Association of DRD4 polymorphism with severity of oppositional defiant disorder, separation anxiety disorder and repetitive behaviors in children with autism spectrum disorder
Abstract
The objective was to examine whether a common polymorphism in the dopamine D4 receptor gene (DRD4) might be a potential biomarker for behavioral variation within the autism spectrum disorder clinical phenotype. Children (N = 66) were evaluated with a validated mother- and teacher-completed DSM-IV-referenced rating scale. Partial eta-squared (ηp2) was used to gauge the magnitude of group differences: 0.01−0.06 = small, 0.06−0.14 = moderate and > 0.14 = large. Children who were 7-repeat allele carriers had more severe oppositional defiant disorder behaviors according to mothers’ (ηp2 = 0.10) and teachers’ (ηp2 = 0.06) ratings than noncarriers, but the latter was marginally significant (P = 0.07). Children who were 7-repeat allele carriers also obtained more severe maternal ratings of tics (ηp2 = 0.07) and obsessions–compulsions (ηp2 = 0.08). Findings for maternal ratings of separation anxiety were marginally significant (P = 0.08, ηp2 = 0.05). Analyses of combined DRD4 and dopamine transporter gene (DAT1) genotypes approached significance (P = 0.05) for teachers’ ratings of oppositional behavior and mothers’ ratings of tics. DRD4 allelic variation may be a prognostic biomarker for challenging behaviors in children with autism spectrum disorder, but these exploratory findings remain tentative pending replication with larger independent samples.
Introduction
Challenging behaviors in children with autism spectrum disorder (ASD) impede educational and habilitative efforts; impact social and school functioning (e.g., Gadow et al., 2008b); contribute to out-of-home placement, reduced opportunities for interactions with nondisabled peers (e.g., Aman et al., 1995) and parental stress and family adjustment problems (e.g., Benson & Karlof, 2009; Hastings, 2003; Herring et al., 2006; Lecavalier et al., 2006), and are the primary reasons for psychopharmacotherapy (Aman et al., 2005); nevertheless, relatively little is known about their biologic substrates. For example, although attention-deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) behaviors are relatively common in ASD (Gadow et al., 2005; Simonoff et al., 2008), few studies have explored potential genetic markers (e.g., Brune et al., 2006; Cohen et al., 2003; Gadow et al., 2008c, 2009b; Roohi et al., 2009). In one such study we found a variable number tandem repeat (VNTR) in the 3′-untranslated region (UTR) of the dopamine transporter gene (DAT1, SLC6A3) was associated with the severity of ADHD, anxiety and tics in children with ASD (Gadow et al., 2008c).
Polymorphisms in other dopaminergic genes, such as the 48-bp VNTR within exon 3 of the dopamine D4 receptor gene (DRD4), are also of interest. The 2-, 4- and 7-repeats are the most common, with the 7-repeat allele purportedly resulting in less efficient dopamine binding and diminished receptor sensitivity (Asghari et al., 1995; Cravchik & Goldman, 2000; van Tol et al., 1992). In non-ASD samples, the 7-repeat allele is implicated in ADHD (Durston et al., 2009; Gizer et al., 2009; Kebir et al., 2009; Smith, 2010) and possibly ASD symptoms in youths with severe ADHD combined subtype (Reiersen et al., 2008), ODD (Kirley et al., 2004) and antisocial behaviors (DiLalla et al., 2009; Fresan et al., 2007; Holmes et al., 2002; Schmidt et al., 2002, 2007), but there are no studies of DRD4 and these behavior syndromes in ASD samples. There are, however, two null reports of DRD4 in the etiology of autism (Grady et al., 2005; Yirmiya et al., 2001). The 7-repeat allele shows a clear association with response to pharmacotherapy (McGough, 2005; McGough et al., 2006) and behavioral intervention (Bakermans-Kranenburg et al., 2008) for disruptive behaviors. There is also evidence of pleiotropy for irritability (McGough et al., 2006), an important behavioral feature of the ODD phenotype, and epistasis with the DAT1 3′-UTR polymorphism (Carrasco et al., 2006; Congdon et al., 2008; Martínez-Levy et al., 2009; Roman et al., 2001).
In the present study we predicted 7-repeat carriers would display more severe ADHD and ODD behaviors than children with shorter (2–5) repeats. Because prior studies indicated different gene–behavior associations for mothers’ versus teachers’ ratings (Gadow et al., 2008c; Roohi et al., 2009; Gadow et al., 2009b), we expected the same would be the case for DRD4. Owing to (i) the high rate of co-occurrence of repetitive behaviors with ADHD (Gadow & DeVincent, 2005; Gadow et al., 2006), (ii) their ancient phylogenic relation with emotional expression (Darwin, 1890) and (iii) preliminary evidence for their conjoint association with disruptive behaviors and anxiety for other gene variants in both ASD (e.g., Cohen et al., 2003; Gadow et al., 2008c, 2009b; Roohi et al., 2009) and non-ASD (e.g., Comings et al., 1996; Rowe et al., 1998) samples, we also explored potential associations with obsessive–compulsive behaviors and tics. Lastly, gene–gene analyses were conducted with our previously reported DAT1 data (Gadow et al., 2008c).
Materials and methods
Participants
Participants in this study were recruited from referrals to a university hospital developmental disabilities specialty clinic located on Long Island, New York, USA. All families with at least one child with a confirmed diagnosis of ASD were contacted by mail for participation in a genetic study. A total of 92 individuals were initially recruited but, to maximize homogeneity, the study sample (N = 66) was limited to individuals who were children (4–14 years old) when the diagnostic and behavioral evaluations were conducted. Child participants were excluded from the study if a Rett MECP2 or a Fragile X mutation was discovered. Demographic characteristics were as follows: mean ± SD age (6.9 ± 2.6 years), gender (87% male), ethnicity (96% Caucasian), IQ (79.2 ± 23.2), socioeconomic status (SES) assessed with Hollingshead’s (1975) index of occupational and educational social status (42.4 ± 11.4), single-parent household (1%) and psychotropic medication use (24%). Mothers’ and teachers’ ratings of psychiatric symptoms were available for 62 and 57 of the children, respectively. This study was approved by a university Institutional Review Board; informed consent was obtained; and appropriate measures were taken to protect patient (and rater) confidentiality. Moreover, this study conforms with The Code of Ethics of the World Medical Association (Declaration of Helsinki).
Procedure
Diagnoses of ASD were confirmed by an expert diagnostician and based on five sources of information about ASD symptoms to verify DSM-IV (American Psychiatric Association, 1994) criteria: (i) comprehensive developmental history; (ii) clinician interview with child and caregiver(s); (iii) direct observations of the child; (iv) review of validated ASD rating scales including the Child Symptom Inventory-4 (CSI-4; DeVincent & Gadow, 2009; Gadow et al., 2008d); and (v) prior evaluations; and, additionally (n = 49), with (vi) the Autism Diagnostic Observation Schedule (Lord et al., 2000) and/or Autism Diagnostic Interview-Revised (Rutter et al., 2003). The distribution of ASD subtypes in the study sample approximates recent epidemiological findings (Fombonne, 2005): autistic disorder (40%), Asperger’s disorder (23%), and pervasive developmental disorder-not otherwise specified (37%).
Prior to scheduling their initial clinic evaluation, the parents of potential participants were mailed a packet of materials including behavior rating scales, background information questionnaire and permission for release of school reports, psycho-educational and special education evaluation records. Rating scales included parent and teacher versions of the CSI-4. Genotype status was determined using DNA isolated from peripheral blood cells and polymerase chain reaction (PCR).
Genotyping
PCR was carried out in a total volume of 20 μL with forward (5′- GCGACTACGTGGTCTACTCG -3′) and reverse (5′- AGGACCCTCATGGCCTTG) primers. Each amplification contained 20 ng of genomic DNA, 1 × multiplex master mix (Qiagen, Valencia, CA, USA) and 1 μm each of the primers. Reaction conditions began with an initial denaturation at 98 °C for 15 min, followed by 40 cycles of 98 °C for 30 s, 60 °C for 90 s and 72 °C for 60 s, with a final extension step of 10 min at 72 °C. Products were analyzed on a QIAxcel System (Qiagen) and genotype analysis conducted by an investigator (D.O.) who was blind to the behavioral characteristics of the study sample.
Measures
The CSI-4 (Gadow & Sprafkin, 1986, 2002) is a behavior rating scale that assesses the behavioral symptoms of a broad range of psychiatric syndromes and has both parent and teacher versions. Individual items bear one-to-one correspondence with DSM-IV symptoms (i.e., high content validity). To assess symptom severity, items are scored (never = 0, sometimes = 1, often = 2, and very often = 3) and summed separately for each symptom dimension. The findings of numerous studies indicate that the CSI-4 demonstrates satisfactory psychometric properties in community-based normative, clinic-referred non-ASD, and ASD samples (see Gadow & Sprafkin, 2009). Specifically, individual symptom categories show satisfactory internal consistency (Cronbach’s alpha), test–retest reliability, and convergent and divergent validity with corresponding scales of other measures (Gadow & Sprafkin, 2002; Gadow et al., 2004; Mattison et al., 2003; Sprafkin et al., 2002). In children with ASD, there is evidence for the construct validity of CSI-4-defined ADHD (Gadow et al., 2006, 2008b), ODD (Gadow et al., 2008a,b; Guttmann-Steinmetz et al., 2009), separation anxiety disorder (Gadow et al., 2008b; Guttmann-Steinmetz et al., 2010) and tics (Gadow & DeVincent, 2005; Gadow et al., 2009a). Confirmatory factor analysis supports the internal validity of the DSM-IV model of behavioral syndromes in a large (N = 730) sample of children with diagnosed ASD (Lecavalier et al., 2009). Correlations of CSI-4 scores with age, gender, IQ and SES are modest. As with almost all behavior rating scales, mother and teacher ratings show modest convergence.
Statistical analyses
Prior to conducting our planned analyses, behavioral symptoms were examined for outliers, skewness and kurtosis. Variables not normally distributed were transformed using the square root function. Chi-square tests (categorical variables), correlations (continuous variables) and anovas (combined categorical and continuous variables) were used to test associations of demographic characteristics (age, gender, ethnicity), IQ level (< 70 vs. > 70), SES, single-parent household, psychotropic medication, special education and ASD subtype with genotype groups as well as the dependent variables to identify potential covariates for subsequent analyses.
As the evidence linking the DRD4 polymorphism to disruptive and impulsive behaviors points to the 7-repeat allele as the risk variant (Smith, 2010), we adopted the widely used procedure of comparing 7-repeat carriers with non-carriers. This also reduced the number of potential genotype groups, which had important statistical advantages.
Multivariate anovas (manovas) were conducted separately for each of three behavioral domains: ADHD (inattention, hyperactivity, impulsivity), oppositional behavior (ODD, separation anxiety), and repetitive behaviors (obsessions–compulsions, tics). We limited examination of subsequent univariate analyses to situations where the multivariate F was significant, thereby reducing the risk of Type 1 error for multiple related variables. Follow-up pairwise comparisons were examined to identify specific differences between genotype groups. We calculated partial eta-squared (ηp2) to gauge the magnitude of group differences to address in part some of the inherent limitations of hypothesis testing (Cohen, 1994; Feise, 2002; Perneger, 1998; Rothman,1990; Zhang et al., 1997). A rule of thumb for determining the magnitude of ηp2 suggests the following: 0.01–0.06 = small, 0.06–0.14 = moderate and > 0.14 = large (Cohen, 1988).
Results
To increase sample homogeneity, one child with an 8-repeat allele was excluded from the analyses. The distribution of DRD4 genotypes was 7-repeat allele carriers (n = 20, 31%) and noncarriers (n = 45, 69%), which does not deviate from the Hardy–Weinberg equilibrium (χ2 = 0.01, P = 0.91). Allele frequencies and descriptive characteristics are presented in Table 1. Comparisons between genotype groups did not show statistically significant differences in age, gender, ethnicity, IQ level, SES, single-parent household, psychotropic medication or special education. Moreover, the two genotype groups did not differ in either the distribution of ASD subtypes (Χ2 = 0.98, P = 0.61) or in severity of each of the three core domains of ASD symptomatology (Table 1).
| Characteristic | 7+ (n = 20) | 7− (n = 45) | χ2 | F | P | ||
|---|---|---|---|---|---|---|---|
| F (%) | Mean ± SD | F (%) | Mean ± SD | ||||
| Age | 7.7 ± 2.8 | 6.6 ± 2.5 | 2.35 | 0.13 | |||
| Gender (male) | 18 (90) | 38 (84) | 0.36 | 0.55 | |||
| IQ (n = 58) | 78.8 ± 23.4 | 79.7 ± 23.0 | 0.02 | 0.88 | |||
| Caucasian | 20 (100) | 42 (93) | 1.40 | 0.24 | |||
| Special education | 15 (75) | 39 (87) | 1.34 | 0.25 | |||
| Medication | |||||||
| Current | 6 (30) | 10 (22) | 0.45 | 0.50 | |||
| Ever | 11 (55) | 16 (36) | 2.16 | 0.14 | |||
| SES | 43.4 ± 11.3 | 41.4 ± 11.1 | 0.45 | 0.51 | |||
| ASD Symptoms | |||||||
| Social | 7.2 ± 3.3 | 7.0 ± 3.5 | 0.05 | 0.82 | |||
| Language | 5.3 ± 3.6 | 7.2 ± 3.3 | 3.56 | 0.07 | |||
| Persev. behav. | 6.2 ± 2.9 | 4.7 ± 2.8 | 3.49 | 0.07 | |||
| Allele frequency | |||||||
| 2 | 2 (5) | 14 (16) | |||||
| 3 | 1 (2.5) | 1 (1) | |||||
| 4 | 15 (37.5) | 74 (80) | |||||
| 5 | 0 (0) | 2 (2) | |||||
| 7 | 22 (55) | 0 (0) | |||||
| 8 | 0 (0) | 1 (1) | |||||
- SES, socioeconomic status assessed with Hollingshead’s (1975) index of occupational and educational social status; scores range from 24 (unskilled laborers) to 66 (major business and professionals).
ADHD
manovas did not indicate multivariate effects for ADHD for either mothers’ (F = 0.49, P = 0.69) or teachers’ (F = 1.92, P = 0.14) ratings; therefore, follow-up univariate analyses were not conducted for these variables.
Oppositional behavior
The multivariate effect was significant for mothers’ ratings of oppositional behavior (F = 3.70, P = 0.03), as was the univariate analysis for ODD (F = 6.04, P = 0.02). Findings for separation anxiety were marginally significant (F = 3.09, P = 0.08). In both cases, 7-repeat allele carriers had more severe symptoms than noncarriers (Table 2).
| Variable (CSI-4) | 7+ Mean ± SD† | 7− Mean ± SD† | F | P | ηp2 |
|---|---|---|---|---|---|
| Mothers’ ratings | |||||
| Oppositional, defiant | 9.6 ± 6.8 | 5.8 ± 4.8 | 6.04 | 0.02 | 0.10 |
| Separation anxiety‡ | 3.8 ± 5.3 | 2.1 ± 2.8 | 3.09 | 0.08 | 0.05 |
| Tics | 2.0 ± 1.7 | 1.2 ± 1.4 | 4.23 | 0.04 | 0.07 |
| Obsessions, compulsions | 1.6 ± 1.4 | 0.8 ± 1.0 | 4.96 | 0.03 | 0.08 |
| Teachers’ ratings | |||||
| Oppositional, defiant | 8.8 ± 6.2 | 5.5 ± 5.7 | 3.50 | 0.07 | 0.06 |
- *Planned comparisons (univariate anovas) for variables for which the multivariate effect (manova) was statistically significant. Variables for which the multivariate effect was not significant were mothers’ and teachers’ ratings of attention-deficit hyperactivity disorder and teachers’ ratings of repetitive movements. †Untransformed scores. ‡Included in the parent version of the CSI-4 only. CSI-4, Child Symptom Inventory-4.
Because teachers were not asked to rate separation anxiety, we conducted only a univariate analysis of ODD. Similar to mothers, teachers rated 7-repeat allele carriers as having more severe ODD behaviors than noncarriers (F = 3.50, P = 0.07), but this difference was only marginally significant. Carriers had more severe symptoms than noncarriers (Table 2).
Repetitive behaviors
The multivariate effect was significant for mother’ ratings of repetitive behaviors (F = 3.85, P = 0.03), as were the univariate analyses for both obsessions–compulsions (F = 4.96, P = 0.03) and tics (F = 4.23, P = 0.04. In both cases, 7-repeat allele carriers had more severe symptoms than noncarriers (Table 2).
manovas did not indicate multivariate effects for teachers’ ratings of repetitive behaviors (F = 1.13, P = 0.33); therefore, follow-up univariate analyses were not conducted for these variables.
DRD4*DAT1 combined genotypes
There were two marginally significant interactions between DRD4 and DAT1 genotypes. Children in the DRD4 7+*DAT1 10-10 genotype group (n = 10) were rated by their teachers as having more severe ODD symptoms (P = 0.05, ηp2 = 0.07) than children with neither or only one risk genotype (n = 48; Fig. 1).
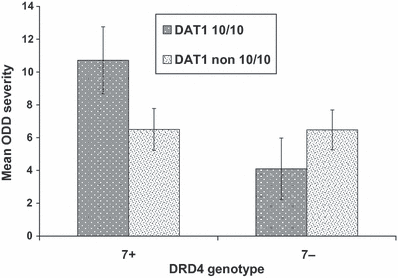
Interaction of DRD4 and DAT1 genotypes for teachers’ ratings of oppositional defiant disorder symptom severity. Results are presented as untransformed scores (mean ± SEM).
Mothers rated the risk genotype group as having more severe tics (P = 0.05, ηp2 = 0.07) than youths with neither or only one risk genotype (Fig. 2).
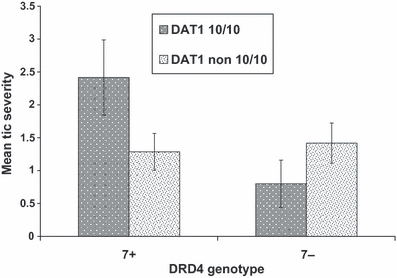
Interaction of DRD4 and DAT1 genotypes for mothers’ ratings of tic severity. Results are presented as untransformed scores (mean ± SEM).
Discussion
Findings of this study suggest that a relatively common DRD4 polymorphism may be a potential biomarker for challenging behaviors in children with ASD. For example, children who were 7-repeat allele carriers were rated as having more severe symptoms of ODD according to both mothers (ηp2 = 0.10) and teachers (ηp2 = 0.06). Although others have reported similar associations with ODD or antisocial behaviors in non-ASD samples (e.g., Fresan et al., 2007; Holmes et al., 2002; Kirley et al., 2004; Schmidt et al., 2002, 2007), these results broaden the net to include children with ASD and in so doing contribute to a decades-old debate about the etiology of behavioral disturbances in children with developmental disabilities. Nevertheless, it is not yet certain whether the pathogenesis of ODD in children with and without ASD involves common mechanisms as top-down approaches indicate both similarities and differences in clinical features (Gadow et al., 2008a; Guttmann-Steinmetz et al., 2009). This suggests that resolution of the many controversies in the nosology of co-occurring neurobehavioral syndromes in ASD is not close at hand and may not necessarily be resolved with bottom-up strategies. One approach that may clarify at least some issues is to examine gene–behavior relations for specific symptoms of ODD in larger samples of youths with ASD as well as non-ASD peers, an important topic for future studies.
Owing to the likelihood that the DRD4 polymorphism is but one of many that contributes to ODD severity, we examined another dopamine system gene variant located on a different chromosome previously genotyped in our sample, a 48-bp VNTR in the 3′-UTR of DAT1 (Gadow et al., 2008c). The 9- and 10-repeat alleles are the most common. Youths with ASD who were homozygous for the 10-repeat allele were rated as having less severe symptoms of ADHD according to mothers’ (but not teachers’) reports versus a group of individuals with all other genotypes. Other investigators have reported a combined DAT1-DRD4 association for disruptive behaviors (e.g., Carrasco et al., 2006; Martínez-Levy et al., 2009; Kebir et al., 2009). In our sample we found marginally significant (P = 0.05) evidence of such a relation for teachers’ (but not parents’) ratings of ODD severity (ηp2 = 0.07). Youths who were both homozygous for the DAT1 10-repeat allele and carriers of the DRD4 7-repeat allele were rated as having more severe ODD than children with neither or only one risk genotype. If replicated, this may represent an example of a source-specific, multiple-gene association.
Anxiety is an important co-occurring feature of ASD (White et al., 2009) and, although we previously found associations between social phobia or generalized anxiety and several gene variants, this is the second polymorphism in our sample to show a possible relation with separation anxiety (Gadow et al., 2010), a disorder that shows considerable overlap with ODD (e.g., Foley et al., 2004; Gadow et al., 2008a). However, the effect size (ηp2 = 0.05) and study sample were small and the significance level was marginal (P = 0.08), all of which increase the probability of a spurious finding. There are, nevertheless, reports indicating that infants with the 7-repeat allele are at greater risk for disorganized attachment (Gervai et al., 2005; Lakatos et al., 2002), which may be more evident when the mother has unresolved loss or trauma (reviewed by Bakermans-Kranenburg & van IJzendoorn, 2007). Moreover, research suggesting that (i) mothers who are carriers of the DRD4 7-repeat allele are differentially more responsive to stress, which appears to influence mother’s parenting behaviors (van IJzendoorn et al., 2008), and (ii) delays in social relatedness are particularly stressful for mothers of children with ASD (Davis & Carter, 2008), provides a possible mechanism whereby maternal genotype, parenting behavior and environmental stressors may interact to influence the behavior of her child with ASD and vice versa.
Attention-deficit hyperactivity disorder
There was no evidence from our multivariate analysis that allelic variation was associated with the severity of ADHD behaviors for this sample. Moreover, even univariate analyses of individual component phenotypes (i.e., inattention, hyperactivity, impulsivity) were also nonsignificant (P > 0.20), and their effect sizes were small (ηp2 < 0.05). In addition, there was no indication of possible interactive effects with DAT1 for ADHD severity. Nevertheless, we note a number of reasons (see Limitations) why the present study may have failed to detect a DRD4-ADHD association. To this list we would add two less frequently discussed possibilities that pertain to the characterization of the clinical phenotype: (i) ASD pathogenic processes may alter the behaviors that typically define the co-occurring syndrome (see Guttmann-Steinmetz et al., 2009), and (ii) a subgroup of youths may possess a form of ADHD peculiar to ASD.
Obsessive–compulsive and tic behaviors
According to maternal report, 7-repeat carriers had more severe obsessive–compulsive behaviors and tics than non-carriers, and the magnitude of this difference was in the moderate range (ηp2 = 0.08 and 0.07, respectively). The differential distribution or transmission of DRD4 alleles in patients with obsessive–compulsive disorder, tic disorder or both has been reported (in some cases tentatively) for samples from Canada (Billet et al., 1998; Díaz-Anzaldúa et al., 2004), France (Millet et al., 2003), Germany (Walitza et al., 2008), Israel (Frisch et al., 2000), Mexico (Camarena et al., 2007; Cruz et al., 1997), South Africa (Hemmings et al., 2004) and the USA (Grice et al., 1996); nevertheless, results are mixed for case-control versus family-based strategies, associations with specific alleles, and patient characteristics (e.g., early onest, co-occurring tics). There are also null findings (e.g., Tarnock et al., 2007).
In this study we also found marginally significant (P = 0.05) evidence suggesting that a combination of DRD4 (at least one 7-repeat allele) and DAT1 (10-10 repeat alleles) risk variants was associated with more severe maternal ratings of tic severity (ηp2 = 0.07). We previously reported that children in the same sample with a combined DAT1 (10-10 repeat alleles) and BDNF (Met66−) genotype were rated by their teachers as having more severe tics (P = 0.02; ηp2 = 0.09; Gadow et al., 2008c, 2009b). Collectively, these results in conjunction with findings from the extant literature are consistent with a polygenic model of symptom modification for certain types of repetitive behaviors in children with ASD, a notion that warrants additional study.
Anger, anxiety, and repetitive behaviors
At present, too little is known about the biological substrates of gene–behavior relations to formulate an explanation as to how anger, anxiety and repetitive behaviors are linked or how ASD disease processes disrupt species-typical neurobehavioral pathways of emotional expression (Nesse, 1999). We do know, however, that both common (Flint & Mackay, 2009) and rare (Cook & Scherer, 2008) gene variants are often associated with multiple and fairly diverse clinical traits. Moreover, for centuries philosophers and scientists have reported on the phylogenic relation of anger and anxiety with seemingly purposeless muscle movements (see Darwin, 1890; Sherrington, 1900). Research suggests that specific types of anxiety are ancient, neurodevelopmentally unique, and associated with both shared and specific gene variants (Belzung & Philippot, 2007; Bracha, 2006; Hovatta & Barlow, 2008). There is also preliminary evidence that the same susceptibility allele may contribute to variation in both emotional expression and repetitive behaviors within the same clinical phenotype (Cohen et al., 2003; Comings et al., 1996; Gadow et al., 2008c, 2009b; Rowe et al., 1998) but, not unexpectedly, relations vary as a function of the type of polymorphism and behavior. For example, Cohen et al. (2003) found associations between the MAOA-u VNTR polymorphism and ratings of stereotypies and social approach but not irritability or fears. Collectively, these converging observations from diverse disciplines plus research indicating that (i) severity of anxiety is more highly correlated with repetitive behaviors than communication or social deficits in children with ASD (Guttmann-Steinmetz et al., 2010), and (ii) anxiety is associated with tic severity in children with chronic multiple tic disorder (e.g., Schneider et al., 2009), even in children with ASD (e.g., Gadow & DeVincent, 2005), all suggest a number of potential pathogenic interrelations for oppositional, separation anxiety, and repetitive behaviors.
Situation and informant specificity
The fact that mothers’ and teachers’ ratings of child behavior displayed different patterns of gene–behavior relations was expected, as they each identify different groups of moderately overlapping individuals. The most parsimonious explanation for this phenomenon is that, like all organisms, children behave differently in different settings (contextual variation), and this is evident in the modest correlation between parent and teacher ratings (Achenbach et al., 1987). Moreover, children with certain neurobehavioral syndromes (e.g., ADHD, ODD) are highly reactive to contextual features (e.g., Zentall & Zentall, 1976), more so than typically developing peers (e.g., Porrino et al., 1983), but nevertheless may demonstrate considerable interindividual variability (e.g., Schleifer et al., 1975), which may be influenced differentially by DRD4 alleles (e.g., Bakermans-Kranenburg et al., 2008). Moreover, some so-called ‘risk’ variants to include the DRD4 7-repeat allele may be better characterized as influencing responsiveness to environmental stimuli where adverse and favorable experiences result in divergent outcomes, even to the point where in the latter scenario 7-repeat allele carriers do better than noncarriers (Belsky et al., 2009).
An alternative model for assessing behavioral deviance and one that is very popular in molecular genetics is to categorize people as either sick or not sick. Here, the diagnosis of childhood neuropsychiatric disorder may be in reality a complex and seemingly idiosyncratic amalgam of behavioral characterizations from multiple informants. These contextual and assessment variables probably contribute in part to inconsistencies in the extant literature.
Limitations and directions for future research
The modest size of the study sample increases the probability of spurious findings, decreases our ability to detect valid gene–behavior associations, and precludes more detailed analyses of various combinations of alleles, particularly the 2-repeat allele, which may be functionally intermediate between the 4- and 7-repeat alleles (Armbruster et al., 2009; Kang et al., 2008; Wang et al., 2004). Therefore, for these and other reasons our findings must be considered tentative pending replication with larger independent samples.
As readers of the molecular biology literature well know there are many aspects of the genetic architecture of behavioral traits (Comings & MacMurray, 2000; Flint & Mackay, 2009; Moore, 2003; Reich et al., 2001) that have important implications for research methodology (e.g., Type 1 and Type 2 error, correction for multiple comparisons, generalization of study findings) and the search for clinically useful prognostic biomarkers. Oppositional, separation anxiety, and repetitive behaviors are each probably impacted by multiple, unique and shared genes (polygeny), one of which may be the 7-repeat allele and whose influence on additional traits may only be evident in the presence of another gene variant (epistasis). Because genetic loci in close proximity are not necessarily independent (linkage disequilibrium), the DRD4 7-repeat allele may not be the relevant risk variant. As is the case with ASD (Happé & Ronald, 2008), it is likely that co-occurring behavioral syndromes such as ODD are comprised of multiple distinct traits (trait heterogeneity) of which the DRD4 7-repeat allele influences but a few. Lastly, environmental variables such as parenting behaviors (Bakermans-Kranenburg et al., 2008; Sheese et al., 2007), which also appear to be associated with maternal DRD4 genotype (Kaitz et al., 2010; van IJzendoorn et al., 2008), are also reported to interact with child DRD4 genotype.
The study of behavioral variation within the ASD clinical phenotype is greatly complicated by the facts that all of the aforementioned concerns also apply to ASD clinical phenotypes and that ASD disease processes probably interact with the progenitors of co-occurring disruptive behaviors. In addition, it now seems evident that rare variants in different genes are independently associated with ASD clinical phenotypes (Cook & Scherer, 2008). Therefore, it is reasonable to hypothesize that DRD4’s possible role in trait augmentation may vary as a function of ASD genotype.
Although the study sample was primarily caregiver-identified Caucasian, it is possible that population stratification may have contributed to spurious findings. However, the seriousness of this threat to internal validity has been questioned (Hutchison et al., 2004), at least with regard to disease risk factors (Cardon & Palmer, 2003), and is supported by meta-analyses of gene–disease associations (Bamshad, 2005; Goldstein & Hirschorn, 2004; Ioannidis et al., 2004). In the present study, controls were children with less severe symptoms from the same sample and were recruited and genotyped in identical fashion and at the same time, which probably reduced the risk that population structure or genotyping error confounded obtained results. Nevertheless, we cannot rule out this possibility.
Summary
Findings suggest that allelic variation in the DRD4 VNTR polymorphism may be a potential prognostic biomarker for ODD symptom severity in both home and school settings for children with ASD as well as for separation anxiety, obsessions–compulsions, and certain repetitive behaviors in the home. Because these behavioral syndromes often result in considerable degree of stress for caregivers and are relatively stable over time and predictive of less favorable long-term outcome, a better understanding of their biologic substrates may lead to more effective interventions as well as address important issues in nosology. Nevertheless, we emphasize the fact that our results are exploratory in that they are intended to indicate future directions for research and should not be interpreted as testing or confirming specific hypotheses.
Acknowledgements
This study was supported, in part, by grants from the National Institutes of Health (GCRC grant No. M01RR10710), the National Alliance for Autism Research, the Matt and Debra Cody Center for Autism and Developmental Disorders, and charitable contributions. The authors wish to thank Dr John Pomeroy for supervising the ASD diagnoses, Mrs Elizabeth Luchsinger for facilitating the genotyping, and anonymous reviewers for providing helpful comments.
Financial disclosures
Kenneth D. Gadow: shareholder in Checkmate Plus, publisher of the Child Symptom Inventory; Carla J. DeVincent: none; Doreen Olvet: none; Victoria Pisarevskaya: none; Eli Hatchwell: none.
Abbreviations
-
- ADHD
-
- attention-deficit hyperactivity disorder
-
- ASD
-
- autism spectrum disorder
-
- CSI-4
-
- Child Symptom Inventory-4
-
- DAT1
-
- dopamine transporter gene
-
- DRD4
-
- dopamine receptor D4 gene
-
- ηp2
-
- partial eta-squared
-
- ODD
-
- oppositional defiant disorder
-
- SES
-
- socioeconomic status
-
- UTR
-
- untranslated region
-
- VNTR
-
- variable number tandem repeat




