Intentional signals during saccadic and reaching delays in the human posterior parietal cortex
Abstract
In the monkey posterior parietal cortex (PPC), there is clear evidence of anatomically segregated neuronal populations specialized for planning saccades and arm-reaching movements. However, functional neuroimaging studies in humans have yielded controversial results. Here we show that the human PPC contains distinct subregions responsive to salient visual cues, some of which combine spatial and action-related signals into ‘intentional’ signals. Participants underwent event-related functional magnetic resonance imaging while performing delayed saccades and long-range arm reaches instructed by visual cues. We focused on activity in the time period following the cue and preceding the actual movement. The use of individual cortical surface reconstructions with detailed sulcal labeling allowed the definition of six responsive regions with distinctive anatomical locations in the PPC. Each region exhibited a distinctive combination of transient and sustained signals during the delay, modulated by either the cue spatial location (contralateral vs. ipsilateral), the instructed action (saccades vs. reaching) or both. Importantly, a lateral and a medial dorsal parietal region showed sustained responses during the delay preferentially for contralateral saccadic and reaching trials, respectively. In the lateral region, preference for saccades was evident only as a more sustained response during saccadic vs. reaching delays, whereas the medial region also showed a higher transient response to cues signaling reaching vs. saccadic actions. These response profiles closely match the behavior of neurons in the macaque lateral and medial intraparietal area, respectively, and suggest that these corresponding human regions are encoding spatially directed action plans or ‘intentions’.
Introduction
The posterior parietal cortex (PPC) is traditionally considered as an association area at the interface between sensory and motor systems, but classical notions of its functioning differ for the relative importance given to its spatial-attentional (Colby & Goldberg, 1999) and action-intentional (Andersen & Buneo, 2002) processing. Single cells in the monkey intraparietal sulcus (IPS) typically combine sensory, attentional, memory, and motor signals (Barash et al., 1991); when a visual cue instructs an eye or arm-reaching response, which must be withheld until a go signal (Hikosaka & Wurtz, 1983), neurons whose receptive fields cover the cue location exhibit an initial burst of activity at cue onset, followed by sustained activation throughout the delay, and possibly by a second burst at movement time (Gnadt & Andersen, 1988). Effector-specific sustained activity is taken as evidence of formation of ‘intentions’, or early movement plans (Andersen et al., 1997), and is observed at the single-neuron and population level in the lateral (LIP) and medial (MIP) intraparietal areas, for saccades and reaching, respectively (Snyder et al., 1997). Anatomical segregation of intentional signals is not absolute, however; some cells are not effector-selective and some reaching-selective cells are also observed in area LIP, and vice versa (Snyder et al., 2000; Calton et al., 2002).
Functional magnetic resonance imaging (fMRI) studies provide evidence for a mosaic of visually responsive regions in the human PPC (Sereno et al., 2001; Schluppeck et al., 2005; Silver et al., 2005; Swisher et al., 2007; Konen & Kastner, 2008). However, the extent to which the human PPC encodes intentions for saccades and reaching is highly controversial. Studies comparing parietal activation during saccades and reaching either report very limited effector specificity (Simon et al., 2002; Medendorp et al., 2005; Connolly et al., 2007; Hagler et al., 2007; Levy et al., 2007; Beurze et al., 2009) or the presence of a reaching-selective but not of a saccade-selective region (Astafiev et al., 2003; Connolly et al., 2003; Fernandez-Ruiz et al., 2007; Filimon et al., 2009).
Failure in detecting intentional signals may indicate that the human PPC primarily subserves spatial-attentional rather than intentional representations, or that intentional signals are not anatomically segregated. Alternatively, it may depend on methodological issues. High anatomical variability in the human PPC might prevent the detection of small regions with selective signals in studies focused on group analysis only. Finger pointing used in most human studies differs from true long-range arm reaching (Filimon et al., 2009). Furthermore, experimental paradigms or data analysis strategies not allowing the disentangling of sustained delay responses may prove unable to detect small effector-related modulations within strong stimulus-driven and movement-related signals (Schluppeck et al., 2006).
Here, we implemented a delayed saccadic/reaching task requiring long-range reaching and closely reproducing monkey experiments (Snyder et al., 1997). Activations during the delay were described relative to individually and rigorously defined anatomical landmarks, and functionally characterized as a combination of spatially and effector-specific transient and sustained response components. This strategy allowed us to detect small but consistent spatially selective intentional signals in subregions possibly corresponding to monkey areas LIP and MIP.
Materials and methods
Subjects
Eight neurologically normal volunteers (six females and two males, mean age 27 years, range 21–33 years) were recruited from the Santa Lucia Foundation (Rome, Italy) community. One was a co-author of this article, whereas the others were naive as to the purposes of the experiment. All subjects were in good health, and were not on medication. All were right-handed, as assessed by a modified version of the Edinburgh Inventory (Oldfield, 1971) (mean index 0.85; SD 0.15), had normal vision, and normal neurological history. The protocol was approved by the Foundation Santa Lucia Ethics Committee, and written informed consent was obtained from each participant before the start of the study.
Tasks
As shown in Fig. 1, a black fixation point (diameter 0.75°) was displayed at the center of the screen on a gray background. Participants were instructed to keep fixation on it and to maintain their right hand still, centrally on their abdomen (hand resting position). A trial started with the appearance of a peripheral cue (blue or green circle, diameter 0.75°) for 400 ms. The cue color instructed the type of movement to be performed (blue circle, eye movement; green circle, hand movement), whereas the cue location indicated the target destination of the impending eye or hand movement (2.5 or 7.5° to the left or right of the fixation circle).
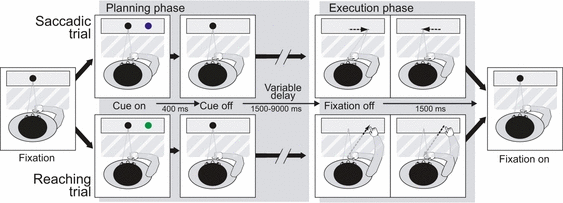
Experimental paradigm and setup. Sequence of events during saccadic and reaching trials. Time flows rightward. Subjects start fixating a central black point (Fixation). A blue or green circle appears at either 2.5 or 7.5° to the left or right of the fixation point (Cue on) and disappears after 400 ms (Cue off). Blue cues instruct the subject to perform a saccade (top row), green cues a reaching movement (bottom row). Subjects keep fixating the central point (Variable delay: 1500–9000 ms) until it disappears (Fixation off). At fixation offset, subjects make a saccade or a direct manual reach to the remembered cue position on a blank screen. They then immediately move back to the starting position. The central fixation point reappears in preparation for the next trial (Fixation on). The hand is covered from view by black cardboard (shown as a dashed semi-transparent area). For interpretation of color references in figure legend, please refer to the Web version of this article.
Participants were instructed to wait for the fixation point offset before actually performing the instructed movement. Thus, during the delay period, subjects retained the instruction about both the effector and the target destination of the movement. After a variable delay (1.5–9 s after cue offset, in steps of 1.5 s intervals), the fixation point disappeared and subjects had to move their eyes or arm to the memorized target location and then immediately back to the starting position. Instructions emphasized movement speed and spatial accuracy, and subjects were encouraged to prepare the movement as soon as the cue appeared. The actual delays, cue color and cue location were pseudo-randomized. The unpredictable duration of the delay was crucial to avoid response anticipation; the subject needed to be ready to respond at any time, but the timing of response could not be predicted. The next trial started after a variable intertrial time (1.5–9 s, in steps of 1.5 s intervals). Naive subjects were falsely informed that we were able to measure response latencies during the fMRI experiment, and that payment for participating in the experiment would be commensurate with the actual performance. At the end of the experiment, we explained the reasons for this deception and always paid the maximum amount agreed upon.
As we had no means of checking eye movements during the fMRI experiment (see below), we performed a preliminary psychophysical experiment on five subjects in a separate laboratory, using the same stimuli and tasks, whereas eye movements were recorded through a video camera and arm movements were controlled by an experimenter. Subjects moved the correct effector with no exception, whereas extra saccades were occasionally recorded at cue onset (< 3% of the trials), irrespective of the type of movement instructed by the cue.
Apparatus
All magnetic resonance (MR) examinations were conducted at the Santa Lucia Foundation on a 1.5 T Vision MR scanner (Siemens Medical Systems, Erlangen, Germany) equipped for echo-planar imaging. Functional MR images were acquired using the Small Flex (Siemens Medical Systems) quadrature receive-only surface radio-frequency coil.
Two features of the surface coil were helpful for this study. First, the surface coil allows a higher signal-to-noise ratio to be obtained with respect to the conventional head coil in brain regions in the proximity of the coil, at the expense of coverage of the rest of the brain. Thus, it is particularly suitable for studies that are focused on a particular brain region, as in the present case. The surface coil was indeed carefully positioned to obtain the maximum signal from the PPC. Second, the head coil completely surrounds the head, so that the screen where visual stimuli are presented is visible only through a mirror. Conversely, there is no need for mirrors when using the surface coil; the subject’s head can be slightly bent ahead so that the screen is directly and comfortably visible. This was of great importance in this study, because it allowed subjects to reach and touch the actual location of the visual target instead of reaching the location where the image of the target is reflected on a mirror (as in most previous studies, but see Beurze et al., 2007, 2009; Filimon et al., 2009; Levy et al., 2007; Stark & Zohary, 2008).
Moreover, our experimental configuration allowed subjects to perform real long-range reaching movements rather than simpler finger/wrist movements as carried out in most previous studies (but see Beurze et al., 2007, 2009; Filimon et al., 2009; Levy et al., 2007; Stark & Zohary, 2008).
Above the coil, the head was supported with a foam doughnut, padded from the sides with more foam, in order to avoid any direct contact between the head and the coil itself. The head was then immobilized using a custom-built bite-bar. A dental impression of each participant was taken outside the magnet, and then attached to a custom-built dual joint plexiglas support, firmly secured to the patient tray. The bite-bar end of the support was flexibly movable, and was freely adjusted by each individual subject to obtain a comfortable position, which was critical to ensure head stability. The support was then locked into place, and subjects were instructed not to forcibly bite the impression but rather to use it as a reference. This system provides a marked reduction in motion artifacts without introducing any discomfort.
Visual stimuli were generated by a control computer (Power Macintosh G3; Apple Computers, Cupertino, CA, USA) located outside the MR room, running in-house software (LabScript) implemented in Matlab (The MathWorks Inc., Natick, MA, USA) using the Psychophysics Toolbox extensions (Brainard, 1997; Pelli, 1997). It allowed the time-locked presentation of visual and auditory stimuli, maintained millisecond timing accuracy over a period of minutes and triggered the acquisition of functional MR images. An LCD video projector with a customized lens was used to back-project visual stimuli to a translucent screen, positioned at the level of the subject’s shoulders and secured to the patient tray. Several expedients have been used to prevent visual stimulations that could create confounds in the reading of the signal. The lower portion of the screen was covered with black cardboard, and visual stimuli were presented immediately above the cardboard top edge. During reaching trials, subjects were trained to move their right hand high and backward (always keeping it behind the cardboard) to touch the screen in the position where the targets appeared. This ensured that the moving hand was never visible to the subject, even if the study could not be conducted in complete darkness, because of the presence of the projector background light. Therefore, we can exclude the possibility that the observed activation was due to the firing activity of parietal cells responding solely to the appearance of the limb in their receptive fields.
A side-effect of using the flexible head coil and direct viewing of the screen was that we could not use an eye-tracker to monitor eye movements during the fMRI experiment, as that would require a mirror to reflect infrared light onto the subject’s eye. However, an experimenter constantly visually monitored subjects’ arm movements during the fMRI experiment to ensure that subjects performed reaching movements when expected. As in the preliminary study reported above, subjects performed a reaching movement when expected with no exception, and did not performed extra reaching movements. Of course we assumed that subjects did perform a saccade in all trials where they did not perform a reaching movement; this assumption was, however, supported by the results of the preliminary study reported above, where this happened with no exception. The preliminary study also indicated that saccades could occasionally be performed at cue onset. As we had no means to detect and exclude from the analysis the specific trials where this happened, these extra saccades may have contaminated blood oxygen level-dependent (BOLD) activity during the cue-delay phase. However, such extra saccades were rare and equally distributed across trial types, so we should not expect this problem to result in any systematic bias, but eventually in a decreased sensitivity in detecting differences between conditions.
Imaging parameters
Blood oxygen level-dependent images were acquired with an echo-planar imaging (EPI) sequence (repetition time, 2 s; echo time, 60 ms; flip angle, 90°) in the axial plane (64 × 64 matrix, 3 × 3 mm in-plane resolution). During each 9 min 40 s scan, we acquired 290 repetitions on each of seventeen 2.5 mm axial slices. Imaging began at the superior convexity and extended ventrally, using a sequential excitation order, with a 0.5 mm gap between slices. Each scan comprised 24 saccadic and 24 reaching trials. Each subject underwent a single acquisition session, and performed either two (for five subjects) or three (for three subjects) scans.
For each subject, we also acquired two high-resolution (1 × 1 × 1 mm) T1-weighted images of the whole brain, using a three-dimensional magnetization prepared rapid gradient echo sequence (TR, 11.4 ms; TE, 4.4 ms; flip angle, 10°; 256 × 256 matrix; 1 × 1 mm in-plane resolution; 220 contiguous 1 mm coronal slices). These structural scans, tuned to optimize the contrast between gray and white matter in brain, were acquired in a separate session using a head coil for full head coverage. Another 1 × 1 × 1 mm magnetization prepared rapid gradient echo image (reference scan) was acquired with the surface coil at the end of each functional session, to aid in registering the functional and anatomical images acquired in different sessions.
Anatomical image processing
Processing of anatomical images was performed using FreeSurfer (Dale et al., 1999; Fischl et al., 1999a). The two high-resolution structural images obtained from each subject were carefully and manually co-registered using blink comparison, and then averaged to enhance the signal-to-noise ratio. The skull was stripped off automatically by a stiff deformable template onto the brain images and the gray/white matter boundary was estimated for each hemisphere with a region-growing method. The result was tessellated to generate a surface that was refined against the magnetic resonance imaging data with a deformable template algorithm. By choosing a surface near the gray/white matter border (rather than near the pial surface, where the macrovascular artifact is maximal) we were able to assign activations more accurately to the correct bank of a sulcus. The surface was then unfolded by reducing curvature and adding an additional local area-preserving term. For a completely flattened cortical surface, the inflated brain was cut along the calcarine fissure and just posterior to the sylvian fissure so that the occipital and parietal lobes were completely detached from the rest of the brain using the algorithm of Dale & Sereno (1993). Furthermore, the unfolded cortical surfaces were transformed to a sphere, and the individual folding patterns were aligned with an average folding pattern in spherical coordinates (Fischl et al., 1999b), in order to provide a way for surface-based intersubject anatomical comparisons (see Heed et al., 2011, for a similar approach in a study on finger pointing).
Additional processing was performed using in-house software (BrainShow), implemented in Matlab (The MathWorks Inc.), which allows the viewing of images and surface reconstructions imported from FreeSurfer to mark specific sulci and gyri, and to superimpose individual statistical maps. BrainShow has been used in previous studies from our and other groups (e.g. Castriota-Scanderbeg et al., 2005; Galati et al., 2008; Pitzalis et al., 2010; Ionta et al., 2011) and is freely available on request for academic usage (E-mail: [email protected]). The parietal lobe of each hemisphere was individually studied, the main sulci were identified and labeled on the basis of a standard atlas of the cortical surface (Duvernoy, 1991) and were traced on the surface reconstructions. This detailed anatomical study served not only for display purposes, but also for describing functional activations relative to the sulcal anatomy.
Anatomical labeling
Figure 2 shows the surface reconstruction of the left hemisphere of a representative subject, and specifies the labels that we used for sulci, according to Duvernoy (1991). On the dorsolateral hemispheric surface (Fig. 2A), the IPS was divided into three segments: (i) an anterior ascending segment (aIPS) (also called the inferior postcentral sulcus), which runs parallel to the central sulcus and divides the postcentral and supramarginal gyri; (ii) a horizontal segment (hIPS), which runs approximately perpendicular to the central sulcus and divides the superior parietal lobule (SPL) and inferior parietal lobule; and (iii) a posterior descending segment (pIPS), continuing the hIPS and entering the occipital lobe. The SPL was defined as the portion of the parietal lobe medial to the IPS, whereas the inferior parietal lobule extended laterally to the IPS. The sulcus intermedius primus of Jensen, when visible, marked the boundary between the supramarginal and angular gyrus in the inferior parietal lobule (Duvernoy, 1991).
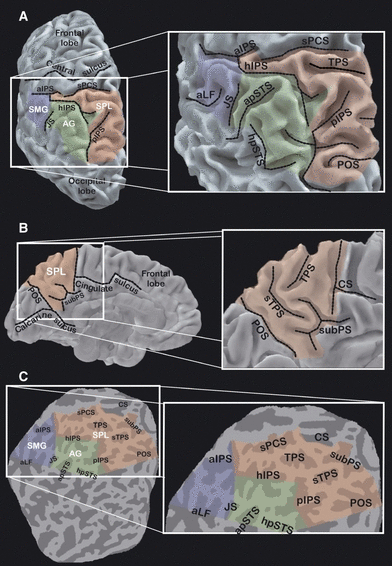
Anatomical reconstruction of the cortical surface of the parietal lobe and sulcal nomenclature. The left column shows a dorsolateral view (A) and a mesial view (B) of the reconstructed cortical surface of the left hemisphere of a representative subject, and a flattened representation (C) of the parietal and occipital lobes. Shadowed areas label the supramarginal gyrus (SMG) (blue), angular gyrus (AG) (green), and SPL (red). For interpretation of color references in figure legend, please refer to the Web version of this article. Dashed black lines in A and B show the main sulci traversing the PPC. In the flattened map, gyri are in light gray and sulci in dark gray. A close-up of the posterior parietal region is shown in the right column for each of the three views. The close-ups show the paths and the names of all sulci traversing the PPC. aIPS, ascending segment of the IPS (also inferior postcentral sulcus); sPCS, superior postcentral sulcus; hIPS, horizontal segment of the IPS; pIPS, posterior segment of the IPS; sTPS, secondary TPS; aLF, ascending segment of the lateral fissure; JS, sulcus intermedius primus of Jensen; apSTS, ascending posterior segment of the superior temporal sulcus; hpSTS, horizontal posterior segment of the superior temporal sulcus; CS, cingulate sulcus; subPS, subparietal sulcus.
Although the aIPS and superior postcentral sulcus, which continues the aIPS dorsally, clearly define the anterior boundary of the PPC, there is not such a clear boundary with either the occipital or temporal lobe. The posterior boundary of the parietal cortex was conventionally considered as a straight line connecting the parieto-occipital sulcus (POS) with the horizontal posterior segment of the superior temporal sulcus (Duvernoy, 1991). A set of straight lines connecting the horizontal posterior segment of the superior temporal sulcus, sulcus intermedius primus of Jensen and inferior tip of the aIPS was taken as the lateral boundary with the temporal lobe.
On the mesial hemispheric surface (Fig. 2B), the anterior boundary of the SPL was defined by the posterior ascending branch of the cingulate sulcus, the ventromedial boundary by the subparietal sulcus and the posterior boundary by the POS, with straight lines drawn between these landmarks.
Functional image processing
Functional images from the event-related experiment were processed in Matlab, using SPM8 (Wellcome Department of Cognitive Neurology, London, UK) and in-house software (BrainShow). First, functional data were manually and carefully registered to the mean structural image imported from FreeSurfer, using the reference scan as a way to establish an initial registration. This step allowed an exact overlay of the functional data onto each cortical surface. Functional scans were then corrected for slice timing (using the first slice acquired in time as a reference) and for head movement occurring during the scan, after discarding the first two volumes of each scan. Both corrections used the algorithms provided by SPM8. Estimated head movements never exceeded 1 mm of translation or 1° of rotation within a scan.
As we were attempting to precisely localize activations in either bank of the IPS, we did not want to introduce blurring by spatially smoothing the functional images. In fact, given the complex convolution of the IPS, adjacent voxels might not represent adjacent portions of the cortical mantle. For example, two neighboring voxels may represent the two opposite banks of the sulcus. Thus, smoothing images in three-dimensional space may result in inaccurate localization of the activation source. To minimize the problem, we oversampled functional images to 1 × 1 × 1 mm and smoothed them with a Gaussian filter at 2 mm full-width-at-half-maximum (FWHM). This procedure ensured a minimum of smoothing, requested as a prerequisite to apply Gaussian field theory to correct for multiple comparison (see below), without losing spatial resolution.
Definition of regions of interest
The first step in the analysis was aimed at defining a set of regions of interest (ROIs) in the PPC of each individual. As we wanted to verify in humans the results of monkey experiments, we defined as ROIs those regions that showed a reliable (i.e. statistically significant) BOLD response to cue onset. In fact, we know from the neurophysiological literature that the neurons showing a sustained response during the delay before action do show a reliable response to cue onset (e.g. Snyder et al., 1997). Because neurons in area LIP show a transient response to both saccadic and reaching cues flashed in their receptive field, even when their sustained responses during the delay following cue offset were selective for saccadic trials (Snyder et al., 1997), we first selected clusters of voxels in each individual’s PPC that exhibited a significant activation time-locked to cue onset. We then searched for evidence of sustained activation in these ROIs during saccadic and reaching delays (see next section).
For the purpose of ROI selection, the time series of functional MR images obtained from each participant was analyzed on a voxel-by-voxel basis, according to the general linear model (Worsley et al., 1992; Friston et al., 1994). Two separate events were modeled for each trial: the cue onset and fixation offset. The hemodynamic response evoked by cue onset represents at least a transient visual-attentional response, and possibly an intentional signal, but this first analysis was not aimed at distinguishing between the two (see below). Distinct predictors were specified for different cue types (saccadic vs. reaching cues) and cue locations (left vs. right), as we expected visual-attentional responses to be modulated by the spatial location of the cue, and possibly even by the cue type. The hemodynamic response evoked by fixation offset represents the actual movement execution; again, distinct predictors were entered to model saccades and arm movements separately. This model allows testing for the presence of responses time-locked to cue onset, having accounted for and removed the contribution of movement execution. Responses to movement execution were always considered as effects of no interest (or confounds). Evoked hemodynamic responses were modeled with a set of gamma functions and their temporal derivatives (Friston et al., 1998), time-locked to cue onset and fixation offset. Other modeled confounds were low-frequency drifts, residual head movement-related effects and overall differences across scans.
The statistical significance of the hemodynamic responses evoked by each type of cue was assessed at each voxel using standard T statistics (P < 10−3 or higher). Standard Gaussian field theory (Worsley et al., 1996) was used to control for multiple comparisons at the cluster level. We restricted our analysis to a limited region, the PPC, as defined in each individual hemisphere on the basis of the cortical surface reconstructions, and we imposed a minimum size for activation clusters, corresponding to a cluster-level P-value < 0.05 corrected over the considered volume (Worsley et al., 1996). For each hemisphere, we generated an image including all clusters significantly activated by at least one type of cue. This constituted the hemisphere-specific ROI for subsequent analysis.
These individual activation images were projected on the folded, inflated and flattened surface reconstructions using BrainShow. The anatomical locations of the activations were carefully studied across the 16 analyzed cerebral hemispheres, on the basis of the individual sulcal anatomy, using the anatomical landmarks previously described. This detailed study allowed us to identify a set of distinct regions with highly consistent sulcal anatomical locations across individuals (described in the first section of the Results). Each individual cluster was thus assigned to the corresponding region, allowing us to perform across-subject ROI-based analysis.
In order to verify the consistency of the assignment of each activated cluster to a given ROI, we projected each assigned cluster onto the individual spherical cortical representation and then superimposed individual clusters assigned to the same ROI in spherical coordinates. We retained surface nodes where at least 30% of superposition was found, and constructed a surface-based average map of the anatomical location of the ROIs, which was then projected back to a representative cortical surface for illustrative purposes.
Analysis on the regions of interest
The second step in the analysis was aimed at characterizing the typical regional responses of the selected ROIs in terms of attentional vs. intentional activity (i.e. activation independent of or dependent on the impending action), transient vs. sustained activity (i.e. activation time-locked to cue onset vs. activation throughout the delay) and ipsilateral vs. contralateral activity (i.e. activation dependent on the cue location).
For each ROI and hemisphere, a representative cluster time course was computed in terms of the first eigenvariate of the preprocessed, unsmoothed fMRI signal in all voxels of the ROI. A general linear model was then fitted to the ROI time courses, which allowed transient vs. sustained responses to be disentangled. For each trial, the model included: a box-car-shaped predictor spanning the (constant) cue period, providing an assessment of transient visual-attentional responses to the cue; a box-car-shaped predictor spanning the (variable) delay period, providing an assessment of sustained delay-related responses; and a predictor time-locked to the fixation offset, representing the actual movement execution. For cue (transient) and delay (sustained) components, separate predictors were provided for each of the four combinations of cue type (saccadic vs. reaching cues) and location (ipsilateral vs. contralateral to the analyzed hemisphere); for the movement component, separate predictors were provided for saccades and arm movements. This resulted in a total of 10 predictors. Low-frequency drifts, residual head movement-related effects, and overall differences across scans were also modeled as confounds.
The resulting parameter estimates (indicating the estimated size of the effect of each predictor in each individual hemisphere where the ROI was detected) were entered into group (across hemispheres) random-effects analyses, allowing inferences to be drawn on the population from which our subjects were extracted. The presence of a reliable sustained response in each region was assessed through a comparison of two models: a restricted model including only parameter estimates for the cue phase (transient responses) and a full model also including parameter estimates for the delay phase (sustained responses). An F-test comparing the variance of the residuals in the two models was used to assess whether the extra sum of squares accounted for by including the delay predictors was significant in the group. For regions exhibiting a reliable sustained response (i.e. in which the extended model explained significantly more variance than the restricted model), 2 × 2 action × space anovas on the parameter estimates of the delay predictors provided information on the selectivity of such sustained responses.
Retinotopic mapping
In a subsample of four subjects, we also mapped the retinotopic organization of the cortical visual areas using phase-encoded stimuli, as described elsewhere (Sereno et al., 1995; Tootell et al., 1997; Pitzalis et al., 2006a). The stimuli consisted of high-contrast flickering colored checkerboards in either a ray- or ring-shaped configuration (polar angle and eccentricity, respectively). These stimuli spared a central 0.75° circular zone of the visual field to avoid ambiguities caused by fixation instability. Retinotopic stimuli were viewed passively, and subjects were only required to maintain stable fixation throughout the period of scan acquisition. Retinotopic mapping was acquired on a separate day using the same apparatus, setup, and coil as for the main experiment. Images were acquired as in the main experiment, but in this case MR slices were 4 mm thick, with an in-plane resolution of 3 × 3 mm, oriented approximately parallel to the calcarine fissure. Each scan took 512 s and included 256 single-shot EPI images per 32 contiguous slices. To increase the signal-to-noise ratio, data were averaged over three scans for each stimulus type (eccentricity and polar angle). Retinotopic data were analyzed using FreeSurfer (Dale et al., 1999; Fischl et al., 1999a) based on standard procedures described in many previous studies (Sereno et al., 1995; Tootell et al., 1997; Hagler & Sereno, 2006; Pitzalis et al., 2006a, 2010). Briefly, P values were estimated on a voxel-by-voxel basis by constructing an F ratio between ‘signal’ (response amplitude at stimulus frequency) and ‘noise’ (amplitude at other frequencies excluding second and third harmonics) with degrees of freedom equal to the number of time points. The phase of the signal at the stimulus frequency was used to map retinotopic coordinates (polar angle or eccentricity). The boundaries of the retinotopic visual areas were defined in each participant on the basis of the field signs calculated from the maps of polar angle and eccentricity (Sereno et al., 1995).
Results
Anatomical location of cue-related activations
The first analysis step was aimed at identifying in each subject the parietal regions showing at least a reliable response to cue onset in either saccadic or reaching trials. On the basis of the study of the location of individual activations relative to the PPC sulcal anatomy and of intersubject anatomical comparisons, we grouped individual activation clusters into six anatomically distinct regions. The six regions, as described below, were detected with a variable degree of consistency across the 16 individual hemispheres. Figure 3 shows representative hemispheres from four different subjects, where the six regions have been rendered using different colors. Four of the six regions were located on the lateral surface of the hemisphere, inside or just around the IPS: an anterior region [lateral-anterior (LA)] (colored in magenta) in the aIPS; a ventral region [lateral-ventral (LV)] (colored in red) in the hIPS; a dorsal region [lateral-dorsal (LD)] (colored in blue) at the interface between the hIPS and pIPS; and a posterior region [lateral-posterior (LP)] (colored in light blue) in the pIPS. Two other regions were best visible on the medial cerebral surface: a dorsal region [medial-dorsal (MD)] (colored in green) was located in the SPL and a posterior region [medial-posterior (MP)] (colored in yellow) was close to the anterior bank of the POS.
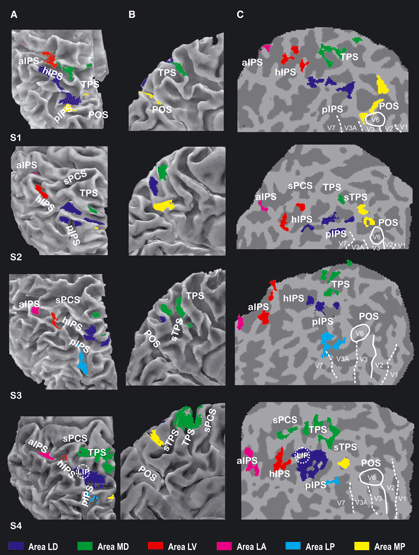
Location of the posterior parietal regions activated after cue onset in four representative subjects. The figure shows an enlarged dorsolateral (A), mesial (B), and flattened (C) view of the reconstructed cortical surface of the PPC in four representative left hemispheres. Colored patches show the location of the regions showing at least a transient activation after either saccadic or reaching cues in the experimental task. Regions are colored according to their anatomical location with respect to sulcal landmarks. Blue, LD; green, MD; azure, LP; yellow, MP; red, LV; magenta, LA. On the flat maps, dashed and solid white lines indicate vertical and horizontal meridians defining the borders of early visual regions, as retinotopically defined, and the white solid circle shows the position of human V6. The dashed circle shows the position of human area LIP, as retinotopically defined in one subject (S4). See Fig. 2 for abbreviations. For interpretation of color references in figure legend, please refer to the Web version of this article.
The LD (blue) was located in the cortical region joining the hIPS and pIPS and was detected in 16/16 hemispheres. No hemispheric asymmetry was evident in either the location or size of the activation (in terms of number of activated voxels). The detailed anatomy of the cortical region between the hIPS and pIPS was quite variable across subjects (see examples in Fig. 3). The hIPS–pIPS interface was always well within the boundaries of the PPC, i.e. well anterior to the POS. In 3/16 hemispheres, the posterior end of the hIPS and the anterior end of the pIPS were perpendicularly oriented and converged into a T-shaped connection. In these cases, area LD was invariably located inside the medially oriented branch of the T-shaped connection. In the remaining 13 hemispheres, the horizontal and posterior segments of the IPS were two clearly distinct sulci, resulting in a narrow bridging lobule connecting the SPL and inferior parietal lobule. In these cases, the shape of the IPS at the interface of the two segments was highly variable, with the ends of the two segments oriented both approximately parallel to the central sulcus in 6/13 cases, and both approximately perpendicular to the central sulcus in the other 6/13 cases, with the pIPS running sometimes medially and sometimes laterally to the hIPS. In these cases, area LD was located on the anterior bank of the pIPS (5/13 hemispheres), posterior bank of the hIPS (4/13 cases) or in an intermediate position, spanning both the hIPS, pIPS and the bridging lobule (4/13 cases). In all cases, the location of area LD on flattened maps was always closer than 1 cm from the bridging lobule (see Fig. 3C).
The location of LD is strikingly consistent with the putative human homologous area LIP, which was described by Sereno et al. (2001) in a position that, in our terminology, corresponds to the anterior end of the pIPS. As noted above, this was the location most often found in our cases. Moreover, the positions of areas LD and LIP can be directly compared on the hemispheres of a subject who has been studied here and in Sereno et al. (2001). The left hemisphere of this subject is shown as S4 in Fig. 3, and the location of area LIP, as retinotopically defined in Sereno et al. (2001), is shown as a dashed white circle to aid comparisons. Although the two experiments were performed in laboratories using different MR scanners, experimental paradigms and analysis procedures, the two maps are strikingly consistent. This region is also similar to the retIPS region as described by Medendorp et al. (2005) and should correspond to the IPS3 field (Hagler et al., 2007; Swisher et al., 2007).
The MD (green) was located in the SPL and was detected in 8/8 left and 6/8 right hemispheres. No hemispheric asymmetry was evident in either the size or location of the activation. In all hemispheres of our sample, the SPL was traversed by a sulcus approximately parallel to the central sulcus, running from the IPS to the medial surface of the hemisphere, called the transverse parietal sulcus (TPS) (Duvernoy, 1991). In some hemispheres, a second smaller sulcus parallel and posterior to the TPS was also observed (see Fig. 2), that we have labeled as the secondary TPS. Area MD was located fully inside the TPS in 4/14 cases, whereas in 7/14 cases it extended from the TPS to the convexity of the SPL surface. In the remaining three cases, area MD was found on the SPL convexity, just posterior to the TPS, and anteriorly to the secondary TPS, when present. In all cases, the location of area MD on flattened maps was always closer than 1 cm from the TPS (see Fig. 3C).
The location of MD falls within the territory of the human parietal reach region as first described by Connolly et al. (2003). Subsequent studies have identified multiple reaching-selective fields in the medial parietal cortex (Beurze et al., 2007; Tosoni et al., 2008; Filimon et al., 2009) and area MD closely corresponds to the most anterior one (preCUN: Beurze et al., 2007; aPCU: Filimon et al., 2009; aPRR: Tosoni et al., 2008). MD may also be coincident with the retinotopically defined fields SPL1 (Konen & Kastner, 2008) and/or mPC (Hagler et al., 2007).
The LP (blue) was invariably located inside the posterior segment of the IPS, in the most posterior section of the PPC, clearly distinct from area LD. It was found in 4/8 left and 6/8 right hemispheres.
The MP (yellow) was found in 5/8 left and 5/8 right hemispheres. Area MP was located on the medial surface of the brain, anterior and superior to the dorsal portion of the POS. In 5/10 cases, the area was located on the anterior bank of the POS, whereas in the other 5/10 cases it was located anteriorly and dorsally to the POS, on the medial bank of a small medially oriented branch of the posterior IPS. This region also falls within the territory of the human parietal reach region (Connolly et al., 2003) and may correspond to the most posterior medial parietal reaching region described in several studies (PO: Beurze et al., 2007; sPOS: Filimon et al., 2009; pPRR: Tosoni et al., 2008; SPOC: Gallivan et al., 2009; Cavina-Pratesi et al., 2010). No retinotopical fields have yet been described in this location.
The LV (red) was found in 6/8 left and 4/8 right hemispheres and was located in the horizontal segment of the IPS, approximately half way between the pIPS and aIPS. It was clearly anterior and ventral with respect to area LD and, unlike LD, was invariably located inside the IPS.
The LA (magenta) was a very small region, located in the anterior segment of the IPS (or inferior postcentral sulcus). This region was found in 6/8 subjects in the left hemisphere, but was never detected in the right hemisphere.
Location of cue-related activations relative to retinotopic areas
Retinotopic mapping was performed to demarcate dorsal areas V1-V7, so as to illustrate the exact relationship between activated regions and the known early visual cortical areas. Retinotopic areas V1, V2, V3, V3A, V6, and V7 were successfully identified by mapping the visual field sign (see Materials and methods), and boundaries of visual areas were rendered on a flattened version of each participant’s reference anatomy. Figure 3 shows four cases where the six regions have been overlaid with a set of lines representing the boundaries of early visual areas that we defined in these subjects. The only area that can be further characterized through retinotopy was LP, which is posterior enough to overlap the known retinotopic boundaries. Area LP overlapped in an equal number of cases the anterior end (superior on flat maps) of areas V3A (50%) and V7 (50%), whose anatomical landmark is indeed the posterior segment of the IPS (Tootell et al., 1997, 1998). The location of area MP relative to retinotopic areas is also interesting in that it did not overlap area V6 but was located very close to its anterior border.
Average anatomical location of the activated regions
Figure 4 shows the average location of the six regions described so far, projected on the surface reconstruction of the left hemisphere of a representative subject (the same as depicted in Fig. 2). Dashed black lines indicate the paths of the sulci traversing the PPC, which, although variable in shape, are almost invariably present (see Ono et al., 1990; Duvernoy, 1991). The colored patches represent the six demonstrated regions. All of the individual activations assigned to a given region on the basis of the individual sulcal pattern were projected and superimposed in spherical coordinates (as described in Materials and methods) and then projected back to the representative cortical surface shown in Fig. 4. Each patch includes the portion of the PPC that was assigned to a given region in at least 30% of the hemispheres. It is evident from the figure that no superposition at all exists between the six patches, thus providing evidence that the anatomical segregation between the six regions is consistent across subjects and survives at group level.
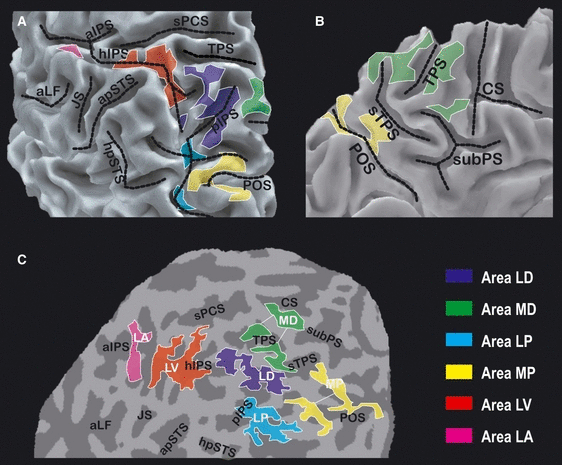
Average location of the six posterior parietal regions. The figure shows the left PPC of the representative subject shown in Fig. 2 in the same three views as in Fig. 2 (A–C). The colored patches represent the six regions showing cue-related activation. The portion of the cortex inside each given patch belongs to the corresponding region in at least 30% of the hemispheres. See Fig. 2 for abbreviations. For interpretation of color references in figure legend, please refer to the Web version of this article.
Functional profiles of the activated regions
The six regions described so far were selected on the basis of the evidence of a reliable response time-locked to the cue onset in either saccadic and/or reaching trials. Subsequent analyses were aimed at characterizing such responses as a function of the impending movement (Action – saccadic vs. reaching) and cue location (Space – ipsilateral vs. contralateral) in order to distinguish visuo-attentional (i.e. action-independent and space-dependent) from intentional (i.e. action-dependent) responses.
The bar plots in Fig. 5A show the amplitude of transient, cue-related responses in the six areas, separately for saccadic and reaching, and for ipsilateral and contralateral trials. A robust effect of Space, with higher responses for contralateral than ipsilateral trials, was found in LD (F1,15 = 6.38, P = 0.023), MD (F1,13 = 16.28, P = 0.001) and LP (F1,9 = 7.72, P = 0.021). A similar, although not statistically significant, effect was present in MP (F1,9 = 3.80, P = 0.083). No effect of Space was instead found in the two most anterior regions (LV –F1,9 = 1.44, P = 0.261; LA –F1,5 = 0.09; P = 0.779). The effect of Action was significant in the two medial regions (MD –F1,13 = 10.40, P = 0.007; MP –F1,9 = 12.21, P = 0.007) and in LV (F1,9 = 10.02, P = 0.011), and tendentially significant in LA (F1,5 = 5.44; P = 0.067). In these four regions, responses were higher for reaching than saccadic cues. Regions LD (F1,15 = 2.32, P = 0.148) and LP (F1,9 = 2.84, P = 0.126) did not systematically prefer either saccadic or reaching cues. The Space by Action interaction was not significant in any region.
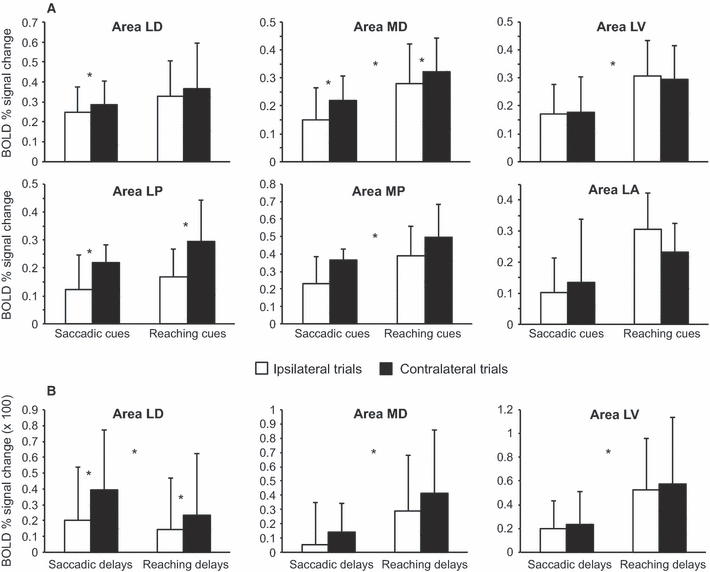
Estimated amplitude of transient and sustained BOLD responses during the cue-delay phase. The bar plots show the estimated amplitude of the transient (A) and sustained (B) components of the fMRI response in the six regions. Amplitudes of sustained responses are only shown for the three areas in which sustained responses were reliably identified. Error bars represent between-hemispheres SD. Trials starting with an ipsilateral or a contralateral cue are shown as white and black bars, respectively. Asterisks in the center of each panel identify significant main effects of Action; asterisks between white and black bars identify significant main effects of Space.
Sustained activity during the delay
In electrophysiological studies on monkeys, area LIP and MIP neurons respond with an initial burst of activity to cues instructing both saccadic and reaching movements, but critically differ for their sustained activity during the following delay, which is effector-specific (Snyder et al., 1997). The further step of our analysis was thus aimed at characterizing the fMRI signal during the cue-delay phase as a combination of transient and sustained components, and evaluating the presence and degree of effector selectivity of the sustained components.
The reliability of sustained delay-related responses was assessed by comparing the variance explained by an extended general linear model, where a constant neural activity during the delay was explicitly modeled in addition to a transient response to the cue, with respect to a restricted model including only transient cue-induced activation. The extended model explained significantly more variance in LD (F1,15 = 11.92, P = 0.004), MD (F1,13 = 13.06, P = 0.003), and LV (F1,9 = 16.05, P = 0.003). Inspection of the parameter estimates of the predictors encoding sustained responses confirmed that they had a positive sign for all conditions in all three regions (i.e. the longer the delay, the higher the estimated activity). There was no evidence for a sustained response in the most posterior regions, LP (F1,9 = 0.08, P = 0.789) and MP (F1,9 = 0.20, P = 0.664), and in the most anterior region, LA (F1,5 = 3.27, P = 0.130).
Further analyses were thus restricted to LD, MD, and LV. The bar plots in Fig. 5B show the amplitude of sustained, delay-related responses in these three areas, separately for saccadic and reaching, and for ipsilateral and contralateral trials. In area LD, sustained activity was affected by Space (F1,15 = 4.66, P = 0.047), with more activity during contralateral than ipsilateral delays, and by Action (F1,15 = 7.82, P = 0.014), with more activity during saccadic than reaching delays. Conversely, in area MD there was more sustained activity during reaching than saccadic delays (F1,13 = 9.80, P = 0.008), with a marginal effect of Space (contralateral > ipsilateral delays –F1,13 = 3.36, P = 0.090). Sustained activity in area LV was also higher for reaching than saccadic delays (F1,9 = 12.13, P = 0.007), with no effect of Space (F1,9 = 0.77, P = 0.403).
In conclusion, only areas LD and MD fulfill all of the criteria expected for regions exhibiting ‘intentional’ signals, i.e. encoding stimuli as a function of the type of spatially-directed actions for which they are potential targets. Indeed, they encode the position of the cue (main effect of Space), and their sustained responses are selective for saccades and reaches, respectively. Area LV, on the other side, exhibits sustained responses selective for reaching but, unlike LD and MD, does not represent the spatial location of the cue (no effect of Space). To further corroborate the selectivity of LD and MD for saccadic and reaching delays, respectively, we directly compared the sustained response estimates in the two areas through a Region by Action by Space repeated-measures anova conducted on the 14 hemispheres where both LD and MD were detected. This analysis yielded a significant Region by Action interaction (F1,13 = 7.15, P = 0.019), confirming a different specificity of the two regions for the two actions. Posthoc analyses (Duncan test, P < 0.05) revealed that, in LD, sustained responses were stronger during saccadic than reaching delays, but only after contralateral cues, whereas no preference for either saccades or reaching was present after ipsilateral cues. Furthermore, sustained responses during saccadic delays were stronger after contralateral than ipsilateral saccadic cues, whereas there was no effect of cue location during reaching delays. In other words, the sustained response pattern in area LD was highly selective for contralateral saccadic delays. In MD, sustained responses were stronger during reaching than saccadic delays both after ipsilateral and contralateral trials, thus confirming a high selectivity for reaching delays.
Figure 6 shows the cue-delay responses as estimated by the extended model in the three regions exhibiting a reliable sustained response, and in one region (MP) not showing sustained responses, for comparison. Response amplitudes are color-coded and plotted as a function of time from cue onset (on the horizontal axis) and delay length (on the vertical axis, with delay lengths continuously interpolated within the range used in the present experiment). The transient component can be visually evaluated by comparing the colors of the response peaks following the cue. The sustained component of the response can be visually evaluated by assessing the right, falling edge of the activated (hot) area. When the sustained component is negligible, the decay of the hemodynamic response will not depend on delay length and thus the falling edge will be approximately vertical. A significant sustained response will instead result in a diagonal falling edge oriented top-rightward, indicating that the longer the delay length, the longer the BOLD responses. Visual inspection of these plots confirms that area LD shows a slightly higher peak in reaching trials, but a more pronounced sustained response in saccadic trials, whereas area MD shows both a higher peak and a more pronounced sustained response in reaching trials.
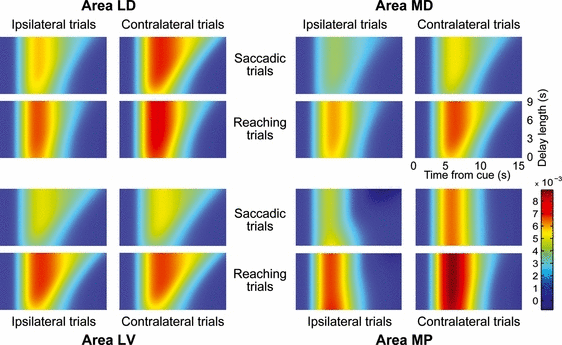
Estimated BOLD responses during the cue-delay phase. Each colored box visually represents the estimated BOLD response during the cue-delay period in a given region and combination of instructed action and cue location, as a function of time and delay length. BOLD responses are reconstructed on the basis of the between-hemispheres averages of the parameter estimates shown in Fig. 5, and interpolated for trials with delays continuously ranging from 1.5 to 9 s (the range used in the present experiment). Data are shown for the three regions (LD, MD, and LV) showing a reliable sustained response and, for comparison, for a region not showing a reliable sustained response (MP). In each panel, time flows rightward starting from the cue onset. Each row represents a possible trial with a given delay length, with delay lengths represented on the vertical axis. The color at each point indicates the intensity of the estimated BOLD response at a given time point of a trial with a given delay length (see color bar, expressed in BOLD percent signal change). For interpretation of color references in figure legend, please refer to the Web version of this article.
Discussion
In this fMRI study, we compared PPC activity during delay periods in which the subject was planning either saccadic or reaching movements in the absence of any visual stimulation. We used an event-related protocol to demonstrate sustained delay-period activity in small and localized areas of the human PPC. We showed that six distinct parietal fields are responsive to visual cues and/or involved in planning actions, with unique functional profiles depending on the modulation by instructed action and cue spatial location. However, only areas LD and MD fulfill all of the criteria expected for regions exhibiting ‘intentional’ signals, i.e. encoding stimuli as a function of both the cue spatial location and the type of action for which they are potential targets. Crucially, an opposite response pattern was observed in the two areas. The sustained response in area LD was greater during saccadic than reaching delays, whereas the reverse was true for area MD. This happened in particular during delays following cues that appeared in the contralateral visual hemifield.
The presence of a significant modulation by the type of the impending action is an unequivocal signature that LD and MD are encoding the subject’s intention to move their eyes or arms. The presence of a cue location effect could indicate that these regions encode the direction of the impending movement. In other words, LD could preferentially code the preparation (and the subsequent execution) of contralateral eye movements, in partial agreement with monkey data (Barash et al., 1991). Alternative explanations of the results in terms of visual or attentional modulation by the visual target can be excluded given that both hypotheses would indeed predict the same response across the two actions. Note that the proposed ‘intentional’ interpretation does not exclude the possibility that the sustained activation contains a selective working memory component, acting as an integrator of all multisensory information relevant for the execution of the planned movement (see Sereno et al., 2001 for a similar interpretation).
Areas LD and MD – comparison with monkey
The response profiles of areas LD and MD closely match the functional properties of neurons in the macaque areas LIP and MIP, respectively (Snyder et al., 1997; Calton et al., 2002). Single cells in these two macaque areas respond to the onset, in their receptive field, of visual cues indicating the request of a delayed motor response. If the motor response required by the task is a saccadic eye movement, the neural activity is maintained for several seconds after the cue offset in area LIP but less in area MIP; the reverse is true for reaching movements. The enhanced response during the delay preceding saccadic or reaching movements is not due to the level of attention (Snyder et al., 1998), or to the spatial location of the target (Calton et al., 2002), but is suggestive of coding of the intention to move either the eye or arm (see Calton et al., 2002).
We tried to closely replicate the rationale of single-neuron recording experiments at the level of data analysis. We first selected a group of PPC regions, based on the presence of a reliable activation time-locked to the visual cue. This would be equivalent to selecting neurons showing a burst of activity when the cue is presented in their receptive fields. We then set apart the activity time locked to the cue into a transient (signal-related) component and a sustained component, accounting for the activity during the delay periods (for similar approaches, see e.g. Toni et al., 2002; Curtis & d’Esposito, 2006; Macaluso et al., 2007). We then looked specifically at the presence of sustained activity during the delay period in these regions, and examined whether it depended on the spatial location of the cue and/or on the nature of the instructed movement. In this way, we were able to detect small differences in sustained activity that could otherwise be masked by a strong undifferentiated transient response to the cue. Indeed, as shown above, monkey area LIP and MIP neurons do show a transient response to both saccadic and reaching cues, whereas their dissociation is manifested in the sustained firing rate during the delay period. The fact that the sustained delay activity found here for areas LD and MD was both action-related and spatially selective makes our results highly consistent with monkey data.
The relative anatomical arrangement of LD and MD is also in agreement with monkey anatomy. Area MD is consistently located in the SPL, just medial to area LD, which is located in the IPS, or more often on the bridging lobule at the interface between the posterior and horizontal sectors of the IPS. In macaques, area MIP is located medially with respect to area LIP, although both are located inside the IPS. Thus, the relative position of the two areas found here in humans parallels that of area LIP and MIP in the macaque, but both areas are medially displaced. Actually, many monkey studies use the functionally defined term ‘parietal reach region’ to refer to the zone where reaching-selective neurons are found, which typically includes both the medial bank of the IPS (i.e. the anatomically defined area MIP: Pesaran et al., 2006; Gail & Andersen, 2006) and the adjacent region on the exposed surface of the caudal SPL (Snyder et al., 1997; Calton et al., 2002). Thus, it remains possible that our area MD also includes parietal subregions that are homologues of parts of the monkey parietal reach region outside area MIP.
Areas LD and MD – previous human data
Previous fMRI studies have described similar regions in the human parietal lobe and/or have proposed human homologues for both area LIP and MIP. As already pointed out, the location of area LD is consistent with the putative human area LIP, identified as the parietal region holding a retinotopic map of remembered saccadic targets (Sereno et al., 2001). More recent fMRI studies at higher field strength have revealed several retinotopic maps in the IPS (IPS1, IPS2, IPS3 and IPS4: Schluppeck et al., 2005; Silver et al., 2005; Swisher et al., 2007). It is most probable that our area LD, as well as the area LIP of Sereno et al. (2001), corresponds to IPS3 (see Hagler et al., 2007; for a comparison in terms of Talairach coordinates). Note that the presence of a retinotopic map of remembered saccadic targets fits well with our finding that this area is responsive during the delay period of a delayed saccade task and that this delay activity is spatially selective. A compatible location is activated in many studies of saccadic eye movements and spatial attention, and often described as the posterior IPS or ‘parietal eye field’ (e.g. Corbetta & Shulman, 2002; Culham & Valyear, 2006).
Similarly, the location of our area MD corresponds well to the superior parietal foci described in many neuroimaging studies on arm-reaching or finger-pointing movements (e.g. Astafiev et al., 2003; Beurze et al., 2007; Fernandez-Ruiz et al., 2007; Tosoni et al., 2008), and already proposed as the human area MIP/parietal reach region (Astafiev et al., 2003; Connolly et al., 2003). MD also corresponds well to the retinotopic map described as mPC by Hagler et al. (2007) and possibly to SPL1 (Konen & Kastner, 2008). Hagler et al. (2007) showed that this retinotopic map was evident not only during delayed pointing, but also during a delayed saccadic task. This is compatible with our results; MD shows a consistently higher transient response for contralateral vs. ipsilateral cues in both saccadic and reaching trials. This result does not challenge the idea that MD encodes intentions to reach; simply, the impending action modulates the intensity of response in area MIP/MD, but not the spatial tuning of neurons, which remains biased in favor of the contralateral hemifield also in the case of (weaker) responses to saccadic cues.
Here we described two distinct activation foci for pointing/reaching movements in the medial parietal cortex: MD anteriorly and MP (see below) posteriorly, the latter in the vicinity of the POS. Although some studies report one large activation region encompassing both foci (Connolly et al., 2003) and others report either only the most anterior (Astafiev et al., 2003; Fernandez-Ruiz et al., 2007; Hagler et al., 2007) or only the most posterior (Beurze et al., 2009; Cavina-Pratesi et al., 2010) one, several studies, as in the present case, report two distinct foci (preCUN and PO: Beurze et al., 2007; aPCU and POS: Filimon et al., 2009; aPRR and pPRR: Tosoni et al., 2008).
Previous fMRI studies comparing activation induced by saccadic and pointing/reaching movements have shown in some cases a substantial lack of effector specificity in the PPC (Simon et al., 2002; Medendorp et al., 2005; Hagler et al., 2007; Levy et al., 2007; Beurze et al., 2009). There is, however, a certain agreement on the fact that the superior-medial regions, corresponding to our MD and MP, respond more to pointing/reaching than to saccadic movements (Astafiev et al., 2003; Connolly et al., 2003; Fernandez-Ruiz et al., 2007; Tosoni et al., 2008; Filimon et al., 2009). However, none of these studies reported higher responses for saccades in a region corresponding to our LD or in any other portion of the PPC.
So why did we find specificity for saccades in LD, which previous studies failed to detect? Our specific focus on sustained activity during the delay may prove crucial to understand this discrepancy. Previous studies on saccades and reaching either used immediate responses, without delay (Simon et al., 2002; Levy et al., 2007; Filimon et al., 2009), did not disentangle cue- and movement-related activation (Medendorp et al., 2005; Hagler et al., 2007) or did not explicitly distinguish transient vs. sustained cue-related activation (Astafiev et al., 2003; Connolly et al., 2003; Medendorp et al., 2003; Fernandez-Ruiz et al., 2007; Beurze et al., 2009). Interestingly, a couple of studies explicitly focusing on this distinction (Schluppeck et al., 2006; Macaluso et al., 2007) described sustained activity during delays preceding saccades in LD/area LIP, although they did not employ a pointing/reaching task for comparison.
Thus, selectivity for saccades in LD may be evident only when considering the temporal profile of the response. Indeed, our results show that transient activation is similar in LD for cues instructing saccadic and reaching movements, and the critical distinction regards how long the response is sustained during the delay. Thus, subtle differences in response duration may be masked by strong and undifferentiated transient responses. Intriguingly, in Tosoni et al. (2008), who used ambiguous cues offering a variable degree of evidence in favor of a saccadic vs. reaching response, the degree of evidence provided by the cue affected the poststimulus peak height in MD/area MIP, but only the profile of the response decay in LD/area LIP. Connolly et al. (2007) similarly described a posterior intraparietal region whose activity scaled with delay length more for saccadic than for pointing delays. Also a recent magneto-encephalographic study (Van Der Werf et al., 2010) reported higher gamma-band synchronization during saccadic delays in a lateral parietal region.
Other methodological factors may prove to be crucial in order to detect intentional signals in the PPC. Taking interindividual anatomical differences explicitly into consideration is one example. The anatomical complexity and intersubject variability of the human PPC are hardly comparable to the macaque PPC (this can be well appreciated in 2, 3) (see also Ono et al., 1990). We contend that this extreme anatomical complexity and variability make the classical approach to the analysis of neuroimaging data, based on intersubject averaging after warping individual brains onto a canonical template, particularly weak in the attempt to set apart relatively weak signal modulations in small subregions close to each other. Although the group analysis approach is extremely valuable for characterizing average population responses in tasks of interest, it can yield artifactual overlap of activation when there are two close but functionally distinct regions embedded in a highly variable anatomical structure. The combination of individual analysis and anatomical reconstruction allowed us to detect small but consistent and selective modulations of the BOLD signal in small parietal subregions.
Another important point is that we used arm-reaching movements, such as those performed in reaching tasks by monkeys (Snyder et al.,1997,2000; Batista et al., 1999), rather than finger-pointing movements, such as those used in most previous fMRI studies (Astafiev et al., 2003; Connolly et al., 2003, 2007; DeSouza et al., 2000; Fernandez-Ruiz et al., 2007; Medendorp et al., 2003, 2005; Simon et al., 2002; Tosoni et al., 2008; but see Beurze et al., 2007, 2009; Filimon et al., 2009; Levy et al., 2007; Stark & Zohary, 2008). Fully extending the arm to make physical contact with a target in peripersonal space requires more computations, recruits a larger number of muscles, involves several joints, lasts longer and involves more somatosensory feedback. Note that finger pointing lacks the transport component of the arm, which is fundamental in real-world reach-to-grasp movements; when preparing to point a finger to a target, one does not need to code the exact location and distance of the target, a particularly demanding job especially when the target is no longer under your view. Thus, the type of spatial coding during the preparatory delay is not the same for finger-pointing and arm-reaching movements.
Other regions
In the present study, we also show four additional parietal fields responsive to visual cues, although none of these regions was both modulated by the spatial position of the cue and showed sustained activity selective for either type of action. The two most posterior regions (LP and MP) did not show any significant sustained activity during the delay. Their signal-related activity could be the result of a visual or a spatially selective attentional modulation. Because, in our task, the visual target and the spotlight of attention are spatially coincident, we cannot decide in favor of any of these two effects. Area LP was located within the limits of the human area V3A/V7 (Tootell et al., 1997), and consistently showed stronger responses for contralateral cues, but with no preference for saccadic or reaching cues. This result closely resembles the second retinotopic map of contralateral space observed by Sereno et al. (2001) in correspondence with the dorsal area V3A, and is in line with the representation of the contralateral hemifield in the macaque (Van Essen & Zeki, 1978; Gattas et al., 1988) as well as in human area V3A (Tootell et al., 1997).
Area MP instead showed a significantly stronger response after reaching than saccadic cues. As discussed above, other studies have already described this region in the vicinity of the POS and its selectivity for reaching movements. From an anatomical point of view, area MP is located just anteriorly to the human V6 (Pitzalis et al., 2006a, 2010). We have reported preliminary evidence for a retinotopic trend in this region (Pitzalis et al., 2006b), which closely resembles that found in monkey area V6A (Galletti et al., 1996, 1999). Hence, the hypothesis could be advanced that MP represents the human homologue of monkey area V6A. Area MP shows a marginal effect of cue location (contralateral side slightly preferred). Accordingly, in monkey V6A, there is a complete representation of the contralateral hemifield, but also a large representation of the ipsilateral hemifield (Galletti et al., 1999). In addition, in monkey V6A, reach-related activity is more represented than saccade-related activity (Galletti et al., 2003). It seems reasonable to conceive, although it is not yet proved, that in monkey V6A the visual responses to stimuli that will be targets of reaching movements are more enhanced than the responses to stimuli that will be targets of saccades.
Area LV showed a significant sustained response during delays, with a higher transient and sustained activity during reaching trials, and no effect of cue location, either for the transient or sustained response component. This region may be involved in the preparation of reaching movements regardless of target location; alternatively, all directions of movement could be represented in each hemisphere. Note that the so-called intentional activity could reflect the reiterate activity running back and forth along cortico-cortical loops (e.g. parieto-frontal, parieto-temporal) during the delay period before arm movement, in turn reflecting the process of choosing and remembering the movement to be performed (Rizzolatti & Matelli, 2003). Culham and colleagues have described a region very close to our LV (reviewed in Culham & Valyear, 2006) that responds more during grasping than reaching movements, and is proposed as a homologue for monkey anterior intraparietal area, an area specialized to compute object properties in order to preshape the hand during grasping (Taira et al., 1990). It could be that LV and the anterior intraparietal area (according to Culham’s definition) refer to the same functional area. In fact, although our subjects reached the target without grasping, it has been suggested that the anterior intraparietal area also responds, although to a lesser degree, during reaching-without-grasp movements (for evidence in such a direction, see Culham, 2004).
Area LA may correspond to the region detected by Culham (2004) anteriorly to the presumptive anterior intraparietal area, during both finger movements and somatosensory finger stimulation in addition to grasping (see also Astafiev et al., 2003). A very similar activation was found by de Jong et al. (2001) in a finger-pointing task when finger selection was contrasted to target selection. Interestingly, in all of these cases the region showed a preferential activity in the left hemisphere.
Conclusion
In conclusion, we show that the simple presentation of a task-relevant visual cue yields activation in six different PPC regions. Although each of these human PPC areas may have been described and functionally characterized in previous neuroimaging studies, here they show unique functional profiles, when studied for the effects of three distinct variables: action specificity (saccades vs. reaching), spatial specificity (ipsilateral vs. contralateral) and type of response (transient vs. sustained). Broadly speaking, the different profiles of the six areas support the notion of a functional gradient within the PPC, with more posterior regions showing only transient responses, and more anterior regions showing more sustained activation. Furthermore, spatial specificity decreases, and action specificity increases, when moving from posterior to anterior regions. A similar gradient has recently been described in a grasping task, with the importance of the spatial location of the target gradually diminishing, and the importance of the side of the acting hand gradually increasing, when moving from posterior to anterior in the PPC (Stark & Zohary, 2008). This smooth gradient of selectivity, which may reflect different stages of transformation of visual signals into motor commands, does not, however, challenge the evidence for anatomically and functionally discrete subregions in the PPC.
Of particular interest are the regions at an intermediate level within this gradient, which are both action and spatially specific. In particular, area LD showed a sustained activity that was greater during saccadic than reaching trials, whereas the reverse was true for area MD. This fact, together with their stronger response for contralateral than ipsilateral cues, suggests that these regions provide topographically organized and anatomically segregated intentional signals for saccades and arm-reaching action, respectively. Their anatomical locations corroborate the hypothesis of a homology with monkey areas LIP and MIP, respectively. Although the relevant modulations of their sustained responses during the delay were quantitatively small, their presence has important theoretical implications for the notion that the human PPC subserves not only the deployment of spatial attention, but also the early phases of action plans towards the external space.
The presence of consistent and specific intentional signals in the human PPC does not necessarily imply that the PPC follows a strict effector-specific organization. Saccadic and arm-reaching movements not only involve different effectors, but also subserve different behavioral goals, i.e. exploring the environment and acting upon objects, and require different types of spatial transformations of visual information (Heed et al., 2011). The PPC could be organized in terms not of effectors, but of broader behavioral domains, or in terms of the relevant spatial reference frames (Galati et al., 2010). The recently reported substantial overlap of posterior parietal activation during planning of hand and foot, compared with eye, movements (Heed et al., 2011) supports either of these hypotheses.
Finally, it is important to remember that intentional signals are observed within regions that are, however, activated during both saccadic and reaching delays. Previous fMRI studies have similarly reported a large overlap of activations during the planning of eye and hand movements, and also in monkey, neither area LIP nor MIP responds exclusively to the preferred effector (Andersen & Cui, 2009). This overlap may reflect an automatic preparation of both actions in parallel, elicited immediately after the detection of the peripheral cue, with later selection signals biasing this activity in favor of the instructed action, and is compatible with a general account of parietal functioning in terms of competition between affordances (Cisek, 2007), with intentional signals reflecting biases for or evidence in favor of a specific spatially-directed action (Shadlen et al., 2008; Tosoni et al., 2008).
Acknowledgements
This work was supported by grants from the Italian Ministry of Health to the Santa Lucia Foundation and by Italian Ministry of Education, University and Research grants PRIN-2006055034 and PRIN-2008PBT985.
Abbreviations
-
- aIPS
-
- anterior intraparietal sulcus
-
- BOLD
-
- blood oxygen level-dependent
-
- EPI
-
- echo-planar imaging
-
- fMRI
-
- functional magnetic resonance imaging
-
- hIPS
-
- horizontal intraparietal sulcus
-
- IPS
-
- intraparietal sulcus
-
- LA
-
- lateral-anterior area
-
- LD
-
- lateral-dorsal area
-
- LIP
-
- lateral intraparietal area
-
- LP
-
- lateral-posterior area
-
- LV
-
- lateral-ventral area
-
- MD
-
- medial-dorsal area
-
- MIP
-
- medial intraparietal area
-
- MP
-
- medial-posterior area
-
- MR
-
- magnetic resonance
-
- pIPS
-
- posterior intraparietal sulcus
-
- POS
-
- parieto-occipital sulcus
-
- PPC
-
- posterior parietal cortex
-
- ROI
-
- region of interest
-
- SPL
-
- superior parietal lobule




