Loss of striatal cannabinoid CB1 receptor function in attention-deficit / hyperactivity disorder mice with point-mutation of the dopamine transporter
Abstract
Abnormal dopamine (DA) transmission in the striatum plays a pivotal role in attention-deficit/hyperactivity disorder (ADHD). As striatal DA signalling modulates the endocannabinoid system (ECS), the present study was aimed at investigating cannabinoid CB1 receptor (CB1R) function in a model of ADHD obtained by triple point-mutation in the dopamine transporter (DAT) gene in mice, making them insensitive to cocaine [DAT cocaine-insensitive (DAT-CI) mice]. DAT-CI mice had a marked hyperactive phenotype, and neurophysiological recordings revealed that the sensitivity of CB1Rs controlling GABA-mediated synaptic currents [CB1Rs(GABA)] in the striatum was completely lost. In contrast, CB1Rs modulating glutamate transmission [CB1Rs(Glu)], and GABAB receptors were not affected in this model of ADHD. In DAT-CI mice, the blockade of CB1R(GABA) function was complete even after cocaine or environmental manipulations activating the endogenous DA-dependent reward system, which are known to sensitize these receptors in control animals. Conversely, the hedonic property of sucrose was intact in DAT-CI mice, indicating normal sweet perception in these animals. Our results point to CB1Rs as novel molecular players in ADHD, and suggest that therapeutic strategies aimed at interfering with the ECS might prove effective in this disorder.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a commonly diagnosed neuropsychiatric disorder in children with inattention, impulsivity, and hyperactivity. A long series of studies point to a dysfunctional dopamine (DA) system as being the basis of ADHD, and specific allelic variants of the genes encoding for the DA transporter (DAT) and for the D2 class of DA receptors (D2Rs and D4Rs) have, in fact, been associated with this disorder (Comings et al., 1996; Cook et al., 1995; Durston et al., 2005; Faraone et al., 2005; Gizer et al., 2008, 2009; Thapar et al., 2005; Todd et al., 2005; Waldman et al., 1998). Abnormal activity of D2Rs occurs in the striatum of ADHD patients (Ilgin et al., 2001; Rosa Neto et al., 2002), and in mouse models of ADHD with targeted deletion of the DAT gene (Gainetdinov & Caron, 2003; Madras et al., 2005). As striatal D2Rs play a major role in motor control (Boulay et al., 1999; Usiello et al., 2000), it is conceivable that abnormal D2R-associated signalling mediates some of the symptoms of ADHD.
D2Rs interact extensively with the endocannabinoid system (ECS) in the striatum. Stimulation of these receptors, in fact, increases striatal levels of endocannabinoids (Beltramo et al., 2000; Centonze et al., 2004; Giuffrida et al., 1999), and sensitizes cannabinoid CB1 receptors (CB1Rs) (De Chiara et al., 2010b). Furthermore, endocannabinoids act as downstream effectors of D2R signalling in the control of striatal synaptic transmission (Gerdeman et al., 2002; Yin & Lovinger, 2006). It is not surprising, therefore, that the ECS has been implicated in a number of DA-related disorders, such as schizophrenia (De Marchi et al., 2003; Emrich et al., 1997; Giuffrida et al., 2004), Parkinson’s disease (Gubellini et al., 2002; Pisani et al., 2005), drug addiction (De Vries et al., 2001; Maldonado et al., 2006; Centonze et al., 2007b), and ADHD (Centonze et al., 2009).
In the present study, we investigated signalling through CB1Rs at both GABA (CB1Rs controlling GABA-mediated synaptic currents [CB1R(GABA)]) and glutamate (CB1Rs modulating glutamate transmission [CB1R(Glu)]) striatal synapses in a model of ADHD, obtained following triple point-mutation in the cocaine-binding site of DAT in mice [DAT cocaine-insensitive (DAT-CI) mice] (Chen et al., 2006; Napolitano et al., 2010). When compared with DAT-knockout mice, DAT-CI mice more closely mimic human ADHD, in which only DAT polymorphisms have been described (Thapar et al., 2005; Gizer et al., 2009). As in human ADHD, these mice display a hyperactive phenotype that can be reversed by administration of psychostimulants (Chen et al., 2006; Tilley & Gu, 2008a,b), and show defective striatal DA transmission (Napolitano et al., 2010).
Materials and methods
DAT-CI male mice (10–12 weeks old) were generated by homologous recombination in 129/SvJ embryonic stem cells as described previously (Chen et al., 2006). The mutant mice have been backcrossed to C57BL/6J mice for 10 or more generations, and therefore they are generally considered to be on the C57BL/6J background (Tilley & Gu, 2008a). DAT-CI mice were used along with their age-matched and sex-matched wild-type (WT) littermates for all experiments. All animals were housed, four per cage with food and water ad libitum, on a 12-h light/dark cycle with lights on at 06:00 h and a controlled (22–23 °C) temperature. All efforts were made to minimize animal suffering and to reduce their number, in accordance with the European Community Council Directive of 24 November 1986 (86/609/EEC).
Motor activity
For measurement of motor activity in DAT-CI and control mice, the open field test (OFT) was used. This protocol was performed as previously reported (De Chiara et al., 2010b). Briefly, mice were placed in the centre of a clear Plexiglas arena (25 × 35 × 20 cm), in which they were allowed to explore for 60 min. Overhead incandescent light bulbs provided 600-lux illumination inside the test chamber. Total distance was recorded with a video tracking system (Videotrack; Viewpoint S.A., Champagne au Mont d’Or, France).
Electrophysiology
Animals were killed by cervical dislocation under halothane anaesthesia, and corticostriatal coronal slices (200 μm) were prepared from tissue blocks of the brain with the use of a vibratome (De Chiara et al., 2010b). Single slices were then transferred to a recording chamber and submerged in continuously flowing artificial cerebrospinal fluid (32 °C, 2–3 mL/min) gassed with 95% O2/5% CO2. The composition of the control solution was: 126 mm NaCl, 2.5 mm KCl, 1.2 mm MgCl2, 1.2 mm NaH2PO4, 2.4 mm CaCl2, 11 mm glucose, and 25 mm NaHCO3.
The striatum could be readily identified under low-power magnification, whereas individual neurons were visualized in situ with a differential interference contrast (Nomarski) optical system. This employed an Olympus BX50WI (Olympus, Tokyo, Japan) or a Zeiss Axioskop (Zeiss, Oberkochen, Germany) upright microscope with a × 40 water immersion objective combined with an infrared filter, a monochrome CCD camera (COHU 4912), and a PC-compatible system for analysis of images and contrast enhancement (winvision 2000; Delta Sistemi, Rome, Italy).
Whole-cell patch clamp recordings were made with borosilicate glass pipettes (outside diameter, 1.8 mm; 2–4 MΩ), in voltage-clamp mode, at a holding potential of −80 mV. Recording pipettes were advanced towards individual striatal cells in the slice under positive pressure and, on contact, tight Giga Ohm seals were made by applying negative pressure. The membrane patch was then ruptured by suction, and membrane current and potential were monitored with a Multiclamp 700A/B patch clamp amplifier (Molecular Devices, Foster City, CA, USA). Whole-cell access resistances measured in voltage-clamp mode were in the range of 5–20 MΩ. For detection of evoked inhibitory postsynaptic currents (eIPSCs), spontaneous inhibitory postsynaptic currents (sIPSCs) or miniature GABAA-mediated inhibitory postsynaptic currents (mIPSCs), the intraelectrode solution had the following composition: 110 mm CsCl, K+-gluconate (30 mM) (Centonze et al., 2009), 1.1 mm EGTA, 10 mm Hepes, 0.1 mm CaCl2, 4 mm Mg-ATP, and 0.3 mm Na-GTP. MK-801 (30 μm) and 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μm) were added to the external solution to block, respectively, N-methyl-d-aspartate and non-N-methyl-d-aspartate glutamate receptors. For kinetic analysis of sIPSCs, events with peak amplitudes between 10 and 50 pA were grouped, aligned by half-rise time, normalized by peak amplitude, and averaged to obtain rise times, decay times, and half-widths.
Only data from putative medium spiny projection neurons were included in the present study. These neuronal subtypes represent over 95% of the entire population of striatal neurons, and were identified by their morphological and electrophysiological properties. In fact, whereas putative cholinergic interneurons were easily recognized in striatal slices because of their large somata (40–60 μm in diameter as compared with 25–35 μm for projection neurons), we identified medium spiny projection neurons from fast-spiking or low-threshold spike GABAergic interneurons by evaluating their firing response (typically tonic, with little or no adaptation) to the injection of depolarizing current immediately after rupture of the GΩ seal, and before the manifestation of the channel-blocking effects of intraelectrode cesium. In some cases, the membrane response to dihydroxyphenylglycine (DHPG) was evaluated to identify and discard GABAergic interneurons, because interneurons but not projection cells are depolarized by a brief pulse of this agonist (30 mM) (Centonze et al., 2007a).
For the study of evoked excitatory postsynaptic currents (eEPSCs) and spontaneous glutamate-mediated excitatory postsynaptic currents (sEPSCs), the recording pipettes were filled with an internal solution composed of 125 mm K+-gluconate, 10 mm NaCl, 1.0 mm CaCl2, 2.0 mm MgCl2, 0.5 mm BAPTA, 19 mm Hepes, 0.3 mm, and 1.0 mm Mg-ATP, adjusted to pH 7.3 with KOH. Bicuculline (10 μm) was added to the perfusing solution to block GABAA-mediated transmission. sIPSCs and sEPSCs were stored by using p-clamp 9 (Molecular Devices, Sunnyvale, CA, USA) and analysed off-line on a personal computer with Mini Analysis 5.1 (Synaptosoft, Leonia, NJ, USA). The detection threshold of excitatory and inhibitory events was set at twice the baseline noise. The fact that no false events would be identified was confirmed by visual inspection for each experiment. Off-line analysis was performed on sIPSCs recorded during fixed time epochs (2–3 min, 5–10 samplings), sampled every 2–3 min. Only cells that exhibited stable frequencies in control samplings (< 20% changes during the control samplings) were taken into account.
Evoked synaptic events (eEPSCs and eIPSCs) were elicited at 0.1 Hz by the use of bipolar electrodes located either in the white matter between the cortex and the striatum (to activate corticostriatal glutamatergic fibres) or within the striatum (to stimulate intrastriatal GABAergic terminals). p-clamp 9 (Molecular Devices, Sunnyvale, CA, USA) was used to store the data. In distinct neurons, eEPSCs or eIPSCs of similar amplitude were obtained with variable intensities of stimulation, mainly depending on the distance between the stimulating and recording sites. eEPSCs normally ranged between 50 and 400 pA, and eIPSCs ranged between 30 and 200 pA (Rossi et al., 2009; De Chiara et al., 2010b).
Drugs applied on brain slices were first dissolved in dimethylsulfoxide (AM251 and HU210) or in water (bicuculline, CNQX, and MK-801), and then in the bathing artificial cerebrospinal fluid to the desired final concentration. The concentrations of the drugs were chosen according to previous in vitro studies on corticostriatal brain slices (Rossi et al., 2008, 2009; De Chiara et al., 2010a,b) and were as follows: AM251, 10 μm; CNQX, 10 μm; HU210, 1 μm; 3,5-DHPG, 50 μm; MK-801, 30 μm; tetrodotoxin, 1 μm (all from Tocris, Bristol, UK); and bicuculline, 10 μm (Sigma-RBI, St Louis, MO, USA).
In vivo treatments
In some experiments, cocaine (15 mg/kg, in 200 μL of saline) was injected intraperitoneally for 5 consecutive days in control (n = 6) and DAT-CI (n = 6) mice. Cocaine-treated mice were killed for the electrophysiological evaluations 24 h after the last intraperitoneal injection (De Chiara et al., 2010a).
To study the effects of natural reward on CB1R sensitivity, some mice were allowed to consume ad libitum a drinking fluid containing sucrose (3% in tap water) for 7 days (n = 6 for DAT mice; n = 6 for control mice) before the electrophysiological experiments (De Chiara et al., 2010b).
Sucrose preference
The test was performed as previously described (Sonnier et al., 2007; De Chiara et al., 2010b). DAT-CI and control mice (n = 8 per sucrose concentration), placed in individual cages 2 weeks before the test, were subjected to a water vs. sucrose two-bottle preference test. Increasing sucrose solutions were used (0.75 and 3%). Each concentration was presented in consecutive 2-day blocks. The solutions were available for 23 h/day. During the remaining 1 h, the volumes consumed were measured and the bottles were refilled. The left–right positions of the sucrose and water were alternated for each concentration (to control for the preference of some mice for a particular side). Total intakes (in millilitres) were averaged.
Statistical analysis
The significance level of the results was established at P < 0.05. Data are presented as the mean ± standard error of the mean. Throughout the text, n refers to the number of cells, unless otherwise specified. For the electrophysiological experiments, statistical analysis between two groups was performed with a paired or unpaired Student’s t-test or Wilcoxon’s test. Multiple comparisons were analysed by one-way anova followed by Tukey HSD. Five to six mice were employed for a single electrophysiological experiment. Two to six neurons per animal were recorded.
In the OFT, horizontal distances travelled were analysed by two-way anova with repeated measures or by one-way anova.
In the sucrose preference test, preference for sucrose (%) was calculated as millilitres of sucrose solution drunk over the total drink intake [(sucrose/sucrose + water) × 100]. Significance was calculated by two-way anova, followed by Fisher’s post hoc analysis.
Results
Motor activity in DAT-CI mice
In order to evaluate locomotor activity in DAT-CI mice, we analysed mutant (n = 8) and WT mice (n = 8) over a 60-min test session in an OFT. Two-way anova with repeated measures indicated a significant genotype effect, with DAT-CI mice displaying higher horizontal activity than controls (F1,70 = 17.754, P = 0.0009), but no significant treatment × time interaction (F5,70 = 8.828, P = 1.1960). Analysis of total distance confirmed the basal horizontal hyperactivity of DAT-CI mice (one-way anova: F1,14 = 17.754, P = 0.0009) (Fig. 1).
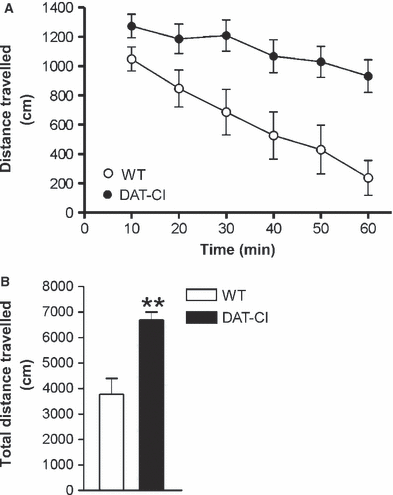
Locomotor activity in WT and DAT-CI mice. (A) Time course of locomotor activity in WT and DAT-CI mice with 10-min intervals over a 60-min test. (B) Total distance travelled in 60 min for mutants and WT mice. **P < 0.01 vs. WT mice.
Sensitivity of CB1Rs(GABA) in DAT-CI mice
Both the frequency (P > 0.05) and the amplitude (P > 0.05) of striatal sIPSCs recorded from DAT-CI mice were indistinguishable from those recorded in the respective controls (n = 5 for both groups) (Fig. 2A). As previously reported (Rossi et al., 2009; De Chiara et al., 2010a,b), application of the CB1R agonist HU210 (10 min) significantly reduced the frequency of striatal sIPSCs in controls (n = 6, P < 0.01 as compared with pre-drug values). The selective antagonist of CB1Rs AM251 prevented this effect (n = 4, P > 0.05 as compared with pre-drug values). In striatal neurons from DAT-CI mice, HU210 effects were abolished (n = 8, P > 0.05), indicating loss of sensitivity of CB1Rs(GABA) in this model of ADHD (Figs. 2B and C).
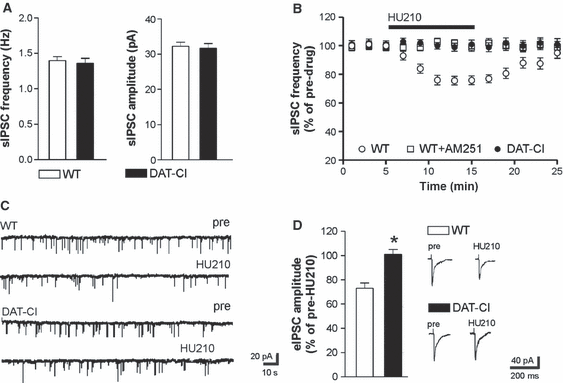
Sensitivity of CB1Rs(GABA) in DAT-CI mice. (A) Both the frequency and the amplitude of striatal sIPSCs recorded from DAT-CI mice were indistinguishable from those recorded from WT mice. (B) HU210, an agonist of CB1Rs, reduced sIPSC frequency in control mice; this effect was fully prevented by preincubation with the CB1R antagonist AM251. The depressant effect of HU210 was completely abolished in neurons from DAT-CI mice. (C) The electrophysiological traces are examples of sIPSCs recorded from striatal neurons of WT and DAT-CI mice. (D) Stimulation of CB1Rs with HU210 also failed to alter eIPSCs, whereas it caused the expected inhibition in the WT counterparts. Traces on the right are examples of eIPSCs from WT (upper traces) and DAT-CI (lower traces) striatal neurons before and during the application (10 min) of HU210.*P < 0.05 vs. WT mice.
Because some of the GABAergic inputs causing sIPSCs may be different from those activated by electrical stimulation, we also tested the effects of CB1R stimulation on eIPSCs recorded from DAT-CI to WT mice. Also in these experiments, HU210 (10 min) failed to affect eIPSCs in DAT-CI mice (n = 5, P > 0.05), whereas it caused a depression of eIPSC amplitude in controls (n = 5, P < 0.05), as previously reported (Rossi et al., 2009; De Chiara et al., 2010b) (Fig. 2D).
GABAB receptors and CB1Rs(Glu) are unaltered in DAT-CI mice
Striatal GABA synapses are modulated by many receptor subtypes inhibiting transmitter release. Thus, we investigated the effects of the GABAB receptor agonist baclofen on sIPSC frequency in DAT-CI mice, to determine whether receptor subtypes other than CB1Rs(GABA) were dysfunctional in this ADHD model. Application of the GABAB receptor agonist baclofen (n = 7) significantly (P < 0.01) reduced striatal sIPSC frequency in controls (pre-baclofen sIPSC frequency in controls: 1.65 ± 0.30 Hz) (De Chiara et al., 2010a,b; Rossi et al., 2008). In slices from DAT-CI mice, the inhibitory effect of baclofen was similar to that seen in controls (pre-baclofen sIPSC frequency in DAT-CI slices: 1.58 ± 0.50 Hz) (n = 8, P > 0.05 as compared with controls) (Fig. 3A).
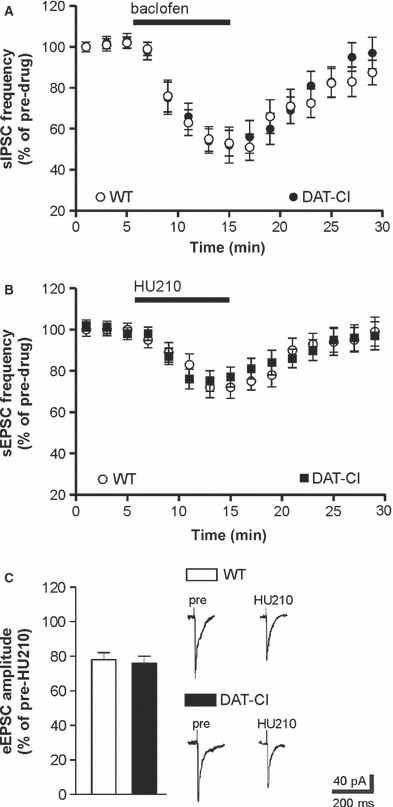
GABAB receptors and CB1Rs(Glu) are unaltered in DAT-CI mice. (A) The depressant effect of the GABAB receptor agonist baclofen on sIPSC frequency was similar in WT and DAT-CI mice. (B and C) HU210-induced reduction of sEPSC frequency or eEPSC amplitude were comparable in WT and DAT-CI mice. Traces on the right of C are examples of eEPSCs recorded from WT (upper traces) and DAT-CI (lower traces) striatal neurons before and during the application (10 min) of HU210.
CB1Rs also control glutamate transmission in the striatum [CB1Rs(Glu)] by a presynaptic mechanism. In agreement with previous findings (De Chiara et al., 2010a,b; Rossi et al., 2008, 2009), HU210 inhibited glutamate-mediated sEPSC frequency and eEPSC amplitude in controls (pre-HU210 sEPSC frequency in controls: 2.40 ± 0.40 Hz) (n = 7, P < 0.01 for both experimental conditions). In DAT-CI slices, the effects of HU210 on sEPSCs and eEPSCs were indistinguishable from those in controls (pre-HU210 sEPSC frequency in DAT-CI: 2.50 ± 0.20 Hz) (n = 6, P > 0.05 as compared with HU210 in controls for both sEPSCs and eEPSCs), indicating that experimental ADHD selectively alters the CB1Rs regulating GABA synapses (Figs. 3B and C).
Effects of DHPG on striatal mIPSCs
Activation of type 5 metabotropic glutamate receptors (mGluRs) by DHPG mobilizes the endocannabinoid 2-arachidonoylglycerol (2-AG) in the striatum (Jung et al., 2005; Maccarrone et al., 2008, 2009), and this effect results in the inhibition of GABA-mediated mIPSCs or eIPSCs through the stimulation of CB1Rs (Centonze et al., 2007a; Maccarrone et al., 2008, 2009; Rossi et al., 2009; De Chiara et al., 2010b). Thus, to determine whether triple point-mutation of DAT altered the sensitivity of GABA synapses not only to the synthetic cannabinoid HU210 but also to endogenous cannabinoids, we measured the effects of DHPG on striatal mIPSCs recorded from WT to DAT-CI mice. Application of DHPG (10 min, n = 10) significantly inhibited mIPSC frequency in control mice (P < 0.05) but not in DAT-CI mice (n = 7, P > 0.05). It is of note that preincubation of control slices with AM251 fully prevented the DHPG effects on mIPSCs (n = 5, P > 0.05), confirming that they were mediated by the mobilization of endocannabinoids acting on CB1Rs (Fig. 4).
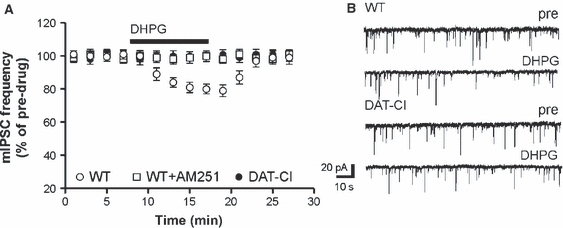
Effects of DHPG on striatal mIPSCs in WT and DAT-CI mice. (A) The reduction of mIPSC frequency caused by the application of the group I mGluR agonist DHPG was inhibited in slices from DAT-CI mice. Preincubation with the CB1R antagonist AM251 prevented the depressant action of DHPG. (B) The traces are examples of voltage-clamp recordings before and during the application of DHPG in WT and DAT-CI mice.
Effects of cocaine and sucrose on CB1Rs(GABA) in DAT-CI mice
Cocaine consumption (Di Chiara & Imperato, 1988; Pontieri et al., 1996) and palatable food such as sucrose activate the DA-dependent reward pathway (Hajnal et al., 2004; Mark et al., 1991), and sensitize striatal CB1Rs(GABA) through D2Rs (De Chiara et al., 2010a,b). Thus, we wanted to determine whether, in ADHD mice, the abnormal activity of DAT could affect the brain response to pharmacological and natural rewards by restoring the sensitivity of striatal CB1Rs(GABA) to HU210. As previously reported (De Chiara et al., 2010a,b), both cocaine (n = 9) and sucrose (3% in the bottle containing the drinking fluid) (n = 8) potentiated the effects of HU210 on sIPSCs in controls (P < 0.05 as compared with untreated controls). Conversely, in DAT-CI mice, cocaine (n = 6) or sucrose (n = 9) was unable to alter the HU210 effects on sIPSCs (P > 0.05 as compared with untreated DAT-CI mice) (Fig. 5).
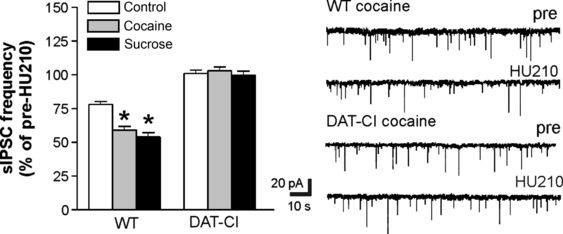
Effects of cocaine and sucrose on CB1Rs(GABA) in DAT-CI mice. The graph shows that cocaine and sucrose normally potentiate the effects of HU210 on striatal sIPSCs, but were not able to rescue the effect of HU210 on sIPSC frequency in DAT-CI mice. The electrophysiological traces on the right are examples of sIPSCs recorded from striatal neurons of cocaine-exposed WT and DAT-CI mice. *P < 0.05 vs. control.
DAT-CI mice are sensitive to sucrose drinking
The lack of effect of cocaine on CB1Rs(GABA) in DAT-CI mice is compatible with the insensitivity of DAT to this psychostimulant (Chen et al., 2006; Napolitano et al., 2010; Tilley & Gu, 2008a,b). To determine whether DAT-CI mice were also insensitive to the hedonic properties of sucrose, we performed a two-bottle preference test in WT mice (n = 6) and DAT-CI mice (n = 6). The behavioural results demonstrated that, at both concentrations tested (0.75 and 3%), sucrose induced a robust hedonic response in WT and DAT-CI mice. Post hoc comparisons showed a similar pleasurable effect of both doses on both groups of mice (water vs. 0.75%, P < 0.0001; water vs. 3%, P < 0.0001; 0.75% vs. 3% sucrose, P > 0.1) (Fig. 6).
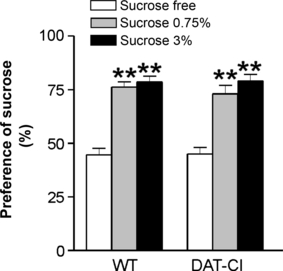
Hedonic effect of sucrose in DAT-CI mice. The graph indicates that 0.75 and 3% sucrose concentrations exert similar preference responses in WT and DAT-CI mice. **P < 0.01 vs. sucrose-free solution.
These data rule out the loss of the pleasurable property of sucrose as the basis of absent CB1R(GABA) sensitization in ADHD mice.
Discussion
The possible involvement of the ECS in ADHD has been scarcely explored. A previous study in ADHD patients demonstrated reduced activity of the anandamide (AEA)-degrading enzyme fatty acid amide hydrolase (Centonze et al., 2009). The resulting increased AEA levels in these patients are possibly implicated in ADHD pathophysiology, because AEA reduces DAT activity in neurons (Chen et al., 2003). Another investigation pointed to altered activity of CB1Rs in ADHD, by demonstrating the association between ADHD and a specific polymorphism of the CB1R gene (Lu et al., 2008).
Here, in a mouse model of the disease obtained by triple point-mutation of the DAT gene, we found selective loss of the sensitivity of CB1Rs controlling GABA transmission in the striatum, suggestive of a complex alteration of the ECS in ADHD. In the striatum, in fact, CB1Rs(Glu) are mostly activated by AEA, because enhancement of AEA tone selectively inhibits sEPSC frequency (Rossi et al., 2010), whereas the other endocannabinoid, 2-AG, is the preferential endogenous agonist of CB1Rs(GABA), because stimulation of 2-AG synthesis with DHPG (Maccarrone et al., 2008, 2009) or following acetylcholine M1 receptor activation (Musella et al., 2010) reduces GABAergic but not glutamatergic transmission. On the basis of the results of the present investigation, and the notion that AEA tone increases in ADHD (Centonze et al., 2009), it can be postulated that AEA-mediated inhibition of striatal glutamate release is enhanced in ADHD, whereas 2-AG-dependent inhibition of GABA synapses is lost (Supporting Information Fig. S1). The combination of these alterations probably causes the inhibition of striatal neuron activity, which is largely dependent on the coordinated activity of glutamate and GABA synaptic inputs (Fisone et al., 2007; Tepper et al., 2007).
Notably, although we did not detect significant alterations in basal glutamate transmission in our model, defective glutamate release has been found in the striatum of a rat model of ADHD (Russell, 2003), and hypoactivity of frontostriatal circuits has been described in subjects with ADHD (Casey et al., 2007; Lou et al., 1989; Rubia et al., 1999; Zametkin et al., 1993). Finally, reduced volume of the caudate nucleus has been found in patients with ADHD (Castellanos et al., 1996, 2002), and an association between caudate volume and severity of hyperactivity symptoms has also been reported (Castellanos et al., 2003; Durston et al., 2005; Schrimsher et al., 2002). We also found that DAT mutation abolished the sensitivity of GABAergic synapses not only to the synthetic CB1R agonist HU210, but also to endocannabinoids (probably 2-AG) (Maccarrone et al., 2008) mobilized in response to the stimulation of type 5 mGluRs. This latter finding suggests that the alteration of CB1R seen in DAT-CI mice may have relevant synaptic consequences during the physiological activity of the striatum, which is mainly driven by glutamate inputs originating from the cortex and the thalamus (Stern et al., 1998).
Striatal CB1Rs have been implicated not only in motor control (Carriba et al., 2007; Martin et al., 2008), but also in emotional processes (Rossi et al., 2009, 2010), suggesting that the loss of CB1R(GABA) function seen in DAT-CI mice might play a role in the hyperactive phenotype of experimental and human ADHD, and also in the anxiety and depression often accompanying ADHD (Cormier, 2008; Klassen et al., 2010; Young, 2008). Loss of CB1R(GABA) function, in fact, occurs concomitantly with anxious–depressive symptoms after stressful events (Rossi et al., 2010), and enhancement of CB1R(GABA) signalling in the striatum mediates the anti-anxiety effects of rewarding experiences (De Chiara et al., 2010a,b). In this respect, sucrose intake has strong rewarding properties in mice, sensitizes CB1Rs(GABA), and blunts the emotional and synaptic consequences of stress (De Chiara et al., 2010b; Rossi et al., 2008). The observation that, despite preserved sweet sensation and preference, striatal CB1Rs(GABA) do not become sensitized in response to sucrose drinking in DAT-CI mice indicates defective mood-enhancing effects of rewarding life experiences in ADHD, probably contributing to the emotional disturbances in these subjects.
DAT-CI mice exhibit severe alterations of DA signalling in the striatum (Napolitano et al., 2010), and striatal CB1R(GABA) sensitivity is under the control of the DA system. Activation of the DA system with cocaine, in fact, enhances the sensitivity of CB1Rs(GABA) to HU210 (Centonze et al., 2007b; De Chiara et al., 2010a), whereas blockade of D2Rs with haloperidol completely inactivates CB1Rs(GABA). Interestingly, CB1R(Glu) sensitivity is unaffected by both cocaine and haloperidol, as we have observed in DAT-CI mice. Together, these observations suggest dysfunctional D2R–CB1R(GABA) coupling in ADHD mice, providing a further piece of evidence supporting striatal DA signalling defects in ADHD. However, D2R-mediated control over the striatal cAMP–protein kinase A–DARPP32 molecular pathway was intact in DAT-CI mice, as was D2R-dependent phosphorylation control of the striatal glutamate receptor one subunit (Napolitano et al., 2010). On the other hand, D2R-mediated autoinhibition of DA neurons was significantly reduced in DAT-CI mice (Napolitano et al., 2010), indicating that only subtle alterations of D2R function take place in this ADHD model. Notably, D2R function is dramatically altered in DAT-knockout mice, which are considered to constitute an extreme model of ADHD (Jones et al., 1999).
Brain-derived neurotrophic factor (BDNF) has been proposed as a critical molecule linking D2R to CB1R(GABA) function in the striatum (De Chiara et al., 2010a). Blockade of D2Rs, in fact, is not able to inhibit CB1Rs(GABA) in mice with reduced brain levels of BDNF, whereas haloperidol increases striatal BDNF levels in control mice, and causes the inhibition of CB1Rs(GABA) via a BDNF-dependent and tyrosine kinase-dependent mechanism. Furthermore, stimulation of D2Rs both reduced striatal BDNF levels and increased CB1R(GABA) activity (De Chiara et al., 2010a). The loss of function of CB1Rs(GABA) in ADHD mice is therefore compatible with enhanced BDNF activity in these mice, and is in tune with the observation that BDNF plasma concentrations are higher in untreated ADHD subjects than in controls (Shim et al., 2008). Furthermore, although not confirmed in a recent meta-analysis (Sánchez-Mora et al., 2010), a moderate association between the valine allele (which favours BDNF maturation and activity) of the Val66Met polymorphism of the BDNF gene and ADHD has been found (Gadow et al., 2009; Kent et al., 2005). At the present stage, however, the hypothesis that BDNF plays a role in ADHD pathophysiology by modulating CB1Rs is merely speculative, and needs to be addressed in future studies.
In conclusion, the present investigation points to CB1Rs as novel molecular players in ADHD, and suggests that therapeutic strategies aimed at interfering with the ECS might prove effective in this disorder.
Acknowledgements
This investigation was supported by grants from the Italian Ministero dell’Università e della Ricerca and from the Italian Ministero della Salute to D. Centonze. A. Usiello represents the Mariano Scippacercola Foundation.
Abbreviations
-
- ADHD
-
- attention-deficit/hyperactivity disorder
-
- AEA
-
- anandamide
-
- 2-AG
-
- 2-arachidonoylglycerol
-
- BDNF
-
- brain-derived neurotrophic factor
-
- CB1R
-
- cannabinoid CB1 receptor
-
- CB1R(GABA)
-
- cannabinoid CB1 receptor controlling GABA-mediated synaptic currents
-
- CB1R(Glu)
-
- cannabinoid CB1 receptor modulating glutamate transmission
-
- CNQX
-
- 6-cyano-7-nitroquinoxaline-2,3-dione
-
- DA
-
- dopamine
-
- DAT
-
- dopamine transporter
-
- DAT-CI
-
- dopamine transporter cocaine-insensitive
-
- DHPG
-
- dihydroxyphenylglycine
-
- D2R
-
- dopamine D2 receptor
-
- ECS
-
- endocannabinoid system
-
- eEPSC
-
- evoked excitatory postsynaptic current
-
- eIPSC
-
- evoked inhibitory postsynaptic current
-
- mGluR
-
- metabotropic glutamate receptor
-
- mIPSC
-
- miniature GABAA-mediated inhibitory postsynaptic current
-
- OFT
-
- open field test
-
- sEPSC
-
- spontaneous glutamate-mediated excitatory postsynaptic current
-
- sIPSC
-
- spontaneous inhibitory postsynaptic current
-
- WT
-
- wild-type