Post-traumatic seizure susceptibility is attenuated by hypothermia therapy
Abstract
Traumatic brain injury (TBI) is a major risk factor for the subsequent development of epilepsy. Currently, chronic seizures after brain injury are often poorly controlled by available antiepileptic drugs. Hypothermia treatment, a modest reduction in brain temperature, reduces inflammation, activates pro-survival signaling pathways, and improves cognitive outcome after TBI. Given the well-known effect of therapeutic hypothermia to ameliorate pathological changes in the brain after TBI, we hypothesized that hypothermia therapy may attenuate the development of post-traumatic epilepsy and some of the pathomechanisms that underlie seizure formation. To test this hypothesis, adult male Sprague Dawley rats received moderate parasagittal fluid-percussion brain injury, and were then maintained at normothermic or moderate hypothermic temperatures for 4 h. At 12 weeks after recovery, seizure susceptibility was assessed by challenging the animals with pentylenetetrazole, a GABAA receptor antagonist. Pentylenetetrazole elicited a significant increase in seizure frequency in TBI normothermic animals as compared with sham surgery animals and this was significantly reduced in TBI hypothermic animals. Early hypothermia treatment did not rescue chronic dentate hilar neuronal loss nor did it improve loss of doublecortin-labeled cells in the dentate gyrus post-seizures. However, mossy fiber sprouting was significantly attenuated by hypothermia therapy. These findings demonstrate that reductions in seizure susceptibility after TBI are improved with post-traumatic hypothermia and provide a new therapeutic avenue for the treatment of post-traumatic epilepsy.
Introduction
A significant, debilitating consequence of traumatic brain injury (TBI) is the development of seizures (Annegers et al., 1998; Vespa et al., 1999). Nearly 40–50% of severe TBI patients develop epilepsy and brain injuries account for 20% of epilepsy (Herman, 2002; Garga & Lowenstein, 2006). Unfortunately, post-traumatic epilepsy is frequently intractable to standard antiepileptic medications (Temkin et al., 2001; Loscher & Schmidt, 2002). Thus, it is important to develop therapeutic interventions targeting the pathological mechanisms that underlie post-traumatic epilepsy.
There are several parallel pathomechanisms of some forms of epilepsy and TBI. Hippocampal epilepsy with mesial temporal sclerosis and TBI both result in a stereotypical neurodegeneration pattern in the hippocampus that includes dentate hilus neuronal loss (Babb et al., 1991; Lowenstein et al., 1992; Bramlett et al., 1997; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004; Swartz et al., 2006). Hilar interneurons exert control on excitation levels in the dentate gyrus and loss of hilar neurons results in hyperexcitability changes contributing to future seizures (Sutula et al., 1989; Lukoyanov et al., 2004).
After both TBI and status epilepticus, cell division in the dentate gyrus is markedly affected. Double-labeling immunocytochemical studies using 5-bromo-deoxyuridine and neuronal nuclear protein (NeuN) have revealed that cell proliferation increases in the dentate gyrus and peaks within 1–2 weeks after TBI (Dash et al., 2001; Braun et al., 2002; Sun et al., 2005; Urrea et al., 2007). Similarly, after status epilepticus, neural progenitor cells proliferate in the dentate gyrus (Parent et al., 1997; Huttmann et al., 2003; Jessberger et al., 2005; Indulekha et al., 2010). Although a subject of debate, some of these cells could develop neuronal phenotypes, and potentially project aberrant axons to the CA3 pyramidal cell region as well as into the dentate hilus (Parent et al., 1999; Jessberger et al., 2007; Shapiro et al., 2007; Nitta et al., 2008).
Another shared feature of hippocampal epilepsy and TBI is abnormal sprouting of the mossy fiber pathway in the dentate gyrus. In both human hippocampal epilepsy and TBI, mossy fiber sprouting has been observed at chronic time-points (Sutula et al., 1988; Houser et al., 1990; Babb et al., 1991; Santhakumar et al., 2000; Golarai et al., 2001; Kharatishvili et al., 2006). Although not a cause of epilepsy, sprouting of the mossy fiber pathway increases the number of recurrent connections on dentate granule cells, further increasing hippocampal excitability (Lowenstein et al., 1992; Dudek et al., 1994; Represa et al., 1994; Coulter et al., 1996; Nadler, 2003; Morimoto et al., 2004).
Hypothermia treatment is a highly promising therapy that improves structural and functional outcome measures after experimental and clinical TBI (Polderman, 2008; Dietrich et al., 2009). Lowering brain temperature after a traumatic brain insult dramatically reduces histopathology, and also improves behavioral recovery (Clifton et al., 1991; Lyeth et al., 1993; Dietrich et al., 1994; Bramlett et al., 1995; Suzuki et al., 2003; Gao et al., 2010). In this study, we tested the hypothesis that hypothermia attenuates seizure susceptibility changes after TBI.
Materials and methods
Traumatic brain injury model
Three experimental groups (n = 49) were used for seizure assessment and histopathology analysis – normothermic sham surgery animals (n = 17), normothermic TBI animals (n = 16) and hypothermic TBI animals (n = 16). Male Sprague Dawley rats (270–320 g) were anesthetized with 3% halothane, 70% N2O and 30% O2 and received a 4.8 mm craniotomy (3.8 mm posterior to bregma, 2.5 mm lateral to the midline) to anchor a plastic injury hub (3.5 mm inside diameter) over the right parietal cortex. At 24 h after the craniotomy, the animals were reanesthetized with 1.5% halothane, 70% N2O and 30% O2 and intubated. Pancuronium bromide (0.5 mg/kg, i.v.) was administered to facilitate mechanical ventilation. Arterial blood pressure, blood gases and blood pH were monitored for 30 min prior to and up to 4 h after TBI to maintain physiological ranges of blood pH between 7.35 and 7.45, PCO2 between 35 and 40 mmHg and PO2 between 105 and 140 mmHg. After stabilization, the animals received a moderate (1.8–2.2 atm) fluid-percussion pulse (22 ms pulse duration) or sham injury. Brain temperature was indirectly monitored with a probe placed in the left temporalis muscle and core temperature was monitored with a rectally placed thermistor. The temporalis muscle temperature has been shown in a previous study to be an accurate reflection of brain temperature in the range of 30–40 °C (Jiang et al., 1991). Self-adjusting feedback warming lamps were used to control brain temperature. The brains of normothermic animals were maintained at 36.5–37.0 °C and hypothermic animals were maintained at 33.0–33.6 °C by gently blowing cooled air over the head. Hypothermia was initiated at 30 min post-injury and maintained for 4 h. The animals were allowed to slowly rewarm to normothermia at ambient temperature of 22–23 °C over 2 h. All experiments were conducted according to protocols approved by the University of Miami Animal Care and Use Committee and carried out according to the NIH Guide for the Care and Use of Laboratory Animals.
Seizure susceptibility determination
At 12 weeks post-surgery, animals were allowed to habituate to the animal testing room in a clear plastic cage for 10 min prior to behavioral testing. Animals received pentylenetetrazole (PTZ) (30 mg/kg; Sigma-Aldrich, St Louis, MO, USA) intraperitoneally. This dose is based on previous reports and our preliminary results (not shown) evaluating a dose–response curve of PTZ in naive 3-month-old Sprague Dawley rats to determine a dose that is at the threshold of eliciting seizures in sham animals (Andre et al., 1998; Erakovic et al., 2001). After receiving PTZ, each animal was observed for 1 h by an investigator who was blinded to the treatment groups to measure seizure frequency and seizure class. Seizure class was scored using a modified scale as follows (Racine, 1972): Class 1, myoclonic (brief shock-like jerks of a muscle or group of muscles); Class 2, unilateral clonic (rhythmic, rapidly alternating contraction and relaxation of a muscle or muscle group) lasting < 1 min; Class 3, bilateral clonic lasting < 1 min; Class 4, bilateral clonic sustained, lasting more than 1 min; Class 5, tonic–clonic (all muscles stiffen with loss of postural control alternating with sustained clonic); and Class 6, terminal tonic–clonic (class 5 that results in death).
Electrocorticography recordings
At 12 weeks after sham or TBI surgery, a separate, additional group of animals (sham, n = 5; TBI normothermia, n = 5; TBI hypothermia, n = 5) were analyzed only for seizure susceptibility after PTZ using both behavioral and electrocorticography (ECoG) recordings. Animals were anesthetized (3% isoflurane, 70% N2O and 30% O2, 5 min) and three cortical screw electrodes (Plastics One Inc., Roanoke, VA, USA) were placed into the skull over the parietal cortex, two caudal to the craniotomy made for the TBI surgery (−3.8 mm bregma, 2.5 mm lateral to the midline) and one indifferent electrode over the contralateral hemisphere caudal to the center of the craniotomy. ECoG activity was recorded differentially between the electrodes (E1 and E2) implanted ipsilateral to the injury site. The contralateral indifferent electrode (Ei) was connected to the cable shield and amplifier ground. The screw electrodes and their lead wires were cemented to the skull with dental acrylic. Upon recovery from anesthesia, the animals were placed in a clear plastic cage and the wires from the electrodes were attached to a flexible swivel and a differential pre-amplifier (CWE Inc., Ardmore, PA, USA). The electrical signals were amplified, filtered (1–30 Hz) and stored in digital form using a DI-720 digitizer (DATAQ Instruments, Akron, OH, USA). After 5 min of baseline ECoG recordings, the animals received PTZ (30 mg/kg, i.p.), and were simultaneously recorded for 60 min and scored for seizure number and class. Animals were perfused with saline (2 min, 4 °C, 75 mL), then with 4% paraformaldehyde in phosphate buffer (30 min, 4 °C, 350 mL) at 1 day after ECoG recordings and behavioral analysis. During the perfusion and brain removal, all skulls and brains were inspected to ensure that penetration of the dura mater did not occur. One animal was discarded in the analysis due to an injury to the dura.
Stereology
At 24 h after seizure determination, animals were anesthetized (3% halothane, 70% N2O and 30% O2) and perfused with 0.2% sodium sulfide (80 mL), and then with 4% paraformaldehyde in phosphate-buffered saline (350 mL). The brains were cryoprotected (30% sucrose in phosphate-buffered saline) and sectioned on a freezing microtome (50 μm thick). Serial sections spaced 300 μm apart were immunostained with mouse anti-NeuN (1 : 500; Millipore, Temecula, CA, USA) or goat anti-doublecortin (1 : 500, C-18 and N-19; Santa Cruz Biotechnology, Santa Cruz, CA, USA) (Atkins et al., 2007b; Shapiro et al., 2007). Immunostaining was developed with anti-mouse or anti-goat IgG (1 : 200), ABC Elite (Vector Laboratories, Burlingame, CA, USA) and 2.5% nickel ammonium sulfate acetate–imidasole buffer, 0.05% 3,3′-diaminobenzidine and 0.001% H2O2 (Vector Laboratories). For both antibodies, anti-NeuN and anti-doublecortin, antibody penetration through the entire section for all animals was verified prior to analysis. The dentate hilus and dentate granule cell layers were contoured at 5× using stereoinvestigator software 7.50.1 (MicroBrightField, Williston, VT, USA) with a BX51TRF microscope (Olympus America, Center Valley, PA, USA) by a blind observer. Sections between bregma levels −3.6 and −4.8 mm were chosen for analysis; this focused the cell-counting analysis near the epicenter of the injury (bregma level −3.8 mm), and also these bregma levels were unequivocally identifiable in all animals. A counting grid of 50 × 50 μm was placed over the dentate hilus region, and a counting grid of 75 × 75 μm was used for the dentate granule cell layer. For sections immunostained with anti-NeuN, the section thickness was 35 μm and the optical disector height was 25 μm with 5 μm guard zones. For sections immunostained with anti-doublecortin, the section thickness was 30 μm, the optical disector height was 22 μm and the guard zones were 4 μm. Using a 50 × 50 μm counting frame for the dentate hilus and a 60 × 60 μm counting frame for the dentate granule cell layer, NeuN- or doublecortin-positive cells were counted in 25–90 randomly-placed sampling sites with a 63×, 1.42 NA objective. For dentate hilus cell counts, Q values ranged from 92 to 477, and CE2/CV2 values were 0.11, 0.57 and 0.18 for the sham, TBI normothermia and TBI hypothermia groups, respectively. For the doublecortin-positive cell counts, the Q range was 47–314, and CE2/CV2 values were 0.12 for the sham group, 0.15 for the TBI normothermic group and 0.12 for the TBI hypothermia group.
Images were taken with 20× and 60× objectives on a BX51TRF microscope (Olympus America) and montaged using the virtual slice module in the neurolucida 7.50.1 software program (MicroBrightField).
Timm staining
Sections were developed with 14% gum arabic, 2.5% citric acid, 2.3% trisodium citrate, 1.7% hydroquinone and 0.08% silver nitrate (Seress & Gallyas, 2000). Sections were developed in parallel for each animal treatment, and then stopped simultaneously with 5% sodium thiosulfate. Timm staining was scored by four investigators blinded to the treatment groups at bregma levels −3.3, −4.3 and −5.8 mm as follows: 0, no Timm granules; 1, sparse Timm granules in the supragranular cell layer; 2, continuously distributed Timm granules in the supragranular cell layer; 3, continuously distributed Timm granules in the supragranular cell layer with patches of confluency; 4, a confluent dense band of Timm granules in the supragranular cell layer; and 5, a band as in 4 that extended into the inner molecular layer (Golarai et al., 2001). Images of the dentate gyrus were taken at 20× and 60× magnification at bregma level −4.3 mm.
Statistical analysis
Data presented are mean ± SEM. Results of the seizure number, seizure class and Timm scoring were analyzed using the Kruskal–Wallis anova on Ranks test with post-hoc Mann–Whitney U t-test. Dentate hilar neuronal counts and doublecortin cell counts were analyzed using a one-way anova with post-hoc Tukey HSD t-test. Significance was set at P < 0.05.
Results
Hypothermia reduces seizures after traumatic brain injury
To determine if post-traumatic seizures are improved with hypothermia treatment, we assessed seizure susceptibility in rats after moderate parasagittal fluid-percussion brain injury (FPI) and normothermia or hypothermia treatment. As moderate FPI does not typically elicit behaviorally visible, spontaneous seizures (Golarai et al., 2001; Santhakumar et al., 2001; Kharatishvili et al., 2006), we utilized the GABAA receptor antagonist PTZ to study the seizure threshold by challenging the injured brain with a decrease in inhibition. Normothermic TBI animals exhibited a significant increase (H2 = 6.904, P < 0.05) in the total number of seizures as compared with sham surgery animals. There was a significant decrease (P < 0.05) in the number of seizures observed in animals treated with post-traumatic hypothermia as compared with TBI normothermic animals (Fig. 1). There was also an increase, although not significant, in the highest seizure class reached in TBI normothermic animals as compared with sham surgery animals and this was not reduced with hypothermia treatment. The time of seizure onset was not statistically different for any animal group (sham, 2.45 ± 0.19 min; TBI normothermia, 4.21 ± 1.88 min; TBI hypothermia, 3.12 ± 0.24 min).
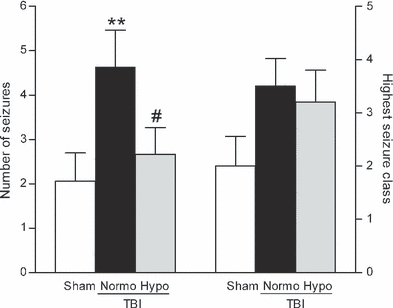
Seizure susceptibility was determined by challenging animals with a decrease in inhibition using PTZ (30 mg/kg, i.p.), a GABAA receptor antagonist, at 12 weeks after recovery from surgery. There was a significant increase in the number of seizures exhibited by each TBI animal (**P < 0.01) at 12 weeks after TBI (n = 16) as compared with sham surgery animals (n = 17). The increase in seizure numbers after TBI was not observed in TBI hypothermic animals (n = 16, #P < 0.05 for seizure number of TBI normothermic vs. TBI hypothermic animals). The highest seizure class reached by each animal was increased, although not significantly, in both TBI normothermic (n = 16) and TBI hypothermic (n = 16) animals as compared with sham surgery animals (n = 17). Data represent mean ± SEM.
To ensure that the behavioral rating scale was accompanied by electrophysiological changes, ECoG recordings were performed in a separate, additional group of animals (Fig. 2). At 12 weeks after sham or TBI surgery, animals were implanted with electrodes in the skull over the parietal cortex. Baseline ECoG recordings were conducted for 5 min prior to PTZ administration (30 mg/kg, i.p.), and for 60 min post-PTZ with simultaneous behavioral assessment. Seizure classes 1–5 identified by a behavioral scorer who was blinded to the ECoG recordings were associated with the ECoG recordings.

Representative ECoG recordings from animals at 12 weeks post-surgery. Electrodes were placed caudally from the craniotomy (E1 and E2) and the indifferent electrode (Ei) was placed in the contralateral hemisphere (A) (adapted with permission from Paxinos & Watson, 2005). A baseline recording was initiated at 5 min prior to PTZ (30 mg/kg, i.p.) and recordings were performed for 60 min (B). Simultaneous blinded behavioral scoring was conducted. For each seizure classification, periods of hyperexcitability consisting of high-amplitude single spikes or combinations of single spikes and repetitive spike discharges were observed on the ECoG records (C).
Dentate gyrus cell loss is not reduced with hypothermia therapy
As the FPI model results in stereotypical cell death in the dentate hilus, an area that exerts inhibitory control over the dentate gyrus, we determined whether hypothermia reduced post-traumatic seizures by reducing dentate hilar neuronal death (Lowenstein et al., 1992; Bramlett et al., 1997; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004; Kharatishvili et al., 2006). After seizure assessment had been performed, the animals were perfused, and serial sections were analyzed by stereology to measure the numbers of NeuN-positive cells in the dentate hilus. As has been reported previously, we observed a significant (F2,40 = 13.39, P < 0.001) decrease in the surviving numbers of dentate hilar neurons in TBI normothermic animals as compared with sham surgery animals (Fig. 3) (Lowenstein et al., 1992; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004; Kharatishvili et al., 2006). However, in agreement with previous studies, we found that hypothermia treatment did not rescue the hilar cell loss observed after trauma (Bramlett et al., 1997).

Dentate hilus neuronal survival was not rescued with hypothermia treatment. The dentate gyrus was immunostained with NeuN to identify surviving neurons (A). Images were taken at 20× magnification and montaged using NeuroLucida. Both TBI normothermic (TBI Normo) and TBI hypothermic (TBI Hypo) animals had fewer remaining NeuN-positive cells in the dentate hilus as compared with sham animals (Sham). Dentate hilar neurons were quantified by stereology (B). Both TBI normothermic and TBI hypothermic animals had fewer surviving dentate hilar neurons at 12 weeks post-injury as compared with sham surgery animals (Sham, n = 15; TBI normothermic, n = 12; TBI hypothermic, n = 16). ***P < 0.001 for sham vs. TBI normothermic or TBI hypothermic animals. Data represent mean ± SEM. Scale bars, 200 μm.
To determine if neurogenesis, another feature of epilepsy, is also affected by brain trauma at a chronic time-point after injury, we examined the numbers of doublecortin-positive cells in the dentate gyrus at 12 weeks after FPI (Parent et al., 1997; Huttmann et al., 2003; Jessberger et al., 2005). There was a significant (F2,40 = 3.415, P < 0.05) decrease in doublecortin-positive cells in both TBI normothermic and hypothermic animals post-seizure as compared with sham surgery animals (Fig. 4). A qualitative comparison suggests that the processes retained more lateral projections and this was observed in both normothermic and hypothermic TBI animals. Together, these findings suggest that early hypothermia treatment does not prevent increases in seizure susceptibility by rescuing dentate gyrus cell loss after TBI.
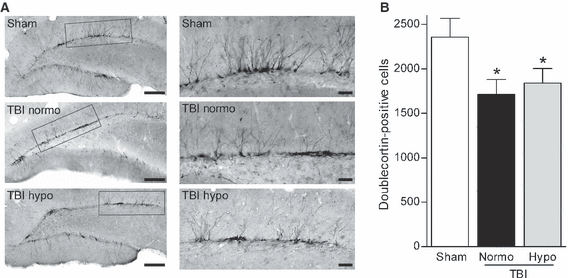
Numbers of doublecortin-positive cells were decreased at chronic time-points after brain injury and this decrease was not rescued by hypothermia therapy. Low-magnification images (20×) of doublecortin immunostaining of the dentate gyrus at 12 weeks post-TBI (A). Higher magnification (60×) of doublecortin-positive cells revealed that the dendritic branches were more laterally oriented in the injured hippocampus as compared with the non-injured hippocampus (B). Quantification by stereology revealed that numbers of doublecortin-positive cells were significantly decreased in both TBI normothermic (TBI Normo) (n = 12) and TBI hypothermic (TBI Hypo) (n = 16) animals as compared with sham animals (n = 15) (C). *P < 0.05 for sham vs. TBI normothermic or TBI hypothermic animals. Data represent mean ± SEM. Scale bars: (A) 200 μm; (B) 50 μm.
Mossy fiber sprouting is attenuated by hypothermia treatment
Mossy fiber sprouting occurs in patients with temporal lobe epilepsy, and is seen in experimental models of brain injury (Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991; Santhakumar et al., 2000; Golarai et al., 2001; Kharatishvili et al., 2006). To determine if hypothermia therapy attenuates mossy fiber sprouting after FPI, we performed Timm staining and scored for the amount of mossy fiber sprouting in TBI normothermic animals as compared with TBI hypothermic and sham surgery animals at 24 h after seizure assessment with PTZ (Fig. 5). Previous work has shown that PTZ at 30 mg/kg does not induce mossy fiber sprouting within 24 h of administration (Golarai et al., 2001). We found that mossy fiber sprouting was present at 12 weeks after brain injury, and this traumatic consequence was attenuated in TBI hypothermic animals (H8 = 29.93, P < 0.001). These results indicate that hypothermia treatment may reduce the increases in seizure susceptibility by reducing aberrant axonal sprouting in the dentate gyrus.
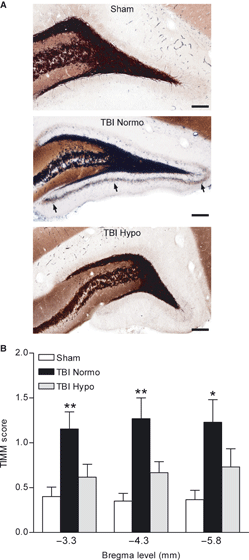
Timm staining of the dentate gyrus of the hippocampus. The mossy fiber pathway is clearly delineated in black. Mossy fiber sprouting onto the supragranular cell layer was observed in 12 week TBI normothermic animals (TBI Normo, arrows), and this was attenuated in 12 week TBI animals treated with hypothermia (TBI Hypo). Images (20 or 60×) of the dentate gyrus are shown at bregma level −3.8 mm (A). Timm scores were significantly higher in TBI normothermic animals (n = 13) as compared with sham animals at all bregma levels assessed (B). No significant differences were found between sham (n = 15) and TBI hypothermic (n = 15) animals (*P < 0.05, **P < 0.01 for TBI normothermic vs. sham animals). Data represent mean ± SEM. Scale bars, 200 μm.
Discussion
Developing a therapy to prevent seizure susceptibility after brain injury is of paramount importance given that current antiepileptic medications are not sufficient to prevent post-traumatic epilepsy (Temkin, 2009). However, previous studies of experimental models of post-traumatic epilepsy have been hampered by the need to use a brain injury that is severe and, even with severe brain injury, only a subset of animals eventually develop spontaneous seizures in the months to years after the TBI (Kharatishvili et al., 2006; Hunt et al., 2009, 2010; Kharatishvili & Pitkanen, 2010). These limitations have hindered the testing of therapeutic strategies to reduce the development of post-traumatic epilepsy. In this study, we report that seizure susceptibility can be reliably observed after moderate FPI by challenging the injured brain with a decrease in inhibitory control utilizing the GABAA receptor antagonist, PTZ.
Using this method to assess seizure susceptibility, we observed significant increases in seizure number in moderate TBI animals as compared with sham animals at 12 weeks post-injury. Furthermore, we were able to test the effectiveness of a highly promising therapy currently in TBI clinical trials to determine if post-traumatic seizure susceptibility can be prevented (Polderman, 2008). We found that hypothermia therapy, a modest reduction in brain temperature for only 4 h after brain injury, significantly reduced the number of chronic seizures elicited by PTZ and attenuated a pathological feature of epilepsy, i.e. mossy fiber sprouting. These results indicate that assessing seizure susceptibility may be an effective method to evaluate potential therapeutic strategies for post-traumatic epilepsy and thereby may be an important mechanism by which early cooling may improve the outcome in patients with TBI.
We found that the observed behavioral changes induced by PTZ were associated with abnormal electrical activity with ECoG recordings. However, as depth recordings within the hippocampus were not performed, we could not determine if isolated hippocampal seizures were affected by hypothermia therapy. In addition, frequencies above the β3F band were filtered, and gamma frequencies were not examined (Lehmkuhle et al., 2009).
Although hypothermia treatment reduced seizure frequency after TBI, seizure severity did not improve. Both seizure frequency and seizure severity correlate with poorer quality of life in epileptic patients, and reducing both aspects of epilepsy should be considered when developing a therapy for post-traumatic epilepsy (Bautista & Glen, 2009). The pathomechanisms of post-traumatic seizures are likely to be multifactorial and, given that hypothermia therapy reduced only one pathology feature, i.e. mossy fiber sprouting, our results suggest that a more prolonged duration of cooling and/or a combinatorial therapeutic strategy of hypothermia with a pharmacological agent may be required to target post-traumatic susceptibility to increases in both seizure frequency and severity (Margulies & Hicks, 2009).
As previously reported, hypothermia therapy did not prevent the loss of the vulnerable cell population of dentate hilar neurons that are rapidly and selectively lost after brain injury (Lowenstein et al., 1992; Bramlett et al., 1997; Golarai et al., 2001; Santhakumar et al., 2001; Grady et al., 2003; D’Ambrosio et al., 2004). One caveat of this interpretation is that we assessed dentate hilar neuronal loss at 24 h after seizure induction and assessment. Although none of our animals exhibited status epilepticus, it is possible that additional neuronal loss occurred as a result of the PTZ treatment, compounding the effects of the brain injury on hilar cell death (Ben-Ari, 1985; Buckmaster & Dudek, 1997; Borges et al., 2003). The loss of hilar cells could reflect both interneuronal cells as well as mossy cells as we assessed NeuN-positive cells (Amaral, 1978; Freund & Buzsaki, 1996). Both of these cell populations help the dentate gyrus act as a gatekeeper in preventing excessive excitatory stimulation (Cavazos et al., 1994; Sloviter, 1994; Buckmaster & Jongen-Relo, 1999). It is critical to develop a pharmacological therapy to prevent the loss of both of these hilar cell populations, and possibly to use in conjunction with hypothermia therapy.
Previous reports have demonstrated an increase in cell proliferation using 5-bromo-deoxyuridine labeling, or other markers of immature neurons after brain injury (Dash et al., 2001; Braun et al., 2002; Sun et al., 2007; Urrea et al., 2007). However, this increase does not last in the hippocampus, as other studies have demonstrated that doublecortin-positive cells are decreased from 14 days to 6 weeks post-injury (Rola et al., 2006; Gao et al., 2008; Potts et al., 2009). To our knowledge, this is the first report of a loss of doublecortin-positive cells at 3 months after brain injury and induction of seizures. Although there was no difference in the loss of doublecortin-positive cells between TBI normothermic and TBI hypothermic animals, given that we assessed doublecortin-positive cells at 24 h after a period of seizure induction, we cannot rule out that this loss is due, in part, to the induced period of seizures elicited by PTZ. Qualitatively, we observed that the remaining doublecortin-positive cells exhibited an immature morphology in TBI animals as compared with sham animals (Walter et al., 2007). After status epilepticus, one hypothesis is that newly generated granule cells could potentially project ectopically to the hilus, creating recurrent excitatory circuitry (Ribak et al., 2000; Austin & Buckmaster, 2004; Shapiro et al., 2007; Walter et al., 2007). It remains to be determined whether the surviving doublecortin-positive cells in the injured hippocampus develop hilar basal dendrites.
A common feature of hippocampal epileptogenesis is aberrant mossy fiber sprouting of dentate granule cells to the supragranular cell layer of the dentate gyrus (Sutula et al., 1989; Houser et al., 1990; Babb et al., 1991). We found that, of all the pathomechanisms analyzed, only mossy fiber sprouting was suppressed by 4 h of early post-traumatic hypothermia therapy. This result suggests that hypothermia selectively attenuated the molecular mechanisms that stimulate mossy fiber sprouting after brain trauma. Although the density of sprouting is modest, previous reports have found that Timm scores ranging from 1 to 2 can correlate with significant hippocampal-dependent cognitive dysfunction (Cilio et al., 2003; Lukoyanov et al., 2004). The molecular determinants of mossy fiber sprouting are still unknown. One potential mechanism that hypothermia may have affected is semaphorin expression. Semaphorins are secreted proteins that are critically involved in neural development by sculpting axon growth by repulsive or attractive effects (Zhou et al., 2008). The mRNA levels of sema3A, F and C are decreased after status epilepticus and temporal lobe epilepsy, and knockout mice of Sema3F are prone to seizure activity (Barnes et al., 2003; Holtmaat et al., 2003; Sahay et al., 2005). Hypothermia therapy has been reported to regulate transcription factors within the hippocampus and may have regulated gene transcription of semaphorins (Atkins et al., 2007a). Another possibility is that hypothermia altered brain-derived neurotrophic factor and trkB receptor signaling and we have previously observed significant effects of hypothermia on downstream targets of the trkB receptor (Dinocourt et al., 2006; Atkins et al., 2007a). However, mossy fiber sprouting is thought to be a consequence, not a causal factor, in the development of post-traumatic epilepsy (Cronin & Dudek, 1988; Sloviter, 1992; Zhang et al., 2002; Morimoto et al., 2004). Thus, hypothermia treatment probably had effects on other temperature-sensitive injury mechanisms that have been suggested to underlie seizure susceptibility (Dietrich et al., 2009).
Another pathological aspect of seizure susceptibility that hypothermia could have affected was electrophysiological alterations in the injured hippocampus. After brain injury, the dentate gyrus exhibits hyperexcitability, resulting from changes in voltage-gated ion channels and GABA receptors as well as impaired potassium buffering by astrocytes (Lowenstein et al., 1992; D’Ambrosio et al., 1999; Ross & Soltesz, 2000; Santhakumar et al., 2001; Griesemer & Mautes, 2007; Hunt et al., 2009). Current studies are assessing other electrophysiological alterations that may also have contributed to the reduction in seizure susceptibility by hypothermia therapy.
Given the low seizure threshold of the hippocampus and the many pathological changes that occur in the hippocampus after brain injury, it is likely that the PTZ-induced seizures involved the hippocampus. In human post-traumatic epileptic patients, between 35 and 62% have epilepsy originating in the temporal lobe (Diaz-Arrastia et al., 2000; Hudak et al., 2004). However, the overlying parietal cortex also exhibits neuronal loss, inflammation, astrogliosis and circuit reorganization, suggesting that this damaged region may also be involved (D’Ambrosio et al., 2005; Kharatishvili et al., 2006; Kharatishvili & Pitkanen, 2010). In both injured regions, hypothermia rescues neuronal death as well as potentiates cell survival pathways, and it is unclear if hypothermia reduced seizure frequency by effects on the hippocampus, parietal cortex or other areas (Lotocki et al., 2006; Atkins et al., 2007a). The multiplicity of the effects of hypothermia in reducing pathology is perhaps its strongest therapeutic feature (Dietrich et al., 2009).
Therapeutic hypothermia is one of the few treatments that have been successfully translated to select patient populations (Marion & Bullock, 2009). For example, therapeutic hypothermia has benefited patients following cardiac arrest, post-natal infants with hypoxic insults, and patients with severe TBI in many single-institution clinical TBI studies (Marion et al., 1997; Hachimi-Idrissi et al., 2001; Bernard et al., 2002; Mayer, 2002; Gunn et al., 2005; Shankaran et al., 2005; Jiang et al., 2006; Polderman, 2008). However, hypothermia has not passed Phase III clinical trials for the treatment of TBI, and secondary complications arising from the use of systemic hypothermia require management (Hayashi, 2009; Polderman, 2009; Polderman & Herold, 2009). The translation of hypothermia animal studies to clinical trials remains challenging and further understanding of the therapeutic time window, optimal duration and degree of cooling would greatly facilitate the development of hypothermia as a potential therapy for post-traumatic epilepsy.
The latency period for developing seizures is a time period of variable duration of the scale of months to years; however, the critical time window for therapeutics to attenuate the development of post-traumatic epilepsy has been proposed to be within 3 days of injury (Salazar et al., 1985; Graber & Prince, 2004). Although antiepileptic drugs are often administered prophylactically in the hours to days after brain injury, they do not always suppress the development of chronic seizures (Temkin, 2009). We demonstrated that early post-traumatic hypothermia treatment for only 4 h had a pronounced, long-lasting effect on seizure susceptibility even when tested at 12 weeks after brain injury. Our results demonstrate that hypothermia may be an efficacious and unique antiseizure medication, perhaps in both the acute setting and in chronic stages after injury.
Acknowledgements
This work was supported by the National Institutes of Health (NS030291 and NS042133). We thank Stephanie DaSilva for technical assistance and Dr. Edward Green for helpful discussions.
Abbreviations
-
- ECoG
-
- electrocorticography
-
- FPI
-
- fluid-percussion brain injury
-
- NeuN
-
- neuronal nuclear protein
-
- PTZ
-
- pentylenetetrazole
-
- TBI
-
- traumatic brain injury




