Quantitative demonstration of comparable architectonic areas within the ventromedial and lateral orbital frontal cortex in the human and the macaque monkey brains
Abstract
The orbital and ventromedial frontal cortical regions of the human and the macaque monkey brains include several spatially discrete areas which are defined histologically by their distinctive laminar architecture. Although considerable information has been collected on the function and anatomical connections of specific architectonic areas within the orbital and ventromedial frontal cortex of the macaque monkey, the location of comparable areas in the human brain remains controversial. We re-examined the comparability of orbital and ventromedial frontal areas across these two species and provide the first quantitative demonstration of architectonically comparable cortical areas in the human and the macaque brains. Images of Nissl-stained sections of the cortex were obtained at low magnification. Differences in the typical size of neurons in alternating pyramidal and granule cell layers were exploited to segregate the cortical layers before sampling. Profiles of areal neuronal density were sampled across the width of the cortex. The location of individual cortical layers was identified on each profile by sampling a set of equally sized images on which the cortical layers had been manually traced. The rank order of sampled architectonic features in comparable architectonic areas in the two species was significantly correlated. The differences in measured features between gyral and sulcal parts of the same architectonic area are at a minimum 3–4 times smaller than the differences between architectonic areas for the areas examined. Furthermore, the quantified architectonic features arrange areas within the orbital and ventromedial frontal cortex along two dimensions: an anterior-to-posterior and a medial-to-lateral dimension. On the basis of these findings, and in light of known anatomical connections in the macaque, this region of the human cortex appears to comprise at least two hierarchically structured networks of areas.
Introduction
Human orbital and ventromedial frontal cortex dysfunction has been implicated in a spectrum of emotion-related psychiatric syndromes, including mood disorders, addiction, schizophrenia and psychopathy (Goldstein et al., 1999; Blair, 2004; Everitt et al., 2007; Ressler & Mayberg, 2007). Damage to the human orbital and adjacent ventromedial frontal cortex (Fig. 1) has long been associated with emotional and motivational deficits that, although as yet incompletely understood, have devastating real-life consequences in terms of patients’ interpersonal, occupational and financial behavior (Harlow, 1848/1999; Damasio, 1996). New research, combining clinical and neuroimaging data obtained on the human brain with the results of experimental techniques in the macaque that are not possible in human subjects, has started to reveal the contribution of individual architectonic cortical areas to specific emotion-related processes (Kringelbach & Rolls, 2004; Phelps et al., 2004; Murray et al., 2007). The success of this effort hinges on the ability of researchers to identify accurately comparable areas across species. For example, it has been reported recently that deep brain stimulation of the posterior ventromedial cortex (Fig. 1) had positive therapeutic effects in a patient population suffering from pharmacologically intractable depression (Ressler & Mayberg, 2007). The cortical region targeted by stimulation contains several architectonically distinct areas and it is unknown which areas are critical to the effects of the treatment or how the stimulation operates to alleviate the symptoms. If comparable architectonic areas between the human and the macaque brain were well defined, significant improvement in the design and rationale of this therapeutic procedure (e.g. the placement of electrodes within specific components of the region of interest) could be obtained by studying the effects of precise lesions and recording the electrophysiological activity of relevant neuronal populations within this part of the macaque brain.

Morphology of the ventromedial (A and C) and orbital (B and D) surfaces of the human and macaque frontal cortex, respectively. The conventional orientations of the brain are indicated (synonymous orientations are noted in parentheses). Sulci and gyri are labeled in white and red, respectively. Note that the labeling of the superior rostral sulcus (SRS) and the inferior rostral sulcus (IRS) on the ventromedial surface follows the nomenclature established in previous architectonic studies (Economo, 1929; Vogt et al., 1995). In studies based on morphology alone (Paus et al., 1996; Fornito et al., 2006) the term superior rostral sulcus labels the first major horizontal sulcus (denoted by an asterisk) ventral to the corpus callosum (CC). Abbreviations: AOG, anterior orbital gyrus; APS, anterior parolfactory sulcus; CG, cingulate gyrus; GR, gyrus rectus; HR, horizontal ramus of the sylvian fissure; IMG, inferior medial gyrus; LOG, lateral orbital gyrus; LOS, lateral orbital sulcus; MOG, medial orbital gyrus; MOS, medial orbital sulcus; MPS, medial polar sulci; OLFS, olfactory sulcus; OLFg, olfactory groove; POG, posterior orbital gyrus; RS, rostral sulcus; SMG, superior medial gyrus; TOS, transverse orbital sulcus.
Comparable areas across species should (1) exhibit a common set of distinctive architectonic features (Fig. 2B), and (2) occupy the same relative borders within a fixed constellation of surrounding areas. The controversy over the location of comparable areas in the human and the macaque brains results from disagreement about the qualitative terms used to describe the architectonic features that define areas and which have been interpreted inconsistently by different investigators in the human brain. For example, the qualitative terms agranular, dysgranular and granular, which describe observable degrees of granule cell density in layer IV, have conventionally established meanings in the macaque. Whereas the central posterior part of the macaque orbital frontal cortex, area 13, has uniformly been described as dysgranular (Walker, 1940; Barbas & Pandya, 1989; Morecraft et al., 1992; Carmichael & Price, 1994; Petrides & Pandya, 1994; Cavada et al., 2000; Dombrowski et al., 2001), the cortex on the human posterior orbital gyrus (Fig. 1) has been alternately and incompatibly described as agranular (Hof et al., 1995; Beck, 1949), dysgranular (Petrides & Mackey, 2006) and granular (Ongur et al., 2003). Moreover, as dysgranular macaque area 13 should correspond according to criterion (1) above to a dysgranular cortical area in the human caudal orbitofrontal region, the implied location of a comparable human area 13 as illustrated in Fig. 2A differs substantially between investigators (Petrides & Mackey, 2006; Hof et al., 1995; Ongur et al., 2003; Semendeferi et al., 1998; Beck, 1949), lying in some instances several centimeters apart. In the present study, we remove the uncertainty of interspecies comparisons made on qualitative descriptions by quantifying the architectonic features of interest over a large region of the human and the macaque ventromedial and orbital frontal cortex.
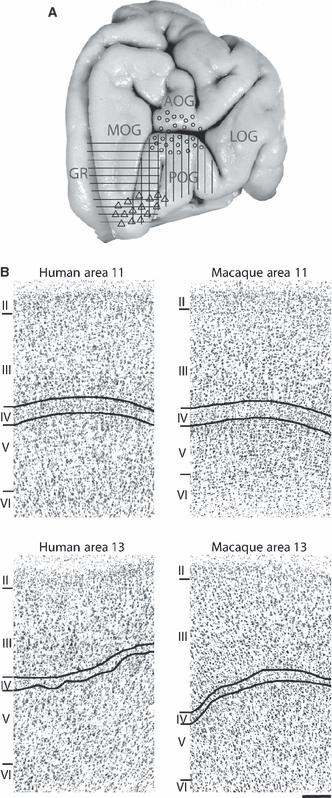
(A) Various parts of the orbital frontal cortex designated by different investigators as an area which would be comparable architectonically to macaque area 13 – vertical bars (Petrides & Mackey, 2006), circles (Hof et al., 1995), horizontal bars (Ongur et al., 2003), triangles (Semendeferi et al., 1998). Major gyri are labeled as in Fig. 1. (B) Digital photomicrographs of the cortical architecture in the central orbital region, areas 13 and 11, in the human and the macaque brains. Cortical layers are identified by Roman numerals. The inner and outer boundaries of layer IV, which is composed of small round granule neurons, is labeled in each photograph by curved black lines. Scale bar = 200μm. In both species, area 13 is a dysgranular cortex (i.e. layer IV is poorly organized and lightly populated with granule cells) with diffusely arranged pyramidal cells in layers V and VI, while area 11 is an example of granular cortex (i.e. layer IV is clearly organized and well populated) with a more clearly defined sublamination of the inner layers V and VI.
In brief, quantitative sampling according to the method described by Mackey & Petrides (2009) was performed on histologically prepared sections obtained from 16 human hemispheres and seven macaque hemispheres. On digital images of the sections, cells were segregated according to their size into one of two areal density images per histological section: a granule cell density image and a pyramidal cell density image (Fig. 3B and C). The cortical ribbon contained in the areal density images was sampled from the outer edge of layer II to the white matter along equally spaced transverse lines (Fig. 3D). The sample along any one transverse represents a profile of the neuronal density at that cortical location (Mackey & Petrides, 2009). Transverse sampling assumes that density profiles sampled from one architectonic area share a common set of features which distinguish them from profiles sampled from spatially adjacent architectonic areas (Ryzen, 1956; Hopf, 1965; Hudspeth et al., 1976; Schleicher et al., 1986, 1999; Mackey & Petrides, 2009). On the density profiles, we calculated the mean density of specific cortical layers which had been manually identified on a separate identically sized set of images (4, 5).

Illustration of (A) general, (B) granule cell and (C) pyramidal cell density images. Shades of gray approaching white indicate higher neuronal density. Note that, in the granule cell density image, there are two bands of higher neuronal concentration which correspond to granule cell layers II and IV. In the pyramidal cell density image, there are three bands of higher neuronal concentration which correspond to pyramidal layers III, Va and VIa. (D) Illustration of transverse lines which span the width of the cortex. The pixel values underlying each transverse line are sampled serially from the outer to the inner contour and represent a profile of neuronal density at that location. This same set of transverse lines is then used to sample seven equally sized images which contain different information about the cortical lamina (A–C and Fig. 4C–F).
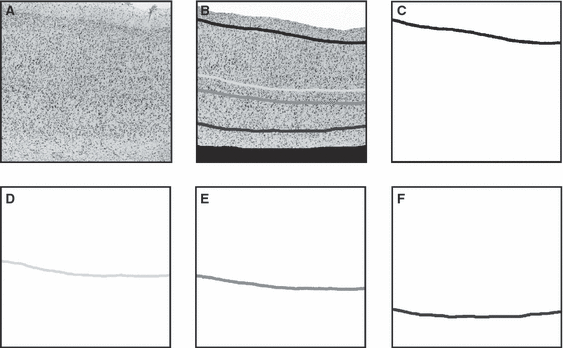
Illustration of the traced neuronal layers II, IV, Va and VIa as well as the inner and outer contour of the cortex. (A) An example of a cortical image. Note the resolution has been reduced for publication. (B) Illustration of manually identified neuronal layers II (black line), IV (light gray line), Va (intermediate gray line) and VIa (dark gray line) as well as the inner and outer contours of the cortex. Layer II is located by drawing a line along its inner boundary with layer III, while layers IV, Va and VIa are identified by tracing a line through the center of each of these layers. Everything outside layer II and outside the brain on the image is masked in white (outer contour) and everything inside layer VI, i.e. the white matter, is masked in black (inner contour). C–F illustrate the four equally sized images that are then generated from the manually traced layers.
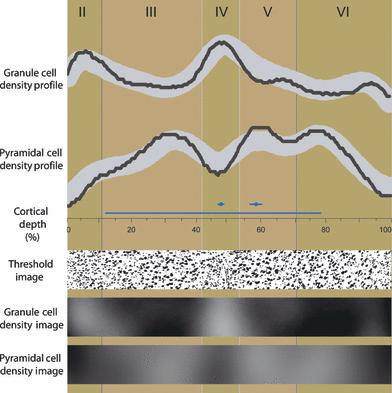
Example of density profiles sampled from a granule cell density image and a pyramidal cell density image to illustrate how the density of layer IV and layer Va are estimated. Alternating background blocks of light and dark brown identify the neuronal layers, which are labeled by roman numerals along the top of the figure. The granule cell density profile and the pyramidal cell density profile are plotted as black lines in the top half of the illustration. The profiles were sampled from the part of the granule cell density image and the pyramidal cell density image displayed in the bottom half of the figure. Also shown is the part of the thresholded image from which the granule cell density image and the pyramidal cell density image were derived. Note the small round granule cell neurons in layers II and IV. Along the horizontal center of the figure, the cortical depth is indicated in per cent. Above this axis, a long blue line denotes the region on the profiles used to calculate the mean density of the profile (from the inner boundary of layer II to layer VIa). Above the long blue line are two short blue lines that indicate the region on the profile defined as representative of layer IV and layer Va. A circle in the center of the short lines mark the location of layer IV and layer Va as coded in the profiles sampled from the image containing the manually traced neuronal layer IV and image containing the manually traced neuronal layer Va, respectively (e.g. Fig. 4D).
Materials and methods
Regions of interest
The ventromedial and lateral orbital frontal cortical regions constitute a large part of the frontal lobe of the primate brain located just above and behind the eyes and extending ventrally and laterally from the inferior edge of the rostrum of the corpus callosum on the medial surface of each hemisphere to the inferior margin of the lateral surface of the frontal cortex (Fig. 1). Although the terms ventromedial and orbital frontal cortex are cited frequently in the literature on the functions of the human brain with little or no distinction, a division of this cortical territory into two separate non-overlapping regions, the ventromedial and lateral orbital frontal regions, is warranted on the basis of comparative architectonic observations in human and macaque specimens (Petrides & Mackey, 2006; present article) and tract tracing experiments in the macaque that indicate the existence of two separate highly interconnected networks of architectonic areas (reviewed in Price, 2007; Barbas, 2007). The lateral orbital frontal region is defined here as the cortical surface of the frontal lobe resting on the orbit of the eye from the medial orbital frontal sulcus to the ventral margin of the lateral frontal surface of the brain. Posteriorly, the orbital region is bordered by the anterior perforated substance and insular cortex. Anteriorly, the lateral orbital region is limited by the frontal pole of the brain. In the macaque this lateral orbital region corresponds closely to the orbital network of Price (2007) and the basoventral network of Barbas (2007). The ventromedial region is defined here as the ventral medial surface of the brain and the medial portion of the orbital frontal cortex up to the medial orbital sulcus (Chiavaras & Petrides, 2000). The ventromedial region is bounded posteriorly by the sub-cortical septal region and terminates anteriorly at the frontal pole of the brain. An arbitrary upper boundary is formed by an imaginary line that runs forward to the front of the brain in the plane of the ventral limit of the genu of the corpus callosum (Fig. 1). The ventromedial region includes the cortex that wraps over onto the ventral surface of the frontal cortex up to the medial orbital sulcus. The ventromedial region in the macaque corresponds to the medial network of Price (2007) and the mediodorsal network of Barbas (2007).
Specimens
The use of macaque and human specimens in this study was approved by, respectively, the McGill University Animal Care Committee in accordance with the Canadian Council for Animal Care guidelines and the Research Ethics Board of the Montreal Neurological Hospital and Institute.
Human specimens were required to meet three criteria: (1) cause of death was not related to neurological factors, (2) there was no history of neurological or psychiatric disease, and (3) the brain was removed within 24 h after death. Eight human brains (16 hemispheres) were included in the study. One brain was photographed, embedded whole in celloidin and sectioned coronally at a thickness of 36 μm. Every tenth section of this brain was mounted on a glass microscope slide and prepared histologically with cresyl violet, which selectively stains cell somata. The two hemispheres in the other seven brains were separated by cutting the corpus callosum and additional connective tissue. All surfaces were then photographed. These 14 hemispheres were blocked, embedded in paraffin and sectioned at a thickness of 12 μm. The location of each block was recorded on a set of photographs generated specifically for this purpose. Every 25th section of the seven paraffin-embedded brains was mounted on a glass microscope slide and prepared histologically with thionin, which stains cell somata. A series of 194 human histological sections was selected for sampling. Each architectonic area was represented by an equal number of sampled sections in every hemisphere. The selection of sections was otherwise random.
Seven hemispheres from five macaque brains that had been histologically prepared for other experimental studies were also used. The macaques were deeply anesthetized with a lethal dose of sodium pentobarbital (100 mg/kg, i.v.; Nembutal, Ceva Santé Animale, Libourne, France), then perfused transcardially with 4% paraformaldehyde in phosphate buffer (pH 7.4). Brains were extracted and cryoprotected by submersion in graded series of dimethylsulfoxide and glycerin in phosphate buffer. Brains were photographed and the hemispheres were separated by severing the corpus callosum and other connecting tracts. The hemispheres were flash frozen in an isopentane bath at −80 °C and sectioned on a cryostat at 35 μm. Three hemispheres were cut in the coronal plane and two hemispheres each in the sagittal and horizontal planes. A one-in-ten series of sections from each hemisphere were mounted on glass microscope slides and prepared histologically with a Nissl cell body stain. Forty-six representative sections were selected for quantitative sampling.
Image acquisition and preprocessing
Acquisition of images and preprocessing and sampling steps were as described previously (Mackey & Petrides, 2009). Images of the selected sections magnified by low-power light microscopy, 10 × 1.00 (DM-RXA2; Leica Microsystems, Wetzlar, Germany), were acquired with a DVC digital camera (1 pixel = 1.315 μm). A motorized microstepper stage attached to the microscope was controlled by imaging software designed for digital microscopy (Northern Eclipse; Empix, Toronto, Canada). By moving the stage in a grid-like fashion, a rectangular raster of adjacent non-overlapping images was acquired from the region of interest on each section. Acquired images were subjected to a seven-step preprocessing protocol: (1) background subtraction, (2) generation of a collage image encompassing the entire region of interest, (3) identification of the cortical layers, (4) thresholding, (5) layer segmentation, (6) smoothing and accentuation of cortical layers by blurring, and (7) generation of an image to guide sampling.
Background subtraction
A background subtraction was performed on each image to attenuate constant image acquisition artifacts, such as dust on the camera aperture or slight inhomogeneities in the illumination of the imaging field.
Generation of a collage image encompassing the entire region of interest
The individual images were aligned by the microscopy software to form a single collage image per histological section. Note that because histological sections have different sizes, the size of the collage images will also be different. Differences in the size of the collage images have no effect on the subsequent preprocessing steps.
Identification of cortical layers
Each collage image was opened in Photoshop in which transparent layers can be placed in digital space on top of a background image. Cortex external to cellular layer II (outer contour), i.e. layer I, and parts of the image external to the brain, were masked with the color white and everything internal to cellular layer VI (inner contour), i.e. the white matter, was masked with the color black. The centers of cellular layers IV, Va and VIa, as well as the inner boundary of layer II, were also traced, interactively. Four images with the same dimensions as the original image of the histological section were subsequently generated. Each of these four images contained the traced location of one cortical layer. The cellular layers and the inner and outer cortical contours were identified before thresholding to ensure the accuracy of their placement as these layers are obscured by subsequent preprocessing steps. Intra- and inter-rater test–retest comparisons indicate that the tracing of cortical contours is highly reproducible and that intra- and inter-rater variability does not have a significant effect on the quantified densities of the identified layers (Mackey & Petrides, 2009).
Thresholding
Each image of a histological section was then thresholded to segment stained cell bodies from the cellular matrix. Segmenting cell bodies from the cellular matrix reduces the influence of artifacts in the staining of the cellular matrix and the differential absorption of the Nissl stain by individual neurons, i.e. background staining (Zilles et al., 1978; Wree et al., 1982). In order to increase the contrast between cells and cellular matrix, an estimate of the cortical background without stained cells was subtracted from the original image. Regional adaptive filtering was also applied to the images in preparation for a global threshold. The same threshold level was applied to all images of histological sections collected from the same hemisphere. The thresholding procedure was established on an independent set of images that are not included in the present study (Mackey & Petrides, 2009). The thresholded images, created in grayscale file format, represent pixels above and below threshold by a gray value of 0, absolute black, and 255, absolute white, respectively.
Layer segmentation
The cells in granular layers II and IV are smaller than those in the surrounding pyramidal layers III and V. A spatial four-point-connected binning algorithm (Northern Eclipse) applied to the thresholded image identified the locations of thresholded elements within the size range of typical granule cells in our histological data (26.3–42.08 μm2) and coded these locations on a separate equally sized image. An estimate of pyramidal cell density was created by subtracting the image in which the location of granule-sized cells were coded from the thresholded image, then blurring the resulting image with a median filter of 3-pixel radius to eliminate cells smaller than granule cells.
Smoothing and accentuation of cortical layers by blurring
Two images that target different neuronal populations were created for sampling: a granule cell density image and a pyramidal cell density image. The granule cell density image was created by blurring the image coding the locations of granule cells created in step 5 with a Gaussian blurring kernel of 60-pixel radius. The granule cell density image represents the areal density of granule-sized cells, which emphasizes layers II and IV where granule cells are concentrated. The pyramidal cell density image was created by blurring with a Gaussian blurring kernel of 60-pixel radius the image created in step 5 that estimated pyramidal cell density. This latter image represents the concentration of pyramidal and other large neurons in the specimen and, as a consequence, highlights pyramidal layers III, V and VI. All neurons have been grouped into one of two classes, granule cells or pyramidal cells, on the basis of their size. Although more detailed neuronal classification schemas exist (see, for example, Defilipe et al., 2002), this methodological simplification was justified by the purpose of the present study, namely to resolve the general outline of architectonic areas. Blurring images prior to sampling eliminates the fine spatial detail associated with individual cells and accentuates the grouping of neurons in layers, facilitating analysis of the sampled profiles. The pixel values in the blurred images were then inverted so that local neuronal density in the images as indexed by a range of grayscale intensities (0–255) would intuitively represent areas of higher and lower density with higher and lower values on the grayscale, respectively.
Generation of image to guide sampling
As a guide to the orientation of the sampling transverses, the cortical contour masks and the traced layer IV were superimposed on a copy of the general density image.
The seven image-preprocessing steps described above produce seven different images to sample for each selected histological section: a granule cell density image, a pyramidal cell density image, four images containing the traced cellular layers and an image created to guide the orientation of sampling. All images were resampled to one-tenth of their original dimensions because of computational constraints imposed by the imaging software.
Transverse sampling and profile analysis
On the image created to guide the orientation of sampling, a series of equidistant points was generated at 10-pixel/131.5-μm intervals along layer IV by the sampling software (Northern Eclipse; Empix). A matching series of straight transverses were then added automatically, spanning the shortest distance between the inner and outer cortical contour and passing through one of the software-generated points on layer IV (see Mackey & Petrides, 2009). After transverse placement, the values of the pixels underlying each transverse were sampled serially from the outer to the inner contour and exported to Excel (2000; Microsoft) as a column of values. Each column of data, the sample along one transverse, represents a profile of cortical density at that location. Each of the other seven equally sized images was sampled by the same lattice of transverse lines. Profiles were standardized to a common length by linear interpolation in r (the shareware version of S-Plus), a programming environment designed for statistics. Standardization of the density profiles converts the cortical depth to a scale of 0–100%, where 0% represents the outer contour and 100% represents the inner contour of the cortex. Standardizing the length of profiles permits the comparison of profiles sampled from cortex with different absolute thicknesses (Hudspeth et al., 1976; Schleicher et al., 1986; Mackey & Petrides, 2009).
Profiles sampled from the same architectonic area should, in principle, share common characteristics which distinguish them uniquely from profiles sampled from other architectonically distinct areas (Ryzen, 1956; Hopf, 1965; Hudspeth et al., 1976; Schleicher et al., 1986, 1999; Mackey & Petrides, 2009). The principal profile features we chose to isolate and quantify were the densities of individual layers, which have been shown to differentiate effectively between several architectonic areas within the orbitofrontal region (Mackey & Petrides, 2009). To illustrate the relationship between the data sampled from separate images along an identically placed transverse line, example profiles are represented graphically in Fig. 5. The relative depth of layer IV is established by sampling the image containing the manual tracing of layer IV. This profile records that layer IV is found at a relative cortical depth of 48.2%, which is indicated in the middle of Fig. 5 by a blue dot. A fixed region around this location, −1 to +1% of the total profile length, is defined as representative of layer IV. The region defined as representative of layer IV in the example profile is displayed as a short blue line in Fig. 5. A representative sample of layer Va was defined as the mean value from −2 to +2% of the total profile length around the location of layer Va. The location of layer Va is also indicated in Fig. 5 by a blue dot and the region defined as representative of layer Va is shown as a short blue line. A fixed region was defined as representative of the cortical layers because the absolute and relative width of the cortical layers varies widely due to cortical curvature. The densities of layers IV and Va were standardized against the density of all layers calculated as the mean value from the layer II/II border to layer VIa in profiles sampled from the granule cell and pyramidal cell density images, respectively. Standardization of the density profiles compensates for differences in background histological staining and section thickness. Sub-layer VIb was excluded because the distinctness of its inner boundary is strongly affected by curvature in the cortex (Mackey & Petrides, 2009). Around the fundus of the sulcus, cortical neurons in layer VIb are clearly segregated from the much smaller glial cell bodies that represent the white matter, while the boundary between these two populations of cells is much more diffuse on the convexity of the gyrus. As the manual identification of the inner boundary of layer VIb could be controversial, this sub-layer has been excluded from the analysis.
Cortical folding
Cortical folding modulates the relative depth of the cortical layers. When the cortex is concave, the inner neuronal layers (i.e. layers V and VI) are thinner relative to the outer layers (i.e. layers II and III) and, when the cortex is convex, the reverse relationship holds for the inner and outer layers (Bok, 1959; Hilgetag & Barbas, 2006; Mackey & Petrides, 2009). We have shown that there is minimal correlation between cortical curvature and the granule cell density of layer IV or the pyramidal cell density of layer Va (r = 0.12 and −0.12, respectively, P < 0.01; see Mackey & Petrides, 2009) as measured by the sampling protocol described here. In the present investigation, we asked whether the difference in architectonic features between sulcal and gyral parts within an area would be as large, or larger, than the difference between areas. This possibility was examined in areas 14c, 14r and 11m, which are located within the olfactory sulcus and on the gyrus on either side of the olfactory sulcus. Twenty profiles from four non-overlapping regions (i.e. crown of the gyrus rectus, the medial bank and the lateral bank of the olfactory sulcus, and the crown of the medial orbital gyrus) (Fig. 1) on each image that contained one of these architectonic areas were assigned to this comparison.
Statistical analyses
The quantified features of the density profiles were transformed to z-scores on a hemisphere by hemisphere basis to facilitate the comparison of data between subjects. A z-score transformation controls for differences in tissue shrinkage during histology and differences in section thickness between brains. The mean standardized granule cell density of layer IV and pyramidal cell density of layer Va of each architectonic area were rank ordered in the human and the macaque data, separately. The rankings of the features in the two species were then compared by the Spearman rank order test. Within species, differences in the mean standardized densities of layers IV and Va between architectonic areas were assessed by anova tests and Tukey’s honestly significant difference (HSD) post-hoc tests.
Results
The location of architectonically comparable areas in the lateral orbital and ventromedial frontal cortex of the human and the macaque brains is shown in Fig. 6. Figure 7A and B illustrate the close match in the mean quantified densities of layers IV and Va between comparable areas in the human and the macaque brains. The mean laminar densities per area were rank ordered separately in the two species. A statistical comparison of the area rankings in the two species demonstrates that the architectonic match between comparable areas is significant (Spearman rank test, layer IV: r = 0.976 and layer V: r = 0.988; P < 0.00001). For quick reference, the key criteria which distinguish between neighboring architectonic areas are listed in Tables 1–4. In addition to identifying specific architectonically comparable areas in the two species, the overall ordering of the mean quantified densities of layers IV and Va arrange the areas of interest along two dimensions that mirror their spatial position within the total constellation of areas (Fig. 7A and B). First, there is a medial to lateral decrease in the density of layer Va and, second, there is a posterior to anterior increase in the density of layer IV.
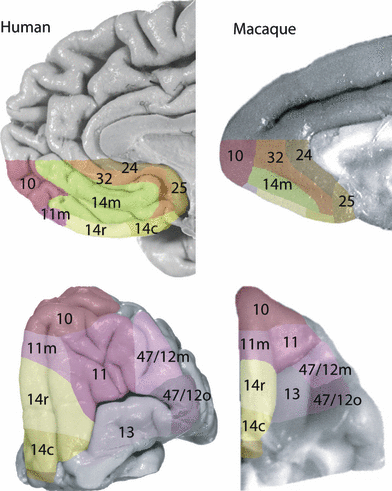
Architectonic parcellation of the human and macaque orbital and ventromedial surface. Comparable areas between species share the same number label and are identified also by matching colors. The boundaries between areas were located on the histological sections by qualitative observation. These boundaries were then matched to macroscopic landmarks, e.g. sulci, and painted manually on the surfaces of the brains in Photoshop.
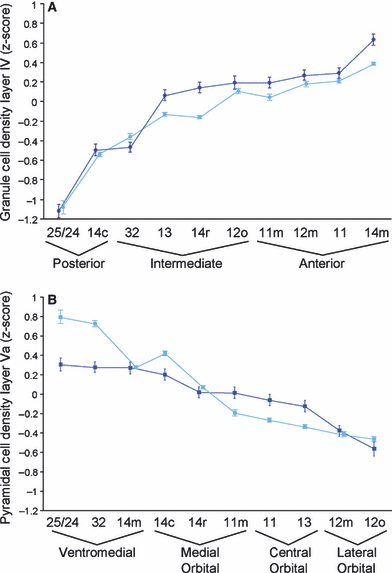
Matching mean density of layer IV (A) and layer Va (B) between comparable architectonic areas in the macaque (dark blue) and the human (light blue) brains. Error bars indicate standard deviation. (A) Architectonic areas are ordered along the x-axis by increasing layer IV density in the macaque. Ordering by the mean density of layer IV arranges areas into groups that mirror their morphological position in a posterior to anterior direction. The range of the spatial groupings, posterior, intermediate and anterior, is indicated by a funnel symbol beneath the relevant architectonic areas. (B) Architectonic areas are ordered along the x-axis by decreasing layer Va density in the macaque. Ordering by the mean density of layer Va arranges areas into groups that coincide with their morphological position in a medial to lateral direction. The range of the spatial groupings, ventromedial, medial orbital, central orbital and lateral orbital, is indicated by a funnel symbol beneath the relevant areas.
| Areas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24/25 | – | < 32 | – | < 14c | – | – | – | – | – | – |
| 32 | > 24/25 | – | < 14m | < 14c | – | – | – | – | – | – |
| 14m | – | > 32 | – | > 14c | > 14r | > 11m | – | – | – | – |
| 14c | > 24/25 | > 32 | < 14m | – | < 14r | – | – | < 13 | – | – |
| 14r | – | – | < 14m | > 14c | – | < 11m | < 11 | > 13 | – | – |
| 11m | – | – | < 14m | – | > 14r | – | < 11 | – | – | – |
| 11 | – | – | – | – | > 14r | > 11m | – | > 13 | – | > 12m |
| 13 | – | – | – | > 14c | < 14r | – | < 11 | – | < 12o | < 12m |
| 12o | – | – | – | – | – | – | – | > 13 | – | < 12m |
| 12m | – | – | – | – | – | – | < 11 | > 13 | > 12o | – |
- Significant differences according to Tukey’s HSD post-hoc tests are in bold.
| Areas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24/25 | – | > 32 | – | > 14c | – | – | – | – | – | – |
| 32 | < 24/25 | – | > 14m | > 14c | – | – | – | – | – | – |
| 14m | – | < 32 | – | > 14c | > 14r | > 11m | – | – | – | – |
| 14c | < 24/25 | < 32 | < 14m | – | > 14r | – | – | > 13 | – | – |
| 14r | – | – | < 14m | < 14c | – | > 11m | > 11 | > 13 | – | – |
| 11m | – | – | < 14m | – | < 14r | – | > 11 | – | – | – |
| 11 | – | – | – | – | < 14r | < 11m | – | > 13 | – | > 12m |
| 13 | – | – | – | < 14c | < 14r | – | < 11 | – | > 12o | > 12m |
| 12o | – | – | – | – | – | – | – | < 13 | – | < 12m |
| 12m | – | – | – | – | – | – | < 11 | < 13 | > 12o | – |
- Significant differences according to Tukey’s HSD post-hoc tests are in bold.
| Areas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24/25 | – | < 32 | – | < 14c | – | – | – | – | – | – |
| 32 | > 24/25 | – | < 14m | > 14c | – | – | – | – | – | – |
| 14m | – | > 32 | – | > 14c | > 14r | > 11m | – | – | – | – |
| 14c | > 24/25 | < 32 | < 14m | – | < 14r | – | – | < 13 | – | – |
| 14r | – | – | < 14m | > 14c | – | < 11m | < 11 | < 13 | – | – |
| 11m | – | – | < 14m | – | > 14r | – | < 11 | – | – | – |
| 11 | – | – | – | – | > 14r | > 11m | – | > 13 | – | > 12m |
| 13 | – | – | – | > 14c | > 14r | – | < 11 | – | < 12o | < 12m |
| 12o | – | – | – | – | – | – | – | > 13 | – | < 12m |
| 12m | – | – | – | – | – | – | < 11 | > 13 | > 12o | – |
- Significant differences according to Tukey’s HSD post-hoc tests are in bold.
| Areas | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24/25 | – | > 32 | – | > 14c | – | – | – | – | – | – |
| 32 | < 24/25 | – | > 14m | > 14c | – | – | – | – | – | – |
| 14m | – | < 32 | – | < 14c | > 14r | > 11m | – | – | – | – |
| 14c | < 24/25 | < 32 | > 14m | – | > 14r | – | – | > 13 | – | – |
| 14r | – | – | < 14m | < 14c | – | > 11m | > 11 | > 13 | – | – |
| 11m | – | – | < 14m | – | < 14r | – | > 11 | – | – | – |
| 11 | – | – | – | – | < 14r | < 11m | – | > 13 | – | > 12m |
| 13 | – | – | – | < 14c | < 14r | – | < 11 | – | > 12o | > 12m |
| 12o | – | – | – | – | – | – | – | < 13 | – | < 12m |
| 12m | – | – | – | – | – | – | < 11 | < 13 | > 12o | – |
- Significant differences according to Tukey’s HSD post-hoc tests are in bold.
In the following section, the areas within the region of interest are described in terms of architectonic features that are present in both species. The areas have been grouped into two regions, a ventromedial region and lateral orbital region, on the basis of tract tracing studies in the macaque which indicate the presence of two separate networks of architectonic areas (Barbas, 2007; Price, 2007) and the present identification of comparable architectonic areas in the human brain. The ventromedial region includes areas 25, 14c, 14r, 14m, 11m and medial part of area 10 (Fig. 6). Area 25 in the posterior-most part of the ventromedial surface of the frontal lobe and extending a short distance onto the orbital surface and area 24, found along the margin of the corpus callosum (1, 6), lacks a granule layer IV and presents a simple arrangement of pyramidal layers with a notably dense layer V. Because both areas are agranular (i.e. lack a granule layer IV) and have an equally dense layer V, they are grouped together in the quantitative data, although several other visible criteria distinguish them (e.g. area 24 has a less dense layer VI). Area 32, which exhibits an emergent though still faint layer IV (i.e. this cortex is dysgranular), forms a buffer between these latter agranular areas and the more anteriorly placed granular area 14 medial (14m). From areas 24 and 25 through area 32 to area 14m, there is a marked increase in laminar complexity, such that sublamination of layers III, V and VI becomes increasingly obvious. Along the medial orbital surface, which includes the gyrus rectus and the medial orbital gyrus (Fig. 1), from the posterior area 25 toward the frontal pole, there is a progressive increase in the density of granule layer IV and a decrease in the density of pyramidal layer Va that is divided into a series of steps labeled 14 caudal (14c), 14 rostral (14r) and 11 medial (11m). Area 14c is almost agranular and contains a prominent layer Va. In dysgranular area 14r, the prominence of layer Va decreases as compared with the density of the other pyramidal layers and decreases again anteriorly in granular area 11m. Like the ventromedial cortex, the sublamination of the pyramidal layers on the medial orbital surface becomes more evident from posterior to anterior. It is notable that all areas in the ventromedial region, areas 24, 25, 32 and all parts of area 14, share a dense layer Va feature (Fig. 7B).
The lateral orbital region includes areas 13, 11, 47/12o and 47/12m (Fig. 6). On the central and posterior part of the orbital surface that is labeled as area 13, the dense layer Va of area 14 is replaced by a diffusely arranged collection of pyramidal neurons and the granule cell layer IV is poorly defined. Anterior to area 13 is a granular cortical area, area 11, located on the anterior orbital gyrus in both the human and the macaque monkey brain (1, 6). Areas 11 and 13 occupy the cortex within the transverse orbital sulcus. In contrast to area 13, area 11 exhibits a clearer sublamination of the inner layers V and VI (Fig. 2B). This trend of increasing laminar complexity in the posterior to anterior direction is continued on the lateral orbital gyrus (Fig. 1) where two parts of area 47/12 were identified, 47/12 orbital (14/12o) and 47/12 medial (47/12m). Area 47/12m is more granular and more clearly sublaminated than its posterior neighboring area 47/12o. Area 47/12o and area 47/12m are set apart from the more medial cortical areas, area 13 and area 11, by the presence of large pyramidal cells in the deep part of layer III and a relatively less dense layer Va.
A significant overall difference between areas in the standardized densities of layers IV and Va was determined by anova in the human (F18,128 454 = 856.19, P < 0.0001) and by a separate anova in the macaque (F18,19 450 = 167.34, P < 0.0001). Further analyses by Tukey’s HSD post-hoc tests demonstrated significant differences between all adjacent areas in the human brain (and most adjacent areas in the macaque brain) on at least one of the measured features (P < 0.01). The specific results of the post-hoc tests are displayed in Tables 1–4. In the macaque, the differences between paired adjacent areas 32 and 14c, 14r and 11m, and 11m and 11 failed to reach significance (Fig. 6) although in each of these cases the direction of the change in density between areas was consistent with the change in density found between comparable areas of the human cortex (Fig. 7). To determine whether the pattern of results was stable in both hemispheres, the data were also coded as right or left hemisphere and the means were compared by cross-factorial anova, which again indicated a difference between areas (human: F18,224 = 30.46, P < 0.0001; macaque: F18,88 = 11.47, P < 0.0001), but no significant difference between hemispheres or an interaction effect.
To investigate variability in the quantified densities between sulcal and gyral parts of the same architectonic area, profiles sampled from the gyrus rectus, the medial and the lateral banks of the olfactory sulcus and the medial orbital gyrus (Fig. 1) were compared in three neighboring architectonic areas: areas 14c, 14r and 11m. Twenty profiles from each of four non-overlapping regions on each image that contained one of the architectonic areas were assigned to this comparison. The mean quantified features in the four morphological regions of each area are presented in Fig. 8. The average deviation of different morphological parts of the same area from the overall area mean is presented in Table 5. For example, the overall mean density of layer IV in area 14c in this sub-sample of the data is −0.446. The average difference between the overall mean density of layer IV in 14c (−0.466) and the mean density of layer IV in the four morphological parts of area 14c is 0.022. In contrast, the difference between the overall mean density of layer IV in area 14c and 14r is 0.277. Table 5 also presents the differences in the overall means of layer IV and layer Va density between areas. The differences between gyral and sulcal parts of the same architectonic area are at a minimum 3–4 times smaller than the differences between architectonic areas.

Graph to illustrate the similarity of quantified architectonic features within areas when sampled from a gyrus or a sulcus: GR, gyrus rectus; MB, medial bank of the olfactory sulcus; LB, lateral bank of the olfactory sulcus; MOG, medial orbital gyrus. Black bars represent the standardized granule cell density of layer IV. Gray bars represent the standardized pyramidal cell density of layer V. Error bars are 95% confidence intervals.
| Layer IV | Layer V | |
|---|---|---|
| Average difference from within area mean | ||
| 14c | 0.022 | 0.079 |
| 14r | 0.047 | 0.050 |
| 11m | 0.061 | 0.032 |
| Difference between area means | ||
| 14c vs. 14r | 0.277 | 0.353 |
| 14r vs. 11m | 0.350 | 0.235 |
Discussion
The present study has provided a quantitative demonstration of comparable architectonic areas in the ventromedial and orbital frontal cortex of the human and the macaque monkey brains (6, 7). It was also demonstrated that the pyramidal density of layer Va increases in a medial-to-lateral dimension and the granule cell density of layer IV increases in a posterior-to-anterior dimension. A discussion of the anatomical and functional significance of these results is preceded by a brief comment on the rationale of sampling layers IV and Va and the effects of cortical curvature on the sampling method.
More than 73 000 areal density profiles were collected from 16 human and seven macaque post-mortem hemispheres. Two features estimated from the profiles, the densities of layers IV and Va, were of primary interest for several reasons. Unlike abstract mathematical features, such as central moments (Schleicher et al., 2005) derived from areal density profiles in previous within-species studies, sampled layer IV and Va densities correspond to unique visible features in the histological specimen. These features are directly comparable with observations in the existing qualitative architectonic literature. As the qualitative descriptions of the macaque frontal cortical architecture are highly consistent (Walker, 1940; Barbas & Pandya, 1989; Morecraft et al., 1992; Carmichael & Price, 1994; Petrides & Pandya, 1994), the standard labeling of areas in the macaque served as an uncontroversial template for the labeling of areas with similar quantitatively sampled architectonic features in the human brain. The densities of layers IV and Va figured prominently in our own visual observations and we were able to distinguish all architectonic areas reported here from their neighbors on the basis of one or both of these features. These features also have known biological significance (e.g. layer IV and the basal dendrites of deep layer III neurons are the target of projections from the thalamus and layer V neurons project to several subcortical structures) (e.g. Negyessy & Goldman-Rakic, 2005; Zikopoulos & Barbas, 2006) which, in turn, may facilitate the integration of our data with emerging theories on the unique computational contributions of particular cortical layers (e.g. Brown et al., 2004). Layer IV and Va densities have also been explicitly cited in hypotheses on the progressive differentiation of the cortex (Sanides, 1964; Barbas & Pandya, 1989), which we explore here in human specimens. Most importantly, these two architectonic features are robust and unambiguously comparable between the human and the macaque brains. Thus, the quantification of layers IV and Va provides a solid basis on which to establish comparability of areas across species in a given region of interest.
The effects of cortical curvature on the sampled density of layers IV and Va were also examined. The curvature of the cortex alters the appearance of the cortical layers independently of changes in laminar architecture between architectonically defined areas. Where the cortex is convex (i.e. on the crown of a gyrus) the infragranular layers (i.e. layers V and VI) are thicker relative to the supragranular layers (i.e. layers II and III) than where the cortex is concave (i.e. around the fundus of a sulcus) (Bok, 1959; Smart & McSherry, 1986; Hilgetag & Barbas, 2006; Mackey & Petrides, 2009). The effect of cortical curvature on the relative position of the cortical layers has a dramatic influence on the shape of areal density profiles. Measurements and illustrations of the effects of cortical curvature on the appearance of the cortical layers and the shape of density profiles are provided in Mackey & Petrides (2009). To avoid the effects of cortical curvature on the shapes of density profiles, the individual layers were identified and sampled separately on the density profiles. The position of the layers on individual profiles was identified by simultaneously sampling images in which the cortical layers had been manually traced. We had previously shown that there is only a small correlation between cortical curvature and the granule cell density of layer IV or the pyramidal cell density of layer Va as measured by the sampling protocol described here (Mackey & Petrides, 2009). In the present study, we have demonstrated that the differences between areas are not masked by changes in the morphological appearance of the cortex. Differences between the sulcal and gyral parts within areas 14c, 14r and 11m are at a minimum 3–4 times smaller than the differences between these areas (see Fig. 8 and Table 5). The sampling method described here is therefore reasonably robust to the effects of cortical curvature on the appearance of the cortical layers.
The present study provides a framework for the comparison of anatomical and functional data collected separately in the human and the macaque brains. For example, considerable effort has been made to define the connectivity of the cortical areas in the macaque ventromedial and orbital frontal regions with the rest of the brain on the assumption that, in the course of evolution, the connectivity of these areas has been largely conserved from the brain of a common ancestor shared with humans. As the cortical areas are defined by their laminar architecture, an area in the human brain that is defined by the same architectonic features that define an area of the macaque brain should exhibit a similar pattern of connectivity. With regard to area 13, there is a dysgranular area in the human posterior orbital gyrus that can be distinguished based on its architecture from neighboring cortex in the same way that area 13 is recognized in the macaque, i.e. it is bordered medially by cortex with a denser layer Va and laterally by an area with a more developed layer IV, a less dense layer Va and larger pyramidal cells in layer III. Since areas 13 in the human and macaque brains are comparably defined by their cortical architecture, human area 13 should possess a pattern of connectivity and a contribution to function similar to that of macaque area 13. It should be noted that, even with modern developments in diffusion tensor imaging and functional connectivity, the methods available for studying connectivity in the human brain cannot provide definitive evidence of precise origins and precise terminations of neuronal projections that are possible in the non-human primate brain. In addition, the value of data derived from other experimental techniques that are not possible in human subjects, such as exhaustive electrophysiological recording of neuronal activity or anatomically precise lesions, is enhanced if the data can be related to comparable areas of the human brain. The present quantitative demonstration of comparable architectonic areas in the two species provides an important framework for future anatomical and functional investigations of the ventromedial and orbital frontal cortex.
The prominence of layer Va in ventromedial areas 25, 24, 32 and 14 in contrast to the less dense layer Va found in the central and lateral orbital areas 13, 11 and 47/12 (Fig. 7B) suggests an architectonic grouping of areas consistent with tract tracing studies in the macaque, which indicate the existence of two separate highly interconnected networks of areas that possess distinct patterns of connectivity with the rest of the brain (Barbas, 2007; Price, 2007). Price and colleagues describe a medial and an orbital network of areas that align approximately with the mediodorsal and basoventral trends observed by Barbas and colleagues (reviewed in Barbas, 2007; Price, 2007). These correspond to the ventromedial and lateral orbital regions, respectively, examined in the present investigation. In the macaque, the lateral orbital network, areas 13, 11 and 47/12, receives projections from olfactory, gustatory, visual, auditory and somatosensory cortex and has been implicated in the rapid flexible coding of rewarding stimuli (Murray et al., 2007). As these cortico-cortical connections are bi-directional, the orbital network is well placed to bias the activity and perhaps reorganize the processing of sensory systems towards currently rewarding stimuli. The ventromedial network (which also includes the medial orbital region), areas 25, 24, 32 and 14, is connected bi-directionally most heavily with non-sensory brain structures such as the amygdala, hippocampus and hypothalamus and thus is appropriately positioned to mediate the relationship between memory and the regulation of the internal milieu (Damasio, 1996). There is evidence also of input to the ventromedial network from auditory association cortex (Barbas et al., 1999, 2005). Probabilistic patterns of connectivity similar to those described for the ventromedial and lateral orbital frontal networks in the macaque have been found in the human brain by in vivo neuroimaging (Croxson et al., 2005). The morphological parcellation of the ventromedial and orbital frontal cortex on which the study by Croxson et al. is based resembles and thus, in part, supports the present identification of architectonically comparable areas in the two species.
The second identified dimension of architectonic variation is a posterior to anterior increase of layer IV density (Fig. 4A), i.e. from the absence of layer IV posteriorly (agranular areas 24 and 25 and almost agranular area 14c) to its emergence (dysgranular areas 32, 13, 14r and 12o) and continued development toward the frontal pole (granular areas 11, 11m, 12m and 14m). These areas have been grouped according to their morphological position as: posterior, intermediate or anterior. Note that intermediate areas 13 and 12o are bordered posteriorly by agranular cortex (Walker, 1940; Barbas & Pandya, 1989; Morecraft et al., 1992; Carmichael & Price, 1994; Petrides & Pandya, 1994) extending from the insular region. The increase in layer IV density is accompanied by greater laminar complexity, which has been shown by tract tracing studies in the macaque to predict the distribution of inter-area connectivity in the frontal cortex (Barbas, 2007). Frontal areas with greater laminar differentiation receive input to layer I from the infragranular layers V and VI of areas with less laminar differentiation and return information to these latter areas by a projection to layers V and VI (Barbas, 1986; Barbas & Rempel-Clower, 1997). The pattern of laminar inputs and outputs in the frontal cortex has been likened to the feed-forward and feed-back pathways found in the visual, auditory and somatosensory streams, which indicate a direction or hierarchy in the flow of information from basic to more abstract processing stages (Shipp, 2005). Here, we indicate the location of a similarly organized flow of information between networks of comparable architectonic areas in the ventral frontal cortex of the human brain. It is noteworthy that there is a complete or almost complete absence of granule layer IV in the frontal lobe of non-primate species (Jelsing et al., 2006). The greater elaboration of the cortex in the primate brain towards the frontal pole (posterior to anterior dimension) in both the ventromedial and the lateral orbital networks (medial to lateral dimension) suggests the possibility that the structurally complex anterior granular cortex is recruited in the control of more specialized emotional acts within the primate behavioral phenotype.
Acknowledgements
This study was funded by the Canadian Institutes of Health Research (MOP-14620) and the Centre of Excellence in Commercialization and Research (CECR). We would like to thank Rhonda Amsel for helpful advice on statistical analyses.




