Obesity-dependent cannabinoid modulation of proliferation in adult neurogenic regions
Abstract
Endocannabinoid signalling participates in the control of neurogenesis, especially after brain insults. Obesity may explain alterations in physiology affecting neurogenesis, although it is unclear whether cannabinoid signalling may modulate neural proliferation in obese animals. Here we analyse the impact of obesity by using two approaches, a high-fat diet (HFD, 60% fat) and a standard/low-fat diet (STD, 10% fat), and the response to a subchronic treatment with the cannabinoid receptor type 1 (CB1) inverse agonist AM251 (3 mg/kg) on cell proliferation of two relevant neurogenic regions, namely the subventricular zone in the striatal wall of the lateral ventricle (SVZ) and the subgranular zone of the dentate gyrus (SGZ), and also in the hypothalamus given its role in energy metabolism. We found evidence of an interaction between diet-induced obesity and CB1 signalling in the regulation of cell proliferation. AM251 reduced caloric intake and body weight in obese rats, as well as corrected plasma levels of cholesterol and triglycerides. AM251 is shown, for the first time, to modulate cell proliferation in HFD-obese rats only. We observed an increase in the number of 5-bromo-2-deoxyuridine-labelled (BrdU+) cells in the SGZ, but a decrease in the number of BrdU+ cells in the SVZ and the hypothalamus of AM251-treated HFD rats. These BrdU+ cells expressed the neuron-specific βIII-tubulin. These results suggest that obesity may impact cell proliferation in the brain selectively, and provide support for a role of CB1 signalling regulation of neurogenesis in response to obesity.
Introduction
The endocannabinoid system (ECS) exerts a regulatory role on energy balance mainly through the activation of CB1 receptors in the hypothalamus and hippocampus (Piomelli, 2003; Pagotto et al., 2006). Accordingly, blockade of central and peripheral CB1 receptors by inverse agonist/antagonists such as SR141716 (rimonabant) has been shown to have an impact on obesity, resulting in weight loss attributable to a reduction in food intake, an increase in whole-body energy expenditure and improvement in multiple metabolic parameters correcting dyslipaemia; there is also a lowering of non-esterified ‘free’ fatty acid levels and a reversal in insulin resistance (Matias & Di Marzo, 2004; Ravinet Trillou et al., 2004; Pavon et al., 2008; Scheen & Paquot, 2009). Thus, CB1 receptor stimulation by an overactive ECS would cause an increase in appetite and food intake together with a series of other metabolic changes that can lead to obesity and related disorders (Engeli et al., 2005).
Besides the neuromodulatory role of the ECS on energy balance, endocannabinoid signalling via CB1/CB2 receptors may also take part in the control of neural proliferation in the hippocampus and the ventricular zone, including cell fate, neural cell death and survival, as part of a neuroprotective response on demand from different brain insults (Aguado et al., 2005; Galve-Roperh et al., 2007; Goncalves et al., 2008). For instance, the central action of CB1 receptor antagonism has been associated with a higher risk of anxiety and depression (Christensen et al., 2007). It has been postulated that energy balance, such as from dietary modulation or physical exercise, may influence cell proliferation in the hippocampus (Stangl & Thuret, 2009), and could also influence proliferation activity in the hypothalamus given its role in energy metabolism (Kokoeva et al., 2005; Pierce & Xu, 2010). Some studies have related a ketogenic diet with disruption of the subventricular and hippocampal neurogenesis (Lindqvist et al., 2006; Valente et al., 2009), and also with inflammation and apoptosis in the hypothalamus (Velloso et al., 2008; Moraes et al., 2009). Together, these data suggest a possible mechanism by which nutrition may also have an impact on mental health. However, it is unclear whether the effect of diet on adult neural proliferation can be influenced by caloric intake, meal frequency, meal texture or meal content (Stangl & Thuret, 2009).
Despite the described role of the ECS on neural proliferation and metabolic control as independent actions, there is still a general lack of knowledge regarding the regulatory role of the ECS on the major sites of adult neurogenesis in obese animals. Here we analyse the impact of obesity defined by two diets, a standard/low-fat diet (10% fat) and a very high-fat diet (60% fat), and the response to a subchronic treatment with the CB1 inverse agonist AM251 (3 mg/kg) on cell proliferation in two relevant neurogenic brain regions, the subventricular zone of the striatal wall of the lateral ventricle (SVZ) and the subgranular zone of the dentate gyrus (SGZ), and also in the hypothalamus. To better understand the effects of energy imbalance as a consequence of obesity and AM251 treatment on cell proliferation, we previously analysed food intake, weight gain and plasma levels of relevant biochemical parameters, such as glucose, triglycerides and cholesterol, and hormones such as insulin, glucagon and leptin.
Materials and methods
Animals
All experimental animal procedures were performed in compliance with the European Communities directive 86/609/ECC and Spanish legislation (BOE 252/34367-91, 2005) regulating animal research. Male Wistar rats (approximately 250 g, 10–12 weeks old; Charles Rivers, Barcelona, Spain) were housed in groups of two in cages maintained in standard conditions (Servicio de Estabulario, Facultad de Medicina, Universidad de Málaga) at 20 ± 2 °C room temperature, 40 ± 5% relative humidity and a 12-h light/dark cycle with dawn/dusk effect.
Diet-induced obesity – measurement of caloric intake and body weight gain
Rats (n = 16 per group) were fed ad libitum for 12 weeks with two different types of diets: a very high-fat diet (HFD, 60% fat diet; D12492, Brogaarden, Gentofke, Denmark) and a standard/low-fat diet (STD, 10% fat diet-D1245B), in order to induce obesity (Fig. 1). The standard diet consisted of 3.85 kcal/g (20% protein, 70% carbohydrates and 10% fat) and the high-fat diet comprised 5.24 kcal/g (20% of the metabolizable energy content was protein, 20% carbohydrates and 60% fat).
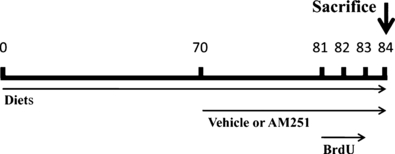
Experimental design used for diet-induced obesity, AM251 administration and 5′-Bromo-2′-deoxyuridine (BrdU) injections. Animals were fed ad libitum for 84 days with two diets: a very high-fat diet and a standard/low-fat diet, in order to induce obesity. After 70 days, when the weight curves diverged and stabilized between the two diets, rats received a daily intraperitoneal injection of AM251 (3 mg/kg) or vehicle (Tocrisolve) for 14 days. Four days before the animals were killed, they received two daily intraperitoneal injections of BrdU (50 mg/kg) at 10-h intervals (08:00, 18:00 h) for three consecutive days. Two hours after the last dose of AM251, rats were killed to collect blood samples and the brains.
The accumulated caloric intake obtained from food intake by each rat and their body weight gain were measured every day for 12 weeks. Treatment was administered when the weight curves achieved a clear divergence and stabilization between the two diets was achieved. This was accomplished after 10 weeks of diet.
Treatment
After 10 weeks (divergence of weight curves), half of each group of diet-fed rats (n = 8 per group) received a daily intraperitoneal (i.p.) injection of, either CB1 receptor inverse agonist AM251 [N-(piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4 dichlorophenyl-4-methyl-1H-pyrazole-3-carboxamide] (Tocris Bioscience, Bristol, UK), at 3 mg/kg in 10% Tocrisolve, or vehicle (1 mL/kg of 10% Tocrisolve in saline) over 14 days, while the diets remained unchanged (Fig. 1). Cumulative caloric intake and body weight gain were measured every day over the 14 days of treatment. Finally, we generated four experimental groups: STD-vehicle, STD-AM251, HFD-vehicle and HFD-AM251.
BrdU administration
5′-Bromo-2′-deoxyuridine (BrdU; Sigma, St Louis, MO, USA) was dissolved at 15 mg/mL in 0.9% saline solution, and administrated at 50 mg/kg i.p. twice per day at 10-h intervals (08:00, 18:00 h), for 3 consecutive days (Fig. 1). The animals were killed 12 h after the last injection of BrdU.
Sample collection
Animals from the four experimental groups were anaesthetized (sodium pentobarbital, 50 mg/kg, i.p.) 2 h after the last dose of AM251 treatment in a room separate from the other experimental animals. Blood samples were briefly collected from the orbital cavity and centrifuged (1600 g for 8 min, 4 °C), and all plasma samples were frozen at −80 °C for biochemical analysis. Animals were then transcardially perfused with 4% paraformadehyde in 0.1 m phosphate buffer (PB), and the brains dissected and kept in the same fixative solution overnight at 4 °C. Brains were cut into 40-μm-thick coronal sections, divided into five parallel series, using a vibratome (Leica VT1000S). Sections were stored at 4 °C in PB with 0.002% (w/v) sodium azide until further used for immunostaining.
Biochemical and hormonal analysis
Plasma metabolites were measured using commercial kits according to the manufacturer’s instructions: glucose (standard glucose oxidase method; Randox Laboratories, Crumlin, UK), and triglycerides (GPO-PAP method; Randox Laboratories). Cholesterol was analysed in the Hematology Service at the Hospital Carlos Haya (Málaga, Spain). Plasma insulin, adiponectin and leptin levels were determined with three different enzyme-linked immunosorbent assay (ELISA) kits from Mercodia AB (Uppsala, Sweden), B-Bridge International, Inc. (Mountain View, CA, USA) and BioVendor (Modrice, Czech Republic), respectively. To perform the ELISA protocols in rat samples, we used 25, 100 and 100 μL of plasma to determine the insulin, adiponectin and leptin concentrations, respectively. A calibration curve and an internal control were included in each plate.
Immunohistochemistry
Antibodies used were as follows (Perez-Martin et al., 2010).
- 1
Mouse anti-BrdU is an IgG1 monoclonal antibody obtained from ascites by using BrdU-bovine serum albumin (72–73 kDa) as immunogen. Dilution = 1 : 2000. Source: Hybridoma Bank, Iowa City, IA, USA; ref. G3G4.
- 2
Biotinylated goat anti-mouse IgG (H+L). Dilution = 1 : 1000. Source: Pierce, Rockford, IL, USA; cat. no. 31800, lot FC799628.
To analyse cell proliferation in the SVZ, the SGZ and the hypothalamus, free-floating coronal sections from −0.30 to −4.16 Bregma levels were selected from one of the five parallel series obtained from each brain of the four experimental groups (Paxinos & Watson, 1998). Sections were first washed several times with PBS to remove sodium azide, and incubated in a solution of 3% hydrogen peroxide and 10% methanol in 0.1 m PB for 45 min at room temperature in darkness to inactivate endogenous peroxidase. After three washes in PBS for 10 min, DNA was denatured by exposing sections to 2 m HCl for 15 min at 37 °C, followed by two washes in 0.15 m borate buffer to neutralize pH. After three washes in PBS for 10 min, sections were incubated in a blocking solution containing 0.3% albumin, 0.3% triton X-100 and 0.05% sodium azide in PBS for 45 min. Sections were then incubated with a mouse monoclonal anti-BrdU diluted 1 : 2000 in PBS containing 0.3% triton X-100 (PBT) overnight at 4 °C with agitation. The following day, sections were washed three times with PBS, incubated in a biotinylated goat anti-mouse immunoglobulin (Amersham, Little Chalfont, UK) diluted 1 : 1000 for 90 min, washed again in PBS, and incubated in avidin-biotin peroxidase complex (Pierce) diluted 1 : 250 in PBT in darkness at room temperature for 45 min. After three washes in PBS in darkness, immunolabelling was revealed with 0.05% diaminobenzidine (DAB; Sigma), 0.05% nickel ammonium sulfate and 0.03% H2O2 in PBS. After several washes in PBS, sections were mounted in slides treated with poly-l-lysine solution (Sigma), air-dried, dehydrated in ethanol, cleared with xylene and coverslipped with Eukitt mounting medium (Kindler GmBH & Co, Freiburg, Germany). Digital high-resolution photomicrographs of the rat brain were taken under the same conditions of light and brightness/contrast by an Olympus BX41 microscope equipped with an Olympus DP70 digital camera (Olympus Europa GmbH, Hamburg, Germany). Representative digital images were mounted and labelled using Microsoft PowerPoint 2007.
Double immunofluorescence
For double immunofluorescence the following antibodies were used: rat anti-BrdU (1 : 2000; Accurate Chemical & Scientific, Westbury, NY, USA), mouse anti-GFAP (1 : 2000; Sigma) and mouse anti-βIII tubulin (1 : 5000; Promega, Madison, WI, USA). Sections were pretreated as described above and incubated overnight at 4 °C with the primary antibodies. After washing in PB, sections were incubated for 2 h at room temperature with the secondary antibodies. The rat anti-BrdU was detected with goat anti-rat IgG labelled with Alexa 488 (1 : 1000; Molecular Probes, Invitrogen, Paisley, UK). The antibodies to GFAP and βIII-tubulin were detected with donkey anti-mouse IgG Alexa 594 (1 : 1000; Molecular Probes). The sections labelled by double immunofluorescence were visualized with a confocal microscope (Leica TCS NT; Leica Microsystems).
Quantification of BrdU-immunoreactive cells
To quantify cell proliferation in the SVZ, the SGZ and the hypothalamus, BrdU immunoreactive (BrdU+) nuclei that came into focus were manually counted using a standard optical microscope with 40× objective. In the SVZ we counted all the BrdU+ cells from the ventral end of the lateral ventricle to the beginning of the rostral migratory pathway (excluding the pathway). Positive nuclei located in the uppermost focal plane were ignored to avoid counting mistakes caused by blur. We considered as BrdU+ nuclei those completely filled with DAB product or showing patches of variable intensity. Twenty-two brain sections were analysed from each animal. BrdU+ nuclei of the SVZ were counted from −0.30 to −0.80 mm Bregma levels, which comprised four 40-μm-thick coronal sections per animal. BrdU+ nuclei of the SGZ and the hypothalamus were counted from −2.12 to −4.16 mm Bregma levels, which comprised 18 40-μm-thick coronal sections per animal. For this analysis we included most hypothalamic nuclei adjacent to the third ventricle, such as the paraventricular hypothalamic nucleus, the anterior hypothalamic area, the dorsolateral and dorsomedial hypothalamic nuclei, and the acuate hypothalamic nucleus. We considered the same number of brain sections per area and animal. Cell counts were performed in a single cerebral hemisphere in all cases. Quantification was expressed as the average number of BrdU+ cells per section for each experimental group (STD-vehicle, STD-AM251, HFD-vehicle and HFD-AM251).
Statistical analysis
Data are represented as mean ± SEM from at least six animals. Statistical analysis was performed using a two-tailed Student’s t-test for unpaired groups, and two-way anova followed by Bonferroni post hoc test for multiple comparisons. A P-value of < 0.05 was considered statistically significant.
Results
Effect of diet and AM251 on caloric intake and body weight gain
To establish the effect of both diets and AM251 treatment on energy balance, we first measured cumulative caloric intake and body weight gain. Figure 2 shows cumulative caloric intake and body weight gain in rats fed with either a STD or with a HFD over the first 10 weeks of the feeding period. Rats fed with the HFD consumed more calories than rats fed with the STD (Fig. 2A) and this difference was statistically significant from the day 1 onwards. Cumulative caloric intake was related to body weight increase (Fig. 2C); interestingly, body weight gain also showed a significant divergence in HFD rats compared with STD rats only from the fourth week onward (30 days). When body weight gain began to stabilize (after 10 weeks of either diet), rats received a daily dose of either vehicle (Tocrisolve) or the CB1 inverse agonist AM251 (3 mg/kg) for 14 additional days. Cumulative caloric intake and body weight gain of rats treated with vehicle were not affected (Fig. 2B and D). Treatment with AM251 reduced cumulative caloric intake in both diets (Fig. 2B) from day 4–5 of treatment (P < 0.05), and this corresponded with a significant body weight decrease (Fig. 2D). Interestingly, body weight decrease was more marked in HFD rats than in STD rats, i.e. there was a significant decrease in HFD rats from the first day of treatment (P < 0.001), whereas we did not detect a significant body weight decrease in STD rats until the eighth day of treatment (P < 0.05). On the last day of AM251 treatment, HFD rats had reduced their weight gain by 82% (−27.6 ± 3.3 g) in comparison with vehicle-treated HFD rats (−4.94 ± 2.14 g; P < 0.001).
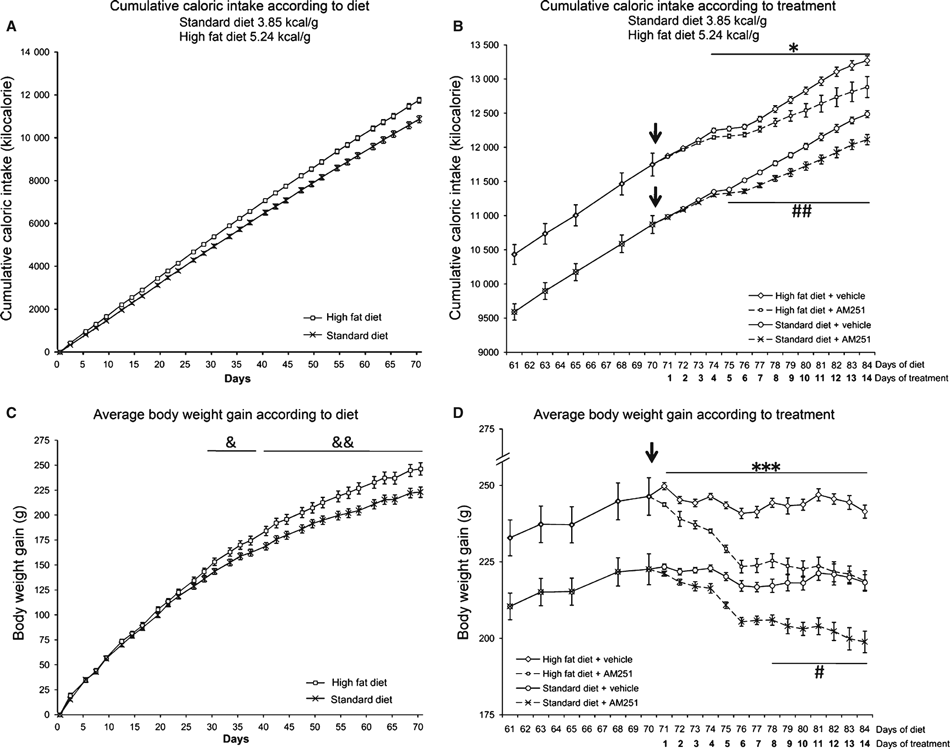
Effect of a standard/low-fat diet (STD) and a very high-fat diet (HFD) on cumulative calorie intake and body weight gain over 10 weeks of diet (A, C) and over 14 days of AM251 treatment (3 mg/kg; B, D). Arrows point to the beginning of AM251 administration. Histograms represent the mean ± SEM (16 animals per diet group; eight animals per treatment group). Student’s t test: &P < 0.05, &&P < 0.01 vs. STD rats. *P < 0.05, ***P < 0.001 vs. HFD rats treated with AM251; #P < 0.05, ##P < 0.01 vs. STD rats treated with AM251.
Effect of diet and AM251 treatment on plasma metabolites and hormones
To further evaluate the effects of energy imbalance as a consequence of obesity and AM251 treatment on cell proliferation, we analysed plasma hormones and metabolites from blood extracted from the rat orbital cavity 2 h after the last dose of AM251 or vehicle administration was given (Fig. 3). No differences in cholesterol levels between groups were found (Fig. 3A). However, a significant decrease was observed in AM251-treated rats in comparison with control rats when the groups were analysed by two-way anova (vehicle vs. AM251, F1,28 = 5.23, P < 0.05). Levels of triglycerides showed a significant decrease in HFD rats (P < 0.05) in comparison with STD rats, which is similar to the decrease observed in AM251-treated STD rats (P < 0.05; Fig. 3B). However, two-way anova revealed decreases related to diet (STD vs. HFD, F1,28 = 10.08, P < 0.01) and treatment (vehicle vs. AM251, F1,28 = 7.20, P < 0.05). Glucose levels showed a significant decrease in HFD rats (P < 0.05) in comparison with STD rats (Fig. 3C). In contrast, glucose levels were increased in both diets after AM251 treatment (both at P < 0.05). Two-way anova revealed a significant decrease related to diet (STD vs. HFD, F1,28 = 13.58, P < 0.001), but a significant increase related to treatment (vehicle vs. AM251, F1,28 = 11.67, P < 0.01; see also Supporting Information Fig. S1 for glucose levels in 24 h-fasted rats).
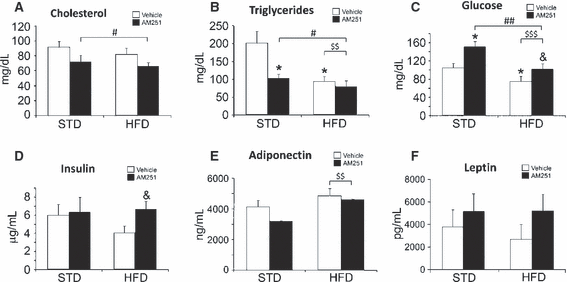
Serum cholesterol (A), triglyceride (B), glucose (C), insulin (D), adiponectin (E) and leptin (F) levels in rats fed with STD or HFD and treated with vehicle or AM251 (3 mg/kg). Histograms represent the mean ± SEM (eight animals per treatment group). Student’s t test: *P < 0.05 vs. STD rats treated with vehicle; &P < 0.05 vs. HFD rats treated with vehicle. Two-way anova and Bonferroni test: #P < 0.05 and ##P < 0.01, between treatments (vehicle vs. AM251); $$P < 0.01 and $$$P < 0.001, between diets (STD vs. HFD).
We also measured levels of three hormones related to glucose homeostasis: insulin, adiponectin and leptin. No difference in plasma insulin levels was observed in STD rats treated with AM251, whereas an increase was detected in AM251-treated HFD rats (P < 0.05; Fig. 3D). Levels of plasma adiponectin and leptin did not show differences when groups were compared (Fig. 3E and F). However, a significant increase of adiponectin was observed in HFD rats in comparison with STD rats when groups were analysed by two-way anova (STD vs. HFD, F1,27 = 7.94, P = 0.01).
Cell proliferation in adult neurogenic regions
To investigate the impact of obesity generated by the two diets (STD and HFD), and AM251 treatment on rat brain neurogenesis, we evaluated newborn BrdU+ cells in the SVZ of the striatal wall of the lateral ventricle, the SGZ of the dentate gyrus and the hypothalamus. In general, no differences in cell proliferation were detected when the two diets were compared. Additionally, no variation in cell proliferation was observed in STD rats treated with AM251. However, opposite effects were observed depending on the neurogenic brain region analysed in HFD rats treated with AM251. Thus, AM251-treated HFD rats displayed a higher number of newborn cells in the SGZ compared with vehicle-treated HFD rats (HFD-vehicle: 118.43 ± 22.22 vs. HFD-AM251: 193.00 ± 24.77; P < 0.05; Fig. 4), which represents an increase of approximately 40%. In contrast, AM251-treated HFD rats displayed a lower number of newborn BrdU+ cells in the SVZ (HFD-vehicle: 484.36 ± 18.81 vs. HFD-AM251: 362.08 ± 53.67; P < 0.05; Fig. 5), representing a reduction of approximately 25%. A highly significant decrease of about 60% in the number of BrdU+ cells was also observed in the ependyma-parenchyma of the hypothalamus (HFD-vehicle: 19.46 ± 3.27 vs. HFD-AM251: 8.51 ± 1.14; P < 0.01; Fig. 6). When groups were analysed by two-way anova, we found a significant AM251 effect in the hypothalamus, suggesting that the number of BrdU+ cells was affected in the same way after AM251 in the two diets (vehicle vs. AM251, F1,23 = 9.36, P < 0.01). No effects of diet were seen on proliferation and no interaction between diet and AM251 was detected. Additionally, we observed no effect of AM251, diet or their interaction in the SGZ. We found a significant interaction effect only between diet and AM251 in SVZ proliferation (interaction, F1,12 = 6.87, P < 0.05), suggesting that the number of BrdU+ cells was affected differently in STD and HFD groups.
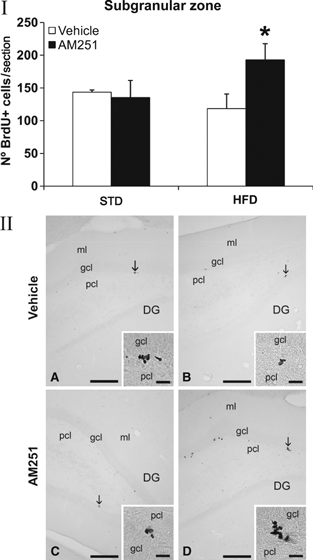
Effect of AM251 on cell proliferation in the subgranular zone of the dentate gyrus in rats fed with STD or HFD by BrdU immunohistochemistry. (I) The histogram represents the mean ± SEM per section of BrdU+ nuclei. The number of positive nuclei is significantly greater in HFD rats treated with AM251. (II) Representative micrographs show low- (A, B) and high- (insets) magnification views of the typical clustering of newborn cells at the inner border of the granular cell layer (arrows). Student’s t test: *P < 0.05 vs. HFD rats treated with vehicle. Scale bars = 200 μm (A–D); 50 μm (insets).
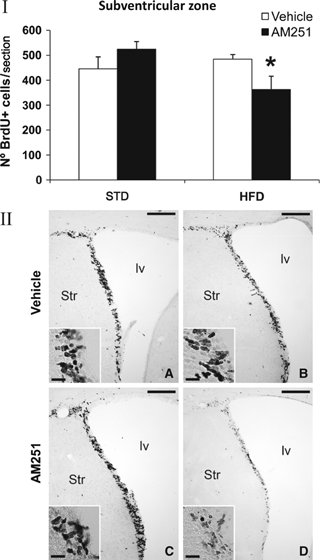
Effect of AM251 on cell proliferation in the subventricular zone of the striatal wall of the lateral ventricle in rats fed with STD or HFD by BrdU immunohistochemistry. (I) The histogram represents the mean ± SEM per section of BrdU+ nuclei. The number of positive nuclei is significantly lower in HFD rats treated with AM251. (II) Representative micrographs show low- (A, B) and high- (insets) magnification views of coronal brain sections at the initial level of the rostral migratory stream. Student’s t test: *P < 0.05 vs. HFD rats treated with vehicle. Scale bars = 200 μm (A–D); 50 μm (insets).
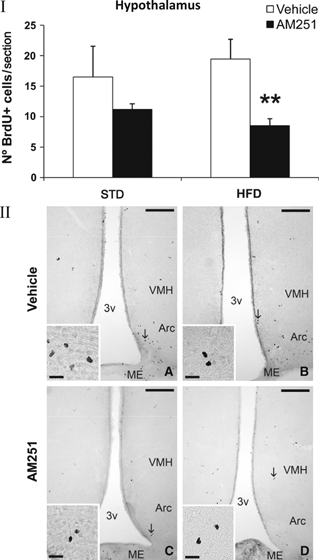
Effect of AM251 on cell proliferation in the ependyma-parenquima of the hypothalamus in rats fed with STD or HFD by BrdU immunohistochemistry. (I) The histogram represents the mean ± SEM per section of BrdU+ nuclei. The number of positive nuclei is significantly lower in HFD rats treated with AM251. (II) Representative micrographs show low- (A, B) and high- (insets) magnification views of BrdU+ nuclei in the periventricular and parenchymal zone of the caudal hypothalamus (arrows). Student’s t-test: **P < 0.01 vs. HFD rats treated with vehicle. Scale bars = 200 μm (A–D); 50 μm (insets).
To characterize the BrdU+ cells, we used specific cell markers such as βIII-tubulin (found almost exclusively in neurons) and GFAP (specific in astrocytes). We found newborn cells that expressed the neuron-specific βIII-tubulin in the SVZ, the SGZ and, for the first time, the hypothalamus (Fig. 7). We did not detect BrdU+/GFAP+ cells.
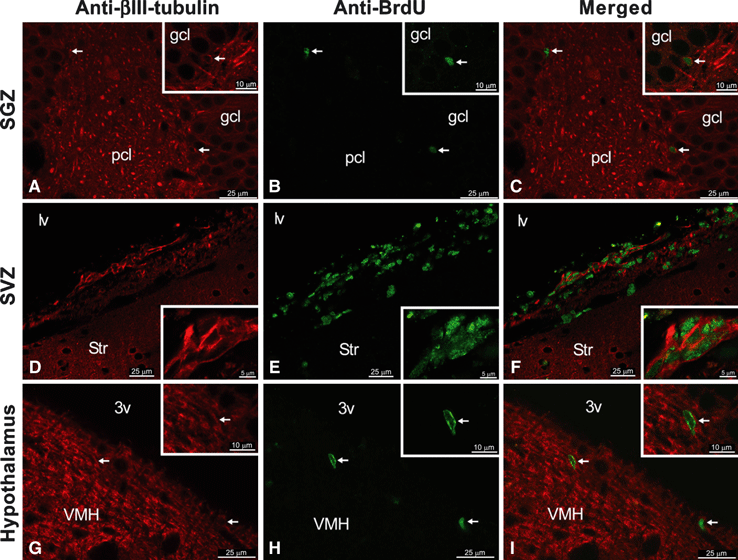
Characterization of newborn cells in the SGZ (A–C), the SVZ (D–F) and the hypothalamus (G–I), showing the co-localization of BrdU (green) and the neuron-specific βIII-tubulin (red) by immunofluorescence. Scale bar details are included for each image.
Discussion
Considering the results obtained here we suggest that inverse agonism of cannabinoid signalling may affect neural proliferation on HFD-obese animals. However, obesity per se does not appear to be a major factor in the control of cell proliferation in either the hippocampus or the extra-hippocampal regions of the brain.
We have not observed any changes on neural proliferation when animals fed with the two diets were compared. Several studies suggest that high-calorie diets rich in fat or cholesterol are environmental factors that can impede energy balance regulation and therefore influence the normal function of the brain (Molteni et al., 2002; Solfrizzi et al., 2003; Morton et al., 2006). Diet-induced obesity resulted in cognitive impairment, the mechanisms of which are associated with vascular damage to the central nervous system and numerous metabolic abnormalities, including dyslipidaemia and glucose intolerance (hyperglycaemia), producing functional resistance to leptin and insulin (diabetes), and preventing physiological anorexigenic activities (Morley, 2004). Triglycerides can impair the transport of leptin across the blood–brain barrier, which in part may account for the peripheral leptin resistance seen in obesity (Banks et al., 2004). For instance, previous reports in humans have suggested that hypertriglycaemia may lead to delirium, and elevated triglycerides in patients with type 2 diabetes mellitus have been associated with poor cognitive performance (Heilman & Fisher, 1974; Mathew et al., 1976; Perlmuter et al., 1988). Additionally, recent studies have suggested that triglycerides could mediate cognitive dysfunction associated with obesity, possibly affecting hippocampal long-term potentiation (Farr et al., 2008), or with diabetes, impairing hippocampal synaptic plasticity (Stranahan & Mattson, 2008; Stranahan et al., 2008a,c). Lindqvist et al. (2006) demonstrated that just 4 weeks of feeding ad libitum a diet rich in fat (42% fat) decreased hippocampal neurogenesis and increased serum corticosterone levels in adult male rats. Several studies have related the increased levels of corticosterone, inhibition of hippocampal neurogenesis (Ambrogini et al., 2002; Darnaudéry et al., 2006, 2007) and SVZ cell proliferation (Lau et al., 2007). These data agree with the effects of a high-cholesterol diet impairing cell proliferation in the dentate gyrus of mice (Kim et al., 2009). In contrast, Strandberg et al. (2008) noted that ketogenic diets (80% fat) do not alter neurogenesis in the rat dentate gyrus, suggesting that not only caloric intake but also other factors such as meal frequency, texture and content can impact adult neurogenesis (Stangl & Thuret, 2009; Yamamoto et al., 2009). Rats fed with a soft diet, as opposed to a solid/hard diet, exhibit decreased hippocampal progenitor cell proliferation (Aoki et al., 2005; Yamamoto et al., 2009). These authors suggested that chewing leading to cell proliferation is related to corticosterone levels and food texture, which has also an impact on adult hippocampal neurogenesis. In the present study, both the STD and HFD had a similar hard texture, and in obese rats no differences in neural proliferation were detected between the two diets. These data also indicate that variations in fatty acid content and caloric intake do not alter the number of BrdU+ cells in the SVZ, the SGZ or the hypothalamus, as has been suggested for ketogenic diets by Strandberg et al. (2008). In addition, we have found no significant differences in the number of BrdU+ cells between obese (present study) and non-obese adult rats (data obtained from others studies with identical BrdU methodology), in the SGZ (Kuhn et al., 1996) or the hypothalamus (Perez-Martin et al., 2010). In contrast, various studies have shown a lower number of BrdU+ cells in the SVZ of non-obese rats (Lau et al., 2007; Wang et al., 2009). Such results suggest that at least in rats, diet-induced obesity may have a selective impact on neural proliferation depending not only on the neurogenic brain region, but also on other factors that affect neuronal plasticity, such as energy expenditure, voluntary exercise and caloric restriction (Stranahan & Mattson, 2008).
A broad range of drugs, such as vitamin E, modulators of neural activity, growth factors, and others anti-inflamatory and anti-oxidant factors, have been found to stimulate neurogenesis (Ciaroni et al., 1999; Anderson et al., 2002; Valente et al., 2009), and this may include cannabinoids. For instance, CB1 receptor signalling can modulate neural stem cell proliferation in vitro (Rueda et al., 2002) and in adult mice (Jin et al., 2004; Aguado et al., 2005; Jiang et al., 2005). Interestingly, Goncalves et al. (2008) showed that the CB1/CB2 receptor agonist WIN 55,212-2 resulted in an increase of proliferation in the SVZ. Such effect contrasted with a reduction in proliferation after inhibition of DAGLα and DAGLβ activity. In contrast, CB1 antagonists (SR141716A and LY320135) did not inhibit progenitor cell proliferation. Goncalves et al. (2008) also demonstrated that the CB1 inverse agonist AM251 reduced the number of COR-1 cells incorporating BrdU in a dose-dependent manner, in contrast to the effect seen with a CB1 agonist. However, they concluded that there was no clear evidence to support a role for the CB1 receptor in adult subventricular neurogenesis. In contrast, the number of BrdU-labelled cells was reduced by approximately 50% in the dentate gyrus and the SVZ of CB1-knockout male mice on a C57Bl/6 background (Jin et al., 2004), whereas BrdU-labelled cells were increased in the dentate gyrus of CB1-knockout female mice on a CD1 background (Wolf et al., 2010), indicating a role of the CB1 receptor on neurogenesis.
Here we found evidence of an interaction between a high-fat diet-induced obesity and CB1 signalling in the regulation of neural proliferation in adult rats. A subchronic treatment over 14 days with the CB1 inverse agonist AM251 in HFD-obese male Wistar rats induced caloric intake reduction, weight loss and improvement in metabolic parameters correcting plasma levels of cholesterol and triglycerides (Chambers et al., 2004; Judge et al., 2009; Kim et al., 2009; Kumar et al., 2009). Interestingly, there are no studies associating this metabolic improvement in obesity and the modulation of cell proliferation depending on the adult neurogenic region analysed. Our results agree with a previous study indicating that AM251 anorectic responses are increased with high-fat feeding (Judge et al., 2009). Moreover, we observed that AM251 treatment significantly reduced the number of BrdU+ cells in the SVZ and the hypothalamus of obese rats fed the high-fat diet only. In contrast, AM251 significantly increased the number of BrdU+ cells only in the SGZ of HFD-obese rats. Therefore, we found no significant variation in cell proliferation in STD rats treated with AM251. Most of these BrdU+ cells express βIII-tubulin in the three neurogenic regions analysed, suggesting that these cells are newborn neurons. We have not observed BrdU+/GFAP+ cells.
The observed effect of AM251, linked to both caloric intake reduction and the increase in SGZ cell proliferation, agrees with previous studies suggesting that a dietary restriction significantly enhances neural proliferation in the rat dentate gyrus (Lee et al., 2000, 2002; Kumar et al., 2009). In addition, acute administration of AM251 alone also stimulates cell proliferation in the dentate gyrus (Hill et al., 2006). Wolf et al. (2010) also demonstrated that AM251 induces cell proliferation up to the first 24 h in the dentate gyrus of female mice, and this increase was largely accounted for by type-2b cells and doublecortin-positive cells. They also observed that BrdU+ cells began to decrease in AM251-treated mice from 48 h onwards. Such results agree with those of our study, in which the increase in cell proliferation was detected 2 h after the last dose of AM251. It is interesting to note that long-term administration of the synthetic CB1/CB2 agonist HU210 also increases neurogenesis in the rat dentate gyrus and stimulates proliferation of neuronal precursors in vitro (Jiang et al., 2005). Long-term administration of HU210 is known to induce a potent desensitization of the CB1 receptor, which is functionally equivalent to receptor antagonism (Rodriguez de Fonseca et al., 1994). These authors also showed that WIN55,212-2 increases the number of BrdU+ cells in cerebral cortical cultures, confirming that the neurogenic effect is mediated through the CB1 receptor. A recent study showed that cannabis-derived extracts Δ9-tetrahydrocannabinol (THC) and cannabidiol have different effects on adult neurogenesis (Wolf et al., 2010). There are two possible explanations for these discrepancies. First, cannabinoid agonists may have biphasic actions, as occur on feeding with low doses being stimulatory and high doses inhibitory of feeding. Second, available cannabinoid agonists do not display the selectivity of the antagonists in terms of CB1 over CB2 selectivity, being active in more than one receptor. This is further complicated by the existence of putative non-CB1, non-CB2 receptors in areas included in the present study, such as the hippocampus (Godlewski et al., 2009).
AM251 treatment reduced the number of BrdU+ cells in the SVZ in obese rats fed the high-fat diet. These data agree with the effect of AM251 on proliferation of the neural COR-1 stem cells observed in vitro by Goncalves et al. (2008). This inverse agonist reduces neural stem cell proliferation in culture, which is in agreement with the opposite effect observed for CB1/CB2 agonists. Thus, subchronic administration (10 days) of WIN55,212-2 or the more specific CB2 agonist JWH133 resulted in a modest increase in subventricular proliferation, and such increased proliferation of both agonists was fully inhibited by the selective CB2 antagonist JTE907 in 6-month-old mice (Goncalves et al., 2008). In contrast to all these data, SR141716A and AM251 paradoxically increased the number of BrdU+ cells in the SVZ of mice, effects that were sustained in CB1-knockout mice but abolished in VR1-knockout mice. These data indicated that non-CB1 receptors might have a role in the regulation of adult neurogenesis. Some studies suggest that neurogenesis may also be modulated by the correlation between the cannnabinoid and the corticosteroid systems. Thus, AM251 blocks the effect of the endocannabinoid reuptake inhibitor AM404 and the endocannabinoid degradation enzyme inhibitor URB597 on the increase of plasma corticosterone levels (Saber-Tehrani et al., 2010). In addition, the CB1 receptor antagonist SR141716A induces an increase in plasma corticosterone levels in both rats and mice (Wade et al., 2006), possibly through the blockade of CB1 receptors. Moreover, corticosterone induces a decrease in SVZ cell proliferation (Lau et al., 2007), suggesting that this effect may be mediated by CB1 receptor signalling. In this context, it should be noted that high-calorie diets and a sedentary lifestyle can also lead to hyperactivation of the hypothalamic–pituitary–adrenal axis, resulting in elevated circulating cortisol levels and lower rates of adult neurogenesis (Stranahan et al., 2008b). These data provide support for the role of AM251 on neurogenesis under conditions of stress in obesity.
In our model, AM251 was capable of reducing triglycerides and cholesterol levels, but not glucose levels, probably due to the heavy insulin resistance produced by these hypercaloric diets. In addition, adiponectin levels tended to be reduced and leptin levels were not modified. Most of these effects were described for rimonabant, which has been shown to restore energy balance by inducing a decrease in circulating levels of fatty acids, cholesterol, triglycerides and leptin, and improved insulin sensibility (Cota et al., 2009; Scheen & Paquot, 2009). To our knowledge the present study is the first to demonstrate a link between the improvement of plasma levels of triglycerides and cholesterol and the modulation of neural proliferation by AM251, the latter mainly by reducing the number of BrdU+ cells in the hypothalamus. Additionally, the consumption of dietary fats is responsible for the induction of inflammation and activation of apoptotic signalling pathways in the hypothalamus (Velloso et al., 2008; Moraes et al., 2009). Thereby, Garris (1989) described that obese (db/db) mutant mice exhibit an increase in the number of degenerated neurons in the hypothalamic ventromedial and arcuate nuclei. Activation of apoptotic signalling pathways in the hypothalamus plays a role in the development of leptin and insulin resistance (Moraes et al., 2009), and hence this process may be counteracted by the anti-apoptotic property of the cannabinoid antagonists/inverse agonists, including AM251 (Pozzoli et al., 2006; Widmer et al., 2008). In this context, note that triglycerides can impair leptin transport across the blood–brain barrier, which in part may account for the peripheral leptin resistance seen in obesity (Banks et al., 2004). Several studies have demonstrated a constitutive neuroproliferative activity in the rodent hypothalamus (Kokoeva et al., 2005, 2007; Xu et al., 2005; Perez-Martin et al., 2010). Moreover, neural proliferation in the hypothalamus is modulated by several growth factors such as BDNF, EGF, bFGF, CNTF and IGF-I (Pencea et al., 2001; Kokoeva et al., 2005, 2007; Xu et al., 2005; Perez-Martin et al., 2010), which may include cannabinoid ligands. Here, we propose that obese states might increase proliferation of hypothalamic neurons involved in the maintenance of energy balance, and that the administration of a drug capable of restoring energy balance could induce proliferation to return to normal. As the standard and high-fat diets used in the present experimental design were hypercaloric, we did not see any differential increase on neural proliferation according to diet. The higher efficacy of AM251 (also found in other studies) on HFD-induced obese animals is associated with a decrease in neural proliferation. Therefore, regardless of whether AM251 blocks or reduces cell death in the hypothalamus, it is not contradictory with AM251 reducing cell proliferation. As occurs with AM251, CNTF also induces weight loss in obese rodents and humans (Lambert et al., 2001). However, in contrast to our results with AM251, CNTF induces cell proliferation in the mouse hypothalamus (Kokoeva et al., 2005).
Understanding the regulation of adult neurogenesis is important given the proposed role of brain recovery from insults such as ageing, ischaemia, stroke, Alzeimer’s disease and Huntington’s disease (Kuhn et al., 1996; Jin et al., 2001, 2004). Hence, the modulation of neural proliferation by CB1 signalling may be one of the adaptive mechanisms that the brain uses to limit functional impairment, including obesity.
Acknowledgements
The anti-BrdU monoclonal antibody developed by Stephen J. Kaufman was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City. This study was supported by grants from the European Union’s 7th Framework Programme (Health-F2-2008-223713, Reprobesity); the following grants from the Spanish Ministry of Science and Innovation: National Institute of Health ‘Carlos III’ (PI07/1226, PI07/0880, SAF2010-19087), UE-ERDF, Red de Trastornos Adictivos (RD06/0001/0000), CIBER OBN and SAF2010-19087; grants from the Spanish Ministry of Education and Science (BFU 2006-11754); grants from the Consejería de Economía, Innovación y Ciencia de la Junta de Andalucía, UE/ERDF (P07-CVI-03079, P07-CVI-01038, CTS433, CVI1038 and 740486); and a grant from the Consejería de Salud de la Junta de Andalucía (PI0232/2008, SAS06-0029 and SAS08-0029), Spain. F.J.B.S. is a recipient of a ‘Miguel Servet’ research contract from the National System of Health (Instituto de Salud Carlos III, grant number CD07/00283). J.S. is a recipient of a ‘Sara Borrell’ postdoctoral contract from the National System of Health (Instituto de Salud Carlos III, grant number CD08/00203).
Abbreviations
-
- BrdU
-
- 5′-bromo-2′-deoxyuridine
-
- CB1/CB2
-
- cannabinoid receptor type 1 and 2
-
- ECS
-
- endocannabinoid system
-
- HFD
-
- high-fat diet
-
- STD
-
- standard/low-fat diet
-
- SGZ
-
- subgranular zone of the dentate gyrus
-
- SVZ
-
- subventricular zone of the lateral ventricle
-
- VMH
-
- ventromedial nucleus of the hypothalamus




