Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment
Abstract
Major depressive disorder is a chronic disabling disease, often triggered and exacerbated by stressors of a social nature. Hippocampal volume reductions have been reported in depressed patients. In support of the neurogenesis theory of depression, in several stress-based animal models of depression, adult hippocampal neurogenesis was reduced and subsequently rescued by parallel antidepressant treatment. Here, we investigated whether repeated social defeat and subsequent individual housing for 3 months induces long-lasting changes in adult hippocampal neurogenesis in rats, and whether these can be normalized by late antidepressant treatment, as would match human depression. Neurogenesis was analysed by stereological quantification of the number of immature doublecortin (DCX)-immunopositive cells, in particular young (class I) and more mature (class II) DCX+ cells, to distinguish differential effects of stress or drug treatment on these subpopulations. Using this social defeat paradigm, the total DCX+ cell number was significantly reduced. This was most profound for older (class II) DCX+ cells with long apical dendrites, whereas younger, class I cells remained unaffected. Treatment with the broad-acting tricyclic antidepressant imipramine, only during the last 3 weeks of the 3-month period after social defeat, completely restored the reduction in neurogenesis by increasing both class I and II DCX+ cell populations. We conclude that despite the lack of elevated corticosterone plasma levels, neurogenesis is affected in a lasting manner by a decline in a distinct neuronal population of more mature newborn cells. Thus, the neurogenic deficit induced by this social defeat paradigm is long-lasting, but can still be normalized by late imipramine treatment.
Introduction
Exposure to stress forms an important risk factor for development of human psychopathologies, such as major depressive disorder (Kessler, 1997; Kendler et al., 2001). In particular, chronic forms of stress, often psychosocial in nature, may predict precipitation of depression (McGonagle & Kessler, 1990). As patients who underwent a first episode are at increased risk to develop recurrent or chronic depression, interest has been raised into the underlying mechanisms that determine particularly longitudinal aspects of the disorder and its maintenance (Solomon et al., 2000).
Of the many brain regions affected in depression, the hippocampus is well known for its role in cognition and stress sensitivity. Volumetric studies have repeatedly found reductions in hippocampal volume in patients suffering from major depression, paralleled by alterations in various neuropsychological and cognitive measures (Sheline et al., 1999; McKinnon et al., 2009). In preclinical models, stress exposure in rodents causes mild volume reductions of the hippocampus as a whole, reduces dendritic complexity of neurons in the CA3 subregion and impairs neurogenesis in the dentate gyrus (DG; Gould et al., 2000; Czeh & Lucassen, 2007). Despite the absence of causal evidence that changes in adult DG neurogenesis are critical to the aetiology of major depression, impaired hippocampal plasticity likely contributes to the cognitive symptoms of depression, as well as clinically effective antidepressant treatment (Sahay & Hen, 2007; Lucassen et al., 2010a,b). Indeed, neurogenesis is affected by many factors, among which treatment with antidepressant drugs – an effect that is age-dependent (Navailles et al., 2008; Couillard-Despres et al., 2009), and is found both in stressed and naive animals (Malberg et al., 2000; Airan et al., 2007; Czeh et al., 2007; Mineur et al., 2007; Oomen et al., 2007).
Most studies examining the effect of stress as a model for depression have measured neurogenesis shortly after exposure to either acute or chronic stressors that notably were often of a physical nature, like restraint (Sahay & Hen, 2007; Lucassen et al., 2010a). Most stressors relevant for depression, however, are chronic and psychosocial in nature (McGonagle & Kessler, 1990), and depression may develop long after the initial stress exposure. Also, the effects of antidepressant drugs have been evaluated, but when used in conjunction with a stress model, they have been administered during or parallel to, and not after, an extended period after stress exposure (Czeh et al., 2007; Kong et al., 2009; Lucassen et al., 2010a). Therefore, we questioned: (i) whether stress induced by social defeat has long-term effects on neurogenesis or corticosterone levels; and (ii) whether neurogenesis can still be normalized by antidepressant treatment starting at a late stage, when depressive-like symptoms are already manifest.
To address this, adult rats were subjected to a long-term social defeat paradigm, consisting of exposure to repeated severe social defeat stress followed by subsequent individual housing for 3 months. This type of paradigm models mainly the maintenance phase of depression, as it results in increases in stress responsivity as well as decreases in social interaction and sensitivity to reward anticipation over time (Von Frijtag et al., 2000, 2002; Buwalda et al., 2005). Social defeat stress followed by subsequent individual housing for 3 months leads to impaired hippocampal long-term potentiation, and this could be restored by late antidepressant treatment (Von Frijtag et al., 2001). In view of the persistent changes in behaviour and hippocampal function in this model, we investigated whether neurogenesis in the DG was affected in the long term. As read-out we quantified the number of doublecortin (DCX)-immunopositive (DCX+) cells. DCX is a microtubule-associated protein selectively expressed in young, immature neurons from approximately 4 to 14 days after birth of a newborn cell (Brown et al., 2003; Filippov et al., 2003). During this period, the temporal course of dendrite maturation can be used to morphologically distinguish younger (class I) and older (class II) DCX-positive cells (Plumpe et al., 2006; Oomen et al., 2010). We used this classification to distinguish between differential effects of our social defeat paradigm or drug treatment on these DCX+ subpopulations.
Materials and methods
Animals
Male Wistar rats (Harlan, Horst, the Netherlands), 8–9 weeks old, weighing 180–200 g at the time of arrival, were initially socially housed (two per cage) in Makrolon class IV cages (Tecniplast, Milan, Italy). Long–Evans male rats (Harlan, UK), weighing 300–350 g were used as residents for social defeat (Von Frijtag et al., 2002). These animals were pair-housed with age-matched sterilized females in plastic cages (63 × 25 × 33 cm) located in a separate room. All animals were housed in a temperature-controlled room (21 ± 1 °C) under regulated lighting conditions (lights on at 19:00 h and off at 07:00 h). Food and water were available ad libitum. All experimental manipulations were conducted during the dark phase (activity period) under a dim red light. The Animal Users Care Committee of the VU University Amsterdam approved all experiments.
Experimental design and treatment
Wistar rats (age ≥ 11 weeks) of the social defeat group were subjected to 5 days of social defeat stress, and were then housed individually for 3 months in Makrolon class III cages from the first defeat onwards, as described before (Fig. 1; Von Frijtag et al., 2001). Control rats were housed in pairs. The social defeat procedure consisted of daily resident–intruder interaction sessions using dominant male Long–Evans rats for five consecutive days. Control animals were handled daily.

Experimental design and treatment groups. The social defeat paradigm (social defeat) in combination with antidepressant treatment was applied in 11-week-old rats. After habituation to the new housing conditions, rats of the social defeat group received daily bouts (5 min) of social defeat during 5 days and subsequent individual housing (3 months). Control animals were handled daily for 5 days and were housed in pairs. Treatment with the tricyclic antidepressant imipramine was applied only during the last 3 weeks (IMI; or no treatment, H2O) of the individual housing period. The length of each period is indicated. All behavioural and immunohistological tests were performed at the end of the paradigm, at the end of treatment (arrow). Independent cohorts of animals were used for behavioural and immunohistochemical analyses.
During the last 3 weeks of this 3-month period, rats were treated by gavage administration of the antidepressant imipramine (20 mg/kg per 0.5 mL water; Sigma-Aldrich, Germany) or water as control (Fig. 1). Subsequently, four experimental groups were generated: (i) control rats with water (Control + H2O); (ii) control animals with chronic imipramine treatment (Control + IMI); (iii) social defeated animals with water (Social defeat + H2O); and (iv) social defeated animals with chronic imipramine treatment (Social defeat + IMI). All behavioural and immunohistological analyses were performed at the end of the treatment period. Independent cohorts of animals were used for behavioural and immunohistochemical analyses.
Reward anticipatory behaviour
A classical Pavlovian conditioning setup was used to investigate anticipatory behaviour, as described earlier (Von Frijtag et al., 2001). To investigate the behavioural response to the conditioning stimulus [repetitive sound (keyboard) and light flashes (three times)], animals were observed before training (trial 0) to determine baseline activity, and again after 35 training trials of pairing with a 5% sucrose reward, using the computer program ‘The Observer’ (Noldus Information Technology, Wageningen, the Netherlands). The researcher who analysed the behavioural data had no knowledge of the experimental groups. Differences in activity (reflected by frequency or transitions of behavioural elements) displayed before training compared with those after training were used as parameter for reward anticipation.
Sucrose preference
The preference for sucrose (5%) was measured in a two-bottle (sucrose and water) consumption test. Consumption was assessed after 24 h by reweighing the pre-weighted bottles. After 2 days, the consumption test was repeated. In case of social housing, consumption for each subject was set to half of the total consumption. Sucrose preference was expressed as the increase in consumption (g) relative to water (g), and this difference was represented as percentage of the total consumption (g) [100% × (Δsucrose – water)/total volume sucrose and water consumed].
Corticosterone assay
Trunk blood samples were collected at the end of the experiment via decapitation (between 09:00 and 11:00 h) into a 7-mL heparin-coated tube (Greiner Bio-One, Monroe, NC, USA) and kept on ice before centrifugation at 1000 g for 10 min. Plasma was decanted and stored at −80 °C until analysis. Levels of plasma corticosterone were assessed using a rat glucocorticoid ELISA kit (Cusabio Biotech, Newark, USA), according to the manufacturer’s instructions.
Tissue preparation and immunohistochemistry
Animals were anaesthetized (between 09:00 and 11:00 h) by injection of pentobarbital sodium salt (Nembutal; 1 mg/kg bodyweight; A.U.V. Cuijk, The Netherlands) and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer (PB; 0.1 m, pH 7.4). Brains were postfixed overnight in the skull at 4 °C, after which they were carefully removed, washed and cryoprotected in 20% sucrose in phosphate-buffered saline. Frozen sections (35 μm thick) were cut using a sliding microtome and collected in PB/azide.
The number of young, differentiating neurons was identified with an antibody against DCX (1 : 800; polyclonal goat anti-DCX; Santa Cruz Biotechnology, Santa Cruz, CA, USA), as described before (Oomen et al., 2007). Amplification was performed with a biotinylated secondary antibody, donkey anti-goat (1 : 500; Jackson ImmunoResearch Laboratories, West Grove, USA), and avidin-biotin complex (1 : 1000; Elite Vectastain ABC kit, Brunschwig Chemie, Amsterdam, The Netherlands) in combination with tyramide (1 : 500; 0.01% H2O2; kindly provided by Dr I. Huitinga, Netherlands Institute for Neuroscience, Amsterdam, the Netherlands). Subsequent chromogen development was performed with diaminobenzidine (20 mg per 100 mL of Tris buffer, 0.01% H2O2).
Stereological quantification and phenotypic analysis
Quantification of cell numbers was performed in every 10th coronal section along the entire rostrocaudal axis of the brain, in a total of nine sections per animal, as described before (Oomen et al., 2010). Total numbers of DCX+ cells per DG were quantified by systematic random sampling performed using the Stereo Investigator System (MicroBrightField) with optical fractionator settings of 180 × 150 grid size and 180 × 150 counting frame, resulting in 200–450 markers per animal. A single examiner unaware of the group codes performed the data collection.
We further distinguished morphologically different subclasses of DCX+ cells, based on an adaptation of the stages of neuronal differentiation described before (Plumpe et al., 2006); the most mature DCX+ cells were named class II; these were characterized by a primary dendrite that was orientated perpendicular to the subgranular zone (SGZ) and radially projecting up into the molecular layer. The younger cells were named class I and were located in the SGZ, without a dendrite, or only a short dendrite reaching no further than the granule cell layer (Oomen et al., 2010; Fig. 3).
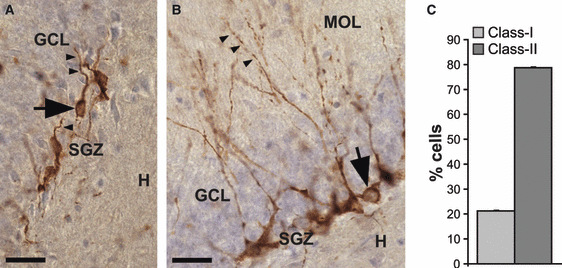
Class I and class II DCX-expressing cells in the DG subgranular zone (SGZ). (A and B) High-power images of clusters of DCX+ cells with different morphology. The granule cell layer (GCL) is seen as a purple cell layer. The hillus (H), SGZ and molecular layer (MOL) are indicated. Scale bar: 30 μm. (A) Class I cells (arrow) with no or short processes reaching no further than the granule cell layer. Note how the initial segment of the dendrite grows parallel to the SGZ (arrowheads). (B) Class II cells (arrow) with at least one dendrite reaching into the molecular layer (arrowheads) and occasionally showing delicate branching with few major branches. (C) Percentage of total amounts of class I and II cells in control animals (mean ± SEM).
Statistical analysis
Statistical analysis was performed using spss 18.0. Results are expressed as group means ± SEM. Treatment effects were assessed with two-way analysis of variance (anova), followed by Student’s–Newman’s–Keuls post hoc analyses for further examination of group differences.
Results
Behavioural and physiological consequences of social defeat
In the present model, we studied consequences of repeated social defeat and subsequent individual housing for 3 months (Fig. 1), and assessed several pathological dimensions of depression (Von Frijtag et al., 2002; Buwalda et al., 2005). We confirmed that socially defeated rats in this paradigm display a depressive-like phenotype (Von Frijtag et al., 2000, 2002), as shown by a reduced anticipation towards a 5% sucrose solution compared with control rats (Fig. 2A). This was reversed to control levels by chronic administration of imipramine (IMI). Two-way anova (stress × treatment) revealed a significant effect of SD stress (F1,20 = 4.77, P = 0.041), and an interaction of stress and treatment (F1,20 = 5.55, P = 0.029). Post hoc comparisons showed a significant decrease after social defeat without treatment compared with controls (P = 0.012), and for social defeat without treatment when compared with the imipramine-treated socially defeated group (P < 0.029). In contrast, no difference was found for the social defeat group that was treated by imipramine when compared with either of the control groups. Imipramine treatment alone in control animals had no significant effect on anticipation towards 5% sucrose. In contrast to sucrose anticipation, neither stress nor imipramine treatment had a significant effect on sucrose preference (Fig. 2B), which is an indicator of more acute stress.
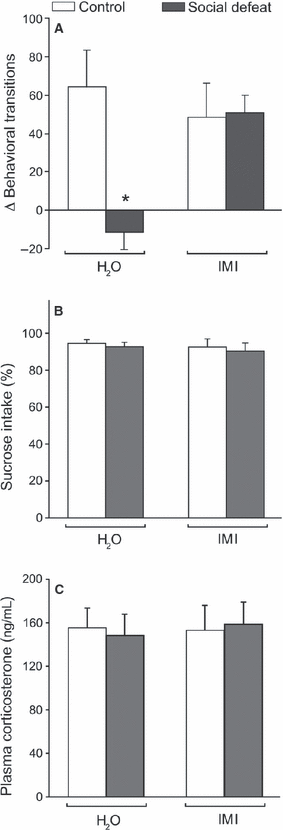
Behavioural and physiological consequences of long-term social stress. (A) Anticipation towards 5% sucrose expressed as the mean difference in activity (# behavioural transitions) in the conditioning stimulus – unconditioned stimulus interval post-training compared with that during pre-training. The social defeat paradigm (social defeat) significantly suppressed reward anticipation, whereas imipramine treatment (IMI) reversed this stress-induced effect. *P = 0.0034 vs. Control + H2O. (B and C) Sucrose preference (sucrose intake − water intake)/total fluid intake (B) and plasma corticosterone level (C) were not affected. All data show mean ± SEM. n = 6 for all experimental groups.
Also, no difference was observed in plasma corticosterone levels between any of the groups (Fig. 2C), indicating that no lasting changes in stress hormone levels had been induced, or that antidepressant treatment had been stressful.
Long-term social stress affects neurogenesis by reducing differentiation and survival of DCX-positive cells
Immunohistological staining of the adult hippocampus revealed numerous DCX+ cell bodies located in the SGZ and only few DCX+ cells within the granule cell layer itself. DCX-expressing cells were classified into class I (Fig. 3A) or class II cells (Fig. 3B; Brandt et al., 2003; Rao & Shetty, 2004; Plumpe et al., 2006). About 70% of all DCX+ cells belonged to class II (Fig. 3C). Hence, at any given time point, DCX identifies a majority of cells with a relatively mature phenotype.
To examine whether adult hippocampal neurogenesis was affected by the social defeat paradigm and/or antidepressant treatment, we first quantified the total population of DCX+ neurons. Two-way anova revealed a significant effect of defeat (F1,30 = 6.17, P = 0.019) on total number of DCX+ neurons (Fig. 4A). Social defeat significantly reduced the total number of DCX+ cells (P = 0.004). This effect was not present anymore after 3 weeks of imipramine treatment, as the social defeat group treated with imipramine differed significantly from the social defeat group (P = 0.048) and was not different from both control groups (CON + H2O, CON + IMI).
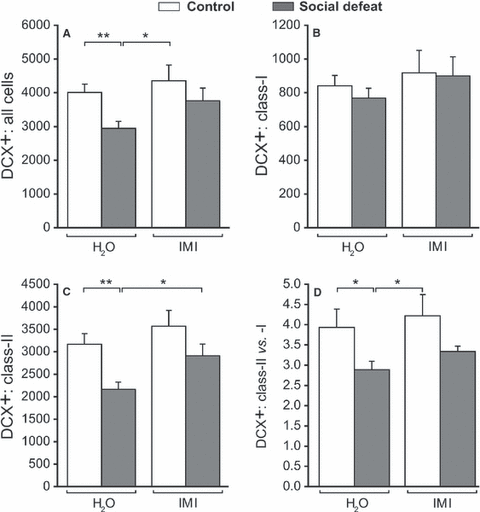
Differences in doublecortin (DCX)-positive cells with different dendritic morphologies after long-term social stress and subsequent imipramine treatment. (A) Total number of DCX+ cells in the SGZ per hemisphere. The social defeat paradigm (social defeat) significantly reduced the total amount of DCX+ cells, whereas imipramine treatment (IMI) normalized this stress-induced effect. (B) No effect of social defeat or imipramine was found on class I DCX+ cell numbers. (C) Social defeat significantly reduced amounts of class II DCX+ cells, whereas this reduction was not found in the imipramine-treated group (IMI). (D) Social defeat significantly reduced the ratio of class I cells over class II cells. Imipramine treatment had no effect on this ratio. All data show mean ± SEM. n = 9 for all experimental groups. **P < 0.01; *P < 0.05.
To further address which subset of the DCX cells was affected, i.e. the relatively younger or older cells, we quantified class I and II DCX+ cells. No effect of defeat or treatment was found on the number of cells belonging to class I cells (F1,30 < 1; Fig. 4B). However, a significant effect of both defeat (F1,30 = 10.41, P = 0.003) and treatment (F1,30 = 4.96, P = 0.034) with no interaction was found on class II DCX+ cells. Social defeat significantly reduced the number of class II cells (P = 0.002), and 3 weeks of imipramine treatment reversed this effect (P = 0.026; Fig. 4C). Imipramine treatment alone had no effect in control rats (P = 0.369).
To confirm whether long-term social stress indeed enforced its strongest effect on class II cells, the ratio of class II cells over class I cells was calculated. This ratio was significantly affected by social defeat (F1,30 = 7.58, P = 0.010). Post hoc comparisons showed that social defeat significantly reduced the ratio of class II/class I cells (P = 0.004). However, imipramine was not able to restore this ratio to basal levels. Together these data demonstrate that: (i) social stress in the long term reduces neurogenesis by specifically reducing class II DCX+ cells, whereas class I cells were unaffected; and (ii) imipramine restored this reduction in neurogenesis by increasing both class I and II DCX+ cells, thereby leaving their ratio unaffected.
Discussion
Here, we showed for the first time that adult hippocampal neurogenesis is still reduced at the end of a 3-month individual housing period that followed a short period of severe social defeat stress. The reduction in neurogenesis was observed, despite that corticosterone levels were normal at the end of this paradigm, and was most profound for the class II DCX+ cells with long apical dendrites, arguing that neuronal differentiation and/or survival of newborn neurons is affected. Treatment with imipramine for the last 3 weeks completely restored this reduction by stimulating both class I and II DCX+ cells.
Validity of the social stress model
The present social defeat model recapitulates several behavioural dimensions of depression (Von Frijtag et al., 2000, 2002). We showed that the depressive-like phenotype of reduced anticipation towards sucrose is associated with a decrease in hippocampal neurogenesis, although sucrose consumption was not changed. This reward-related consummatory response is different from appetitive behaviours measured by anticipation (Berridge & Robinson, 1998). Although consummatory behaviour is known to be reduced shortly after stress exposure (Muscat et al., 1988), we confirmed that in the long term, appetitive behaviour is specifically affected (Von Frijtag et al., 2000, 2002). Because the mesolimbic dopamine reward system is primarily involved in appetitive behaviour and not in the affective component of consumption, we hypothesize that reduced appetitive behaviour is an adequate representative of depressive-like behaviour (Berridge & Robinson, 1998; Von Frijtag et al., 2002). Moreover, anhedonia is commonly observed in depressive patients (Auriacombe et al., 1997).
As the present results were obtained in rats that were 6 months old, it is unlikely that interference with early postnatal development has played a major role (Rakic, 2002; Leventopoulos et al., 2007). The age at which rats experienced stress in our model (adult rats > 11 weeks) is of importance given the protracted period of risk for development of affective disorders into young adulthood (Kaufman et al., 2001).
As compared with most other stress-induced models (Berton et al., 2006; Czeh et al., 2007), the present paradigm was used to study long-lasting effects of social stress on structural plasticity changes that have been implicated in depressive symptoms and maintenance of depression, long after the initial exposure to active stress has occurred, and when the rise in stress hormone levels has normalized (McGonagle & Kessler, 1990; Buwalda et al., 1999; McKinnon et al., 2009).
Neurogenesis – subclasses of DCX+ cells
We quantified DCX+ cell numbers in the DG to examine whether the persistent anhedonic phenotype present long after exposure to social defeat was associated with a reduction in neurogenesis. DCX has been previously established as a reliable marker for young and migratory neurons in the adult DG (Jin et al., 2002; Brown et al., 2003; Rao & Shetty, 2004; Plumpe et al., 2006). It is expressed approximately from Day 4 to Day 14 after a new cell is born, and most likely all DCX+ young neurons originate from cell divisions during the past 3–4 weeks (Rao & Shetty, 2004). DCX expression is further selective for the neuronal lineage, as DCX-positive cells co-express early neuronal antigens like Tuj1, PSA-NCAM or pax-6 (Filippov et al., 2003), but lack specific markers for glia, undifferentiated, stem cells or apoptotic cells, making it a reliable marker of newly generated neurons in the adult DG (Brown et al., 2003; Rao & Shetty, 2004; Knoth et al., 2010).
DCX immunoreactivity in dendrites allowed classification of these immature neurons. DCX is transiently expressed from the proliferative progenitor cell stage to a postmitotic phase with long dendrites (Filippov et al., 2003; Plumpe et al., 2006). Several subclasses of DCX+ cells can be distinguished based on the presence and shape of the apical dendrites (Plumpe et al., 2006; Oomen et al., 2010). Smaller cells without a dendrite that penetrate the granule cell layer are known to reflect progenitor cells (type 2b and type 3 cells; see Plumpe et al., 2006), whereas longer cells with extensions into the molecular layer are considered immature postmitotic neurons.
Here, we used a simplified scheme of this subdivision (Plumpe et al., 2006), and defined class I cells as young cells with no or short processes reaching no further than the molecular layer, and class II cells as mature post-proliferative cells with at least one dendrite reaching into the molecular layer. About 70% of DCX+ cells belong to class II and 30% to class I, and it has been shown that of this latter 30%, about two-thirds are in cell cycle (Plumpe et al., 2006). Interestingly, the ratio of class I and II cells we found was similar to those in rodents that were 2 months old, despite the fact that the total number of DCX+ cells equals about 10% of what is observed in these younger animals. This implies that despite the lower overall DCX+ cell numbers, different stages of the neurogenic process are present in similar proportions both in young adults as well as adult animals.
In a recent paper (Walker et al., 2007), DCX+ cells were sorted using fluorescence-activated cell sorting (FACS), and particularly the younger DCX+ cells with low levels of DCX per cell, comparable with our class I cells, were shown to be capable of dividing again, in contrast to the older types with higher DCX levels. Although we cannot compare FACS cells with the present populations in brain tissue, it is tempting to speculate that as predominantly the class II DCX+ cells were affected in our study, social stress may have affected progression through the cell cycle and thereby limited neuronal differentiation and/or newborn cell survival.
Neurogenesis – social defeat and reversal by imipramine
We found that at the end of the social defeat paradigm total DCX+ cell numbers in the DG were reduced, which was due to a reduction in class II cells, whereas the class I cells remained unaffected. It has been proposed that neurogenic stimuli that act on precursor cells are different from those regulating dendritic maturation and survival of newborn cells. In contrast to chronic unpredictable (physical) stress exposure, after which a rapid recovery of neurogenesis occurs (Heine et al., 2004), our present results imply that social defeat reduces neurogenesis for a prolonged period of time and that it does so by inhibiting specifically the differentiation and survival stage, but leaves progenitor cells unaffected.
A remaining question, however, is whether this long-term reduction in neurogenesis is caused by: (i) long-term effects of social defeat; (ii) by the individual housing; or, most likely, (iii) a combination of both. In adult rats, individual housing by itself is considered to be a social stressor. Adult individual housing induces changes in anxiety- and anhedonia-like behaviour (Wallace et al., 2009), as well as neurochemical alterations (Hall, 1998). However, social isolation in male rats does not result in an increased, lasting expression of stress hormones (McCormick et al., 2005; Stranahan et al., 2006; Wallace et al., 2009), which is in line with our current findings. Also, in the absence of external factors, adult social isolation does not affect hippocampal neurogenesis (Westenbroek et al., 2004; Stranahan et al., 2006). However, individual housing does increase the stress response to external stressors, both on glucocorticoid levels (McCormick et al., 2005; Wallace et al., 2009) and neurogenesis (Westenbroek et al., 2004; Stranahan et al., 2006), and it delays the positive effect of physical activity on neurogenesis (Stranahan et al., 2006). This would argue that individual housing in our paradigm might be involved in the maintenance of the reduction in neurogenesis rather than in its onset.
This additive effect of social isolation is further supported by the observations that depressive-like behaviour (e.g. reduced anticipation to sucrose) induced by social defeat is maintained during individual housing, but can be counteracted by social housing (Von Frijtag et al., 2000).
As social isolation itself cannot reduce anticipation towards 5% sucrose (Von Frijtag et al., 2000), the observed reduction in neurogenesis is most likely induced by repeated social defeat stress, and maintained in the long term due to a lack of social support. This would match human depression in which active stress is often involved in the onset of depression, while passive stress, e.g. in the form of social isolation, has strong precipitating effects on the development of the disease (Heinrich & Gullone, 2006).
The current reduction in hippocampal neurogenesis was associated with an anhedonic phenotype, which is in accordance with the concept of a role for neurogenesis in depression or antidepressant action. This concept originated from animal studies in which stress was shown to inhibit neurogenesis (Alonso et al., 2004; Mineur et al., 2007), and from studies in which various classes of antidepressants were found to promote newborn cell proliferation (Malberg et al., 2000; Santarelli et al., 2003; Warner-Schmidt & Duman, 2006), survival and neurogenesis (Malberg et al., 2000; Wang et al., 2005; Oomen et al., 2007). Furthermore, the 3–4-week therapeutic time lag of antidepressants coincides with the maturational time course of newborn neurons (Jacobs et al., 2000).
However, ablating neurogenesis does not result in a depressive-like phenotype per se (Henn & Vollmayr, 2004). Also, the enhanced survival of newborn cells that occurs upon administration of antidepressants to young mice is age and strain dependent, and is abolished when older mice are studied (Navailles et al., 2008; Couillard-Despres et al., 2009; David et al., 2009; Marlatt et al., 2010). Similarly, in hippocampal tissue of depressive patients, the stimulatory effect of antidepressants (Boldrini et al., 2009) also appears to depend on age (Lucassen et al., 2010b). Hence, both neurogenesis-dependent and neurogenesis-independent mechanisms are likely to contribute to the reversal of depressive-like behaviours by antidepressants (Sahay & Hen, 2007; David et al., 2009).
In this study, treatment with imipramine during the last 3 weeks of a 3-month individual housing period following exposure to severe social defeat stress restored total DCX+ cell numbers back to control levels. Interestingly, whereas the social defeat paradigm affected specifically class II DCX+ cells, the action of imipramine action is not cell-type specific, as it left the ratio of type II over type I cells unaffected. This is in accordance with previous studies in which imipramine increased several stages of the neurogenesis process (Santarelli et al., 2003; Chen et al., 2009), including proliferation, neuronal differentiation, survival as well as integration of adult-generated cells into existing neuronal circuits.
Overall, using a paradigm that models both temporal aspects of a social stress-mediated onset and the maintenance phase of depressive symptoms, we have shown that in the absence of lasting changes in corticosterone levels at the end of the social defeat paradigm, neurogenesis is still significantly reduced. This is accompanied by a reduction of differentiation and/or survival of the newborn neurons, whereas younger cells are unaffected. An interesting outcome of our study is that this form of long-term social stress does not affect all domains of the adult neurogenic process, but appears selective for the differentiation and survival stages. In addition, these neurogenic deficits can still be normalized by late imipramine treatment that increased both classes of DCX+ cells.
Acknowledgements
The authors wish to acknowledge the expert advice on the social defeat paradigm by Dr J. Van Der Harst and Prof. Dr B. Spruyt (Delta Phenomics BV). P.V.B., A.B.S. and W.J.G.H. received funding from the Top Institute Pharma project T5-203. P.J.L. is supported by the Volkswagen Stiftung Germany, the HersenStichting Nederland, the EU and ISAO. The authors report no conflict of interest.
Contributors
P.V.B., W.J.G.H., A.B.S. and S.S. designed the experimental groups of the study. P.V.B., under the assistance of C.A.O. and P.J.L., carried out the neurogenesis experiment. P.V.B. carried out the animal experimental studies. P.V.B. managed literature searches and analyses under the supervision of S.S. and P.J.L. P.V.B. and S.S. undertook the statistical analyses, and created the figures. P.V.B., A.B.S., P.J.L. and S.S. wrote the manuscript, of which the first draft was written by P.V.B. All authors contributed to and have approved the final manuscript.
Abbreviations
-
- DCX
-
- doublecortin
-
- DG
-
- dentate gyrus
-
- FACS
-
- fluorescence-activated cell sorting
-
- PB
-
- phosphate buffer
-
- SGZ
-
- subgranular zone




