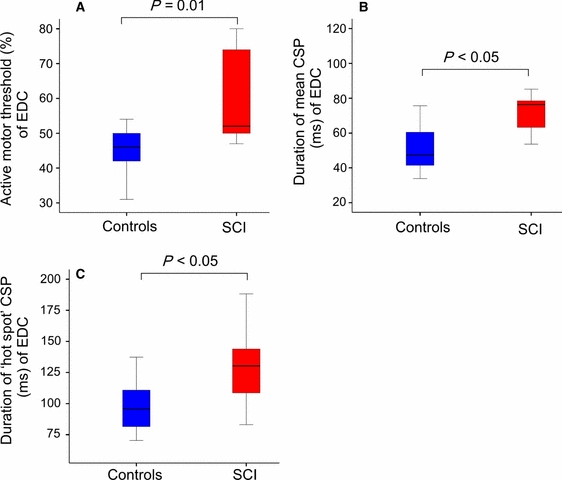
(A) Active motor thresholds (MTs) are shown as the percentage of stimulator output (grey/blue, controls; black/red, SCI participants). (B and C) Increased inhibition. Mean and hot spot CSPs were prolonged in SCI participants (black/red) when compared with controls (grey/blue) (P < 0.05, Mann–Whitney test). For interpretation of color references in figure legend, please refer to the web version of this article.
| AMT (%) | ED of the CoG of the EDC (cm) | CSP at the hot spot (ms) | Mean CSP (ms) | |
|---|---|---|---|---|
| SCI subjects | ||||
| 1 | 48.00 | 1.50 | 100 | 70 |
| 2 | 51.00 | 0.80 | 130 | 80 |
| 3 | 52.00 | 0.90 | 110 | 80 |
| 4 | 55.00 | 0.80 | 200 | 120 |
| 5 | 47.00 | 1.30 | 190 | 90 |
| 6 | 75.00 | 0.70 | 120 | 80 |
| 7 | 50.00 | 1.00 | 140 | 50 |
| 8 | 74.00 | 0.80 | 140 | 60 |
| 9 | 80.00 | 0.40 | 80 | 30 |
| Median | 52 | 0.8 | 130 | 76 |
| IQR | 25.5 | 0.4 | 60 | 20 |
| Controls (n = 14) | ||||
| Median | 46 | 1.45 | 96 | 47 |
| IQR | 12.5 | 0.68 | 30 | 20 |
To account for intersubject anatomical variability, we expressed the location of the individual CoG as the ED between the CoG and the anatomical hand knob (Yousry et al., 1997; Dechent & Frahm, 2003). The ED of the CoG of the EDC–MEP map was smaller in SCI participants than in controls [SCI, median = 0.8 cm (IQR, 0.4) vs. controls, median = 1.45 cm (IQR, 0.68); Mann–Whitney U = 29, nc = 14, np = 9, P = 0.031, two-tailed; Table 2].
Relationship between cervical cord atrophy and TMS physiology
We applied multiple regression analysis including all subjects, as there was no a priori reason why controls would have a different relationship between the dependent parameters (TMS parameters) and independent parameters (SCA and clinical parameters) than SCI participants (see Materials and methods) (Fig. 3A and B). The SCA in this patient cohort was reduced by more than 30%, as reported previously (Freund et al., 2011). Here, we relate these same data to the electrophysiological data derived from the cortical maps acquired in the present study; this, in contrast to our previously published work, allows for direct comparison of an MRI-based measure of cord atrophy and cortical excitability and inhibition. Crucially, we found a negative association between SCA and AMT (r2 = 0.39, P = 0.015), and negative associations between SCA and mean CSP duration (r2 = 0.26, P = 0.022) and hot spot CSP duration (r2 = 0.4, P = 0.005). In other words, AMT was higher and CSP duration longer in SCI participants with greater cord atrophy.
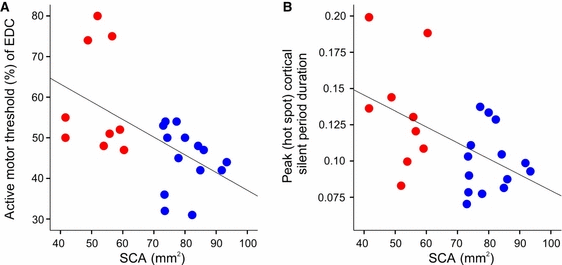
Negative association of SCA and (A) increased AMTs and (B) hot spot CSP duration (grey/blue, controls, black/red, SCI participants). For interpretation of color references in figure legend, please refer to the web version of this article.
Discussion
After experimental SCI, CS axons degenerate (Kalil & Schneider, 1975), axotomized CS neurons shrink (Beaud et al., 2008), and spine density is reduced (Kim et al., 2006). In human SCI, similar to findings from experimental SCI, the spinal cord and cortex are atrophic and axonal integrity is reduced (Wrigley et al., 2009; Cohen-Adad et al., 2011; Freund et al., 2011). Here we report and extend these findings, as we show alterations in the topography and excitability of the corticomotor projection to a forearm muscle following cervical SCI and its association with cervical cord atrophy. In particular, when SCI participants were compared with controls, we found: (i) an increased AMT of the EDC; (ii) an increased duration of the CSP; and (iii) a reduction in the ED between the EDC CoG and the anatomical hand region of M1. Furthermore, the novel findings revealed by multiple linear regression analysis were: (i) a negative association between SCA and AMTs; and (ii) a negative association between SCA and CSP duration. In the following, we discuss these findings with respect to their implications for cortical reorganization.
Corticomotor representation is changed following SCI
In a previous study on the same group of patients, we observed that voluntary movement of the hand was associated with an increased BOLD signal, not only in the somatotopic hand area of M1, but also in the adjacent leg area. We had therefore expected to see a similar shift in the CoG of the CS projection to the EDC when tested with TMS. In fact, this did not occur. Stereotaxic TMS mapping showed that the location of the EDC CoG was shifted posteriorly towards the region of the anatomically defined hand knob in the central sulcus. The CoG is thought to approximate to the location of the highest density of CS projections, and in non-SCI individuals it has been shown to correspond with the CoG identified with fMRI imaging (Ruohonen & Karhu, 2010). Why, then, did it not show a shift in direction similar to that of the BOLD signal after SCI?
The MEP responses evoked by a single TMS pulse are attributable to conduction in large-diameter CS axons that have relatively direct monosynaptic or polysynaptic inputs to spinal motoneurons (Rothwell, 1997). Thus, the small shift in the EDC CoG that we observed might be caused by limited reorganization of the immediately surrounding neuronal networks, which normally innervate the intrinsic hand muscles (Lemon, 2008). The low cervical lesion in our patients had damaged the CS outputs to intrinsic hand muscles to a much greater extent than those to the EDC in the forearm. However, CS axons to intrinsic hand muscles often send branches to forearm muscles, and could therefore contribute to MEPs evoked in the EDC by TMS. If the strength of these connections had increased after SCI, then this would shift the CoG into the M1 hand area, which is predominantly represented in the anterior wall of the central sulcus (Rathelot & Strick, 2009). This type of reorganization of CS neurons might have increased motor output and possibly could improve voluntary movement of the upper limb.
However, this is not the only explanation. For example, the higher TMS thresholds observed in the SCI group might recruit more of the EDC representation located within the sulcus, and shift the balance of representation more posteriorly. Second, the posterior location of the CoG might have been attributable to an increased contribution of the primary sensory cortex to the recovery of hand function (Green et al., 1998, 1999).
However, this does not explain why we did not evoke any MEPs in the EDC when we stimulated over the leg area of M1, when the fMRI images showed such clear activation of that area during volitional hand movement (Freund et al., 2011). One possibility is that the outputs activated during voluntary movement include polysynaptic inputs from the leg area that are not activated strongly by TMS. These could, for example, be unmasked cortico-cortical connections linking neurons in the leg area to CS neurons in the arm area; alternatively, they might involve CS outputs from the leg area that had contacted spinal interneuronal networks with indirect access to the EDC motoneurons. If the excitability of these indirect connections was low, then they would not have been detected by single TMS pulses. Future experiments using multiple TMS pulses to improve conduction through the network might be able to detect such connections. Alternatively, it may be possible to use spinal reflex testing to activate the networks and test whether these are modulated by inputs from TMS over the leg area. Nevertheless, the complete lack of any evidence for fast-conducting CS connections from the leg area to the EDC in SCI humans suggests that any reorganization of motor output may be relatively minor in functional terms.
If this is so, then what is represented by the BOLD signal activation of the leg area in fMRI? One possibility is that this represents sensory input from the hand, which has reorganized to innervate neurons in the leg representation. This would be consistent with the BOLD signal activity in the leg area after electrical stimulation of the median nerve. Another possibility is that, during hand movement, there is a potential for uncontrolled ‘overflow’ of activation to the leg area, which is normally prevented in intact individuals, but in SCI individuals is no longer cancelled because leg movements no longer occur. One way to test for the importance of leg area activation to voluntary hand movement in future experiments would be to use TMS as a ‘virtual lesion’, and examine whether leg area stimulation interferes with hand movement in SCI but not in intact individuals.
Relationship between cord atrophy and physiological measures
Reduced SCA is associated with impaired upper limb function following SCI (Freund et al., 2011). Here, we provide further evidence for this, as we demonstrate a negative correlation between SCA and increased AMT and CSP duration. In other words, greater damage to the spinal cord correlates with decreased CS excitability and prolonged duration of CSP. As the corticomotor system is directly connected to the spinal cord via CS projections (Lemon, 2008), these findings may be caused by retrograde degeneration (Cohen-Adad et al., 2011; Freund et al., 2011) of these projections that might, in turn, lead to physiological remodelling and changes to the output properties of the injured CS neurons, as observed in the rodent model of SCI (Ghosh et al., 2010).
CS motor output is reduced following SCI
The cortical AMT of the EDC was significantly increased in SCI participants. This was not attributable to a difference in scalp–brain distance, which affects the efficiency of TMS (Stokes et al., 2007), as this was equal in both groups of subjects. One possibility is that increased AMT is attributable to a reduced density of motoneurons or interneurons in low segments of the cervical cord that innervate the EDC. However, this seems unlikely to be the only explanation, given the negative association between increased AMT and lower SCA at C2. At this level, the major reason for changes in SCA is degeneration of axons and not motoneurons. Moreover, we accounted for potential influences of changes in excitability of spinal motoneurons or cortical neurons, as this was equalized, as far as possible, across subjects by their maintaining a constant background voluntary contraction. It therefore seems likely to reflect the reduced density of surviving CS neurons, which would require a higher intensity of stimulation to recruit a given CS output (Di Lazzaro et al., 2004). In addition, because the response evoked by TMS results from activation of facilitatory inputs to CS neurons (Amassian et al., 1987; Shimazu et al., 2004), changes in cortical AMT may reflect structural and functional alterations in cortico-cortical connectivity that result from degenerative processes affecting CS neurons (Aguilar et al., 2010).
In line with a previous study on complete SCI (Lotze et al., 2006), we observed increased CSP duration. The CSP is thought to be mediated by GABAB receptors on pyramidal cells (McCormick, 1989). Degeneration of CS axons and soma shrinkage of pyramidal cells (Hains et al., 2003; Beaud et al., 2008) may lead to reduced afferent excitatory cortico-cortical and thalamo-cortical drive to the motor cortex. Consequently, a shift of excitability towards inhibition could occur. However, we cannot exclude the possibility that the increased AMT may also contribute to the prolonged CSP. If SCI reduces the number of CS neurons without changing the number of inhibitory interneurons, then the higher intensity required to recruit sufficient CS output may activate proportionally more inhibitory interneurons within the cortex. Finally, it should be noted that, because of high thresholds in some participants, we were only able to measure CSP at a relatively low intensity of stimulation (110% AMT). At such intensities, the duration of the CSP in controls is short, and this may be partially attributable to changes in excitability of spinal motoneurons that are refractory from having discharged in the preceding MEP (Fuhr et al., 1991). Under normal circumstances, spinal motoneurons become excitable after 100 ms, so that increased duration of the hot spot CSP in patients to 130 ms seems likely to have been caused by changes in cortical excitability. However, as we did not test for changes in spinal motoneuron behaviour in these patients, this must be regarded as a tentative conclusion that requires further testing in future experiments.
Limitations of the study
The present study has limitations that could have contributed to differences in the CoG EDC between the two groups. First, the study was conducted on a relatively small number of SCI participants in the chronic stage of SCI. Thus, longitudinal studies are required to validate the present findings and to investigate the temporal development of parameter changes associated with SCI. Second, the muscle studied may also lead to different results. Initially, we intended to also examine the first dorsal interosseous muscle, but, owing to muscle atrophy, this turned out not to be feasible. Therefore, in future studies, additional proximal arm and trunk muscles should be added to the analysis in order to obtain a more detailed image of changes to the cortical muscle representations that are (relatively) unaffected by the injury.
Summary
We found that the motor output of the forearm muscles was reorganized in SCI participants with severe upper and lower limb motor impairment and reduced SCA (i.e. atrophy). The CoG of the forearm muscles shifted posteriorly towards the location of the intrinsic hand muscles rather than towards the expected denervated leg area. Crucially, we were able to demonstrate that a smaller SCA (i.e. atrophy) was directly linked to reduced excitability and increased intracortical inhibition, possibly reflecting changes in cortico-cortical connections. Therefore, changes in the corticomotor projections and changes within cortico-cortical connections of partially deprived CS neurons innervating the hand neurons may be an underlying mechanism that drives cortical reorganization and provides an additional neuronal substrate contributing to muscle activation of the impaired forearm.
Acknowledgements
We thank all participants for their contribution, Dr Angela Gall for clinical assessment, Dipl. Ing. Alessio Buetti for technical assistance, and Dr Daniel Altman for statistical advice. This study was supported by the Swiss National Science Foundation (Grant No. PBFR33-120920), Schweizerische Stiftung für medizinische und biologische Stipendien (Grant No. PASMP3-124194), Swiss Paraplegic Research (Nottwil), and the Biotechnology and Biological Sciences Research Council (BBSRC).
Abbreviations
-
- AMT
-
- active motor threshold
-
- ARAT
-
- action research arm test
-
- ASIA
-
- American Spinal Cord Injury Association
-
- BOLD
-
- blood oxygen level-dependent
-
- CoG
-
- centre of gravity
-
- CS
-
- corticospinal
-
- CSP
-
- cortical silent period
-
- ED
-
- Euclidian distance
-
- EDC
-
- extensor digitorum communis muscle
-
- EMG
-
- electromyographic
-
- fMRI
-
- functional magnetic resonance imaging
-
- 9HPT
-
- nine-hole peg test
-
- IQR
-
- interquartile range
-
- M1
-
- primary motor cortex
-
- MEP
-
- motor-evoked potential
-
- MRI
-
- magnetic resonance imaging
-
- n c
-
- number of controls
-
- n p
-
- number of participants
-
- SCA
-
- cross-sectional cord area
-
- SCI
-
- spinal cord injury
-
- SD
-
- standard deviation
-
- TMS
-
- transcranial magnetic stimulation




