Fasting biases brain reward systems towards high-calorie foods
Abstract
Nutritional state (e.g. fasted vs. fed) and different food stimuli (e.g. high-calorie vs. low-calorie, or appetizing vs. bland foods) are both recognized to change activity in brain reward systems. Using functional magnetic resonance imaging, we have studied the interaction between nutritional state and different food stimuli on brain food reward systems. We examined how blood oxygen level-dependent activity within a priori regions of interest varied while viewing pictures of high-calorie and low-calorie foods. Pictures of non-food household objects were included as control stimuli. During scanning, subjects rated the appeal of each picture. Twenty non-obese healthy adults [body mass index 22.1 ± 0.5 kg/m2 (mean ± SEM), age range 19–35 years, 10 male] were scanned on two separate mornings between 11:00 and 12:00 h, once after eating a filling breakfast (‘fed’: 1.6 ± 0.1 h since breakfast), and once after an overnight fast but skipping breakfast (‘fasted’: 15.9 ± 0.3 h since supper) in a randomized cross-over design. Fasting selectively increased activation to pictures of high-calorie over low-calorie foods in the ventral striatum, amygdala, anterior insula, and medial and lateral orbitofrontal cortex (OFC). Furthermore, fasting enhanced the subjective appeal of high-calorie more than low-calorie foods, and the change in appeal bias towards high-calorie foods was positively correlated with medial and lateral OFC activation. These results demonstrate an interaction between homeostatic and hedonic aspects of feeding behaviour, with fasting biasing brain reward systems towards high-calorie foods.
Introduction
Obesity is a leading cause of illness and premature death worldwide and the incidence is increasing rapidly (Kopelman, 2000). Body weight and appetite are regulated by homeostatic internal factors, acting through circulating hormones, vagal afferents, brainstem and hypothalamic circuits (Murphy & Bloom, 2006; Parkinson et al., 2009). However, food selection and intake are also influenced by the rewarding properties of foods, which may override homeostatic controls, contributing to weight gain, and also hindering weight loss during reduced caloric intake (Yeomans et al., 2004; Berthoud & Morrison, 2008).
Using functional neuroimaging techniques such as positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), changes in the activity of brain reward systems can be measured. These corticolimbic reward systems include the ventral striatum, insula, amygdala and orbitofrontal cortex (OFC) (Kringelbach, 2004; Volkow & Wise, 2005; Berthoud & Morrison, 2008). Activity in these regions changes in response to the taste, smell, thought and sight of food (Gordon et al., 2000; Morris & Dolan, 2001; Gottfried et al., 2002; Tataranni & Delparigi, 2003; Hinton et al., 2004; Wang et al., 2004; Delparigi et al., 2005; Holsen et al., 2005; Simmons et al., 2005; St Onge et al., 2005; Beaver et al., 2006; Porubska et al., 2006; O’Doherty, 2007; Small et al., 2007). Activity in these brain reward systems also changes with the rewarding properties of the food stimuli, e.g. high-calorie vs. low-calorie foods, foods self-reported as preferentially craved, more appetizing or disgusting, or with food aversion during repeated consumption of the same food (Gordon et al., 2000; O’Doherty et al., 2000; Small et al., 2001; Killgore et al., 2003; Kringelbach et al., 2003; Hinton et al., 2004; Beaver et al., 2006; Calder et al., 2007; Farooqi et al., 2007; Rolls & McCabe, 2007; Rothemund et al., 2007; Stoeckel et al., 2008).
Activity in the brainstem and hypothalamus changes with nutritional state through homeostatic pathways, but their interaction with brain reward systems also influences feeding behaviour (Saper et al., 2002; Kringelbach, 2004; Grill et al., 2007; Berthoud & Morrison, 2008; Figlewicz & Benoit, 2009). Negative energy balance increases the rewarding properties of food (Cabanac, 1971; Stoeckel et al., 2007; Cameron et al., 2008). Activity in brain reward systems at rest changes under conditions of prolonged fasting (Del Parigi et al., 2002). Activity in response to food stimuli also changes with acute (e.g. fasted vs. fed) or chronic negative energy balance, and subjective rating of hunger (LaBar et al., 2001; Morris & Dolan, 2001; Hinton et al., 2004; Holsen et al., 2005; Porubska et al., 2006; Farooqi et al., 2007; Fuhrer et al., 2008; Rosenbaum et al., 2008).
We sought to investigate the interaction between nutritional state and the rewarding properties of food using an fMRI paradigm of visual food stimuli together with measurement of their subjective appeal. We examined whether acute fasting increases the degree to which the brain’s reward circuitry is engaged by high-calorie compared with low-calorie foods, with a priori regions of interest including ventral striatum, amygdala, anterior insula and OFC.
Materials and methods
Participants
Twenty right-handed, healthy non-obese subjects [10 male, 10 female; mean ± SEM age 26 ± 1 years, range 19–35; body mass index (BMI) 22.1 ± 0.5 kg/m2, range 18.2–27.1, with only one subject having a BMI > 24.9], with no history of neurological or psychiatric problems, participated in the study. All subjects had stable body weight (< 5% change in the preceding 3 months), normal eating habits (using the SCOFF questionnaire for eating disorders and restraint score from the Dutch Eating Behaviour Questionnaire) (Wardle, 1987; Luck et al., 2002), and were not vegetarian or vegan, or gluten- or lactose-intolerant. One subject ate breakfast only 3 days per week, two ate breakfast 4 days per week, and fourteen ate breakfast every day. Local research ethics committee approval (Hammersmith and Queen Charlotte’s and Chelsea Research Ethics Committee) and informed consent were obtained, and the study was in accordance with the Declaration of Helsinki guidelines.
Protocol
Subjects were scanned on two separate mornings (between 11:00 and 12:00 h) at least 6 days apart (13 ± 2 days) in a randomized cross-over design after an overnight fast and skipping breakfast (‘fasted’: mean ± SEM 15.9 ± 0.3 h between starting supper and fMRI scan) or after an overnight fast and eating breakfast (‘fed’: 1.6 ± 0.1 h since breakfast). Subjects avoided alcohol and vigorous exercise the day before and on the day of the study, and were told to eat their normal meals the day before the study but eat nothing after supper other than water. On the day of the study, subjects were asked to skip breakfast or eat a filling breakfast of their choice and keep a detailed food diary including saving of food packaging. Breakfast caloric content and macronutrient composition was determined using standard dietary calculators in DietPlan 6 (Forestfield Software Ltd, West Sussex, UK) (Krebs, 2002; Mills & Patel, 2009). Breakfast intake (mean ± SEM) was 724 ± 59 kcal, 47 ± 4% of estimated resting energy expenditure using the Schofield equation (Schofield, 1985), 51 ± 4% carbohydrate, 33 ± 3% fat and 15 ± 1% protein.
Before and after scanning, volunteers completed 10-cm visual analogue scales of appetite (VAS) containing the questions: ‘How hungry do you feel right now?’ (hunger), ‘How full do you feel right now?’ (full), ‘How pleasant would it be to eat right now?’ (pleasant), ‘How sick do you feel right now?’ (nausea) and ‘How much do you think you could eat right now?’ (volume) (Wren et al., 2001). VAS rating scores for before and after fMRI scanning were also averaged for each subject to allow for changes in ratings over the period of the scanning session.
There was no significant difference in mood (positive or negative) between the two scanning visits (P = 0.2 and 0.7, respectively), as assessed by the Positive and Negative Affect Schedules (Watson et al., 1988).
Visual stimuli and design
During the fMRI scan, four types of colour photographs were presented in a block design: (i) high-calorie foods (e.g. burgers, cakes and chocolate), (ii) low-calorie foods (e.g. salads, fruits and vegetables, fish), (ii) non-food-related household objects (e.g. furniture, clothing, electrical equipment) and (iv) Gaussian blurred images of the high-calorie foods, low-calorie foods and object pictures. Pictures were obtained from freely available websites and the International Affective Picture System (NIMH Center for the Study of Emotion and Attention, University of Florida, Gainesville, FL, USA). Food and object pictures were of similar luminosity and resolution. Food images were selected to represent familiar foods which are typical to the modern Western diet. The total caloric load, caloric density and macronutrient composition of the foods were as follows – high-calorie foods: 855 ± 108 kcal, 317 ± 14 kcal/100 g, 42 ± 1% fat, 48 ± 2% carbohydrate, 10 ± 1% protein; low-calorie foods: 274 ± 33 kcal, 87 ± 9 kcal/100 g, 35 ± 3% fat, 43 ± 4% carbohydrate, 23 ± 3% protein; high-calorie vs. low-calorie foods: P < 0.001 for energy content, density and percentage protein; P = 0.03 for percentage fat.
Photographs were presented in 18 s blocks in a single run lasting approximately 17 min. Each block contained six different images from the same category (high-calorie foods, low-calorie foods, household objects), with a total of nine blocks of each type shown in one of two pseudorandom block orders with a randomized picture order within each block. Each high-calorie block consisted of equal numbers of foods containing chocolate, non-chocolate sweet and savoury foods. Each image was displayed for 2500 ms, followed by a 500 ms inter-stimulus interval of a fixation cross. Each food and object block was followed by a similar duration block of six blurred pictures.
Images were viewed via a mirror mounted above an eight-channel RF head coil which displayed images from a projector using the IFIS image presentation system (In Vivo, Wurzburg, Germany) and ePrime 1.1 software (Psychology Software Tools Inc., Pittsburgh, PA, USA). While each image was on display to subjects in the scanner, they were asked to immediately rate how ‘appealing’ each picture was to them at that moment on a scale of 1–5 using a hand-held keypad (1 = not at all, 2 = not really, 3 = neutral, 4 = a little, 5 = a lot). The appeal rating was thus made and recorded simultaneously with the stimulus presentation used for fMRI activation.
A 4-Hz flashing visual checkerboard (alternating with a fixation cross in five blocks of 24 s each) was viewed at the end of each scanning session as a control visual stimulus to look for non-specific changes in fMRI activation between fasted and fed states. This scan lasted around 5 min.
Imaging acquisition
Imaging was conducted on a 3T Philips Intera whole-body scanner. Whole-brain data were acquired with T2*-weighted gradient-echo echoplanar imaging with an automated higher-order shim procedure (44 ascending contiguous 3.25 mm thick slices, 2 × 2 mm voxels); SENSE factor 2 repetition time (TR) 3000 ms; echo time (TE) 30 ms; 90° flip angle; FOV 190 × 219, matrix 112 × 112, slice acquisition angle −30° from the AC–PC line to reduce frontal lobe signal dropout due to the air sinuses, with a z-shim gradient correction to compensate for through-plane susceptibility gradients (Deichmann et al., 2003). The first five volumes of each fMRI run were discarded to allow for equilibrium effects. No neuroanatomical abnormalities were seen on a high-resolution T1-weighted turbo field echo structural scan collected at each visit (TE 4.6 ms; TR 9.7 ms; flip angle 8°; FOV 240 mm; voxel dimensions, 0.94 × 0.94 × 1.2 mm).
Image pre-processing and statistical analysis
SPM5 (update 826, Wellcome Dept of Imaging Neuroscience, UCL, UK) was used for individual pre-processing with motion and slice timing correction, normalization to a standard EPI MNI template, smoothing (8 mm FWHM), and analysis using the general linear model (GLM) by convolution of the individual block onsets with the haemodynamic response function and including the motion parameters as regressors, with subsequent contrast analysis (high-calorie vs. object, low-calorie vs. object, high-calorie vs. low-calorie, object vs. blurred, and visual checkerboard vs. fixation cross) (Beaver et al., 2006).
Second-level group random effects analysis was performed separately on the fasted and fed scans. A statistical threshold of P < 0.001 uncorrected and cluster extent > 5 voxels (2 × 2 × 2 mm) was used for activation using whole-brain analysis with correction for multiple comparisons made using false discovery rate (FDR) and family-wise error rate at P < 0.05. For a priori regions of interest (ROI) threshold was P < 0.005 uncorrected with cluster extent > 5 voxels, with small volume correction for multiple comparisons using family-wise error rate at P < 0.05. Anatomical labelling of activations was checked with reference to neuroanatomical atlases (Duvernoy, 1995, 1999). For visualizing activations, group maps were overlaid on the average of the mean EPI scans for each subject, averaged separately for the 1st and 2nd visits, and then combined. In a separate group analysis, BMI was also included as a covariate in the second-level group random effects analysis to identify whether the activation in any of the ROIs for the high-calorie vs. low-calorie food contrast was influenced by BMI when fasting.
ROIs were ventral striatum, anterior insula, amygdala, and medial and lateral OFC for the picture viewing; and lingual gyrus, calcarine sulcus and lateral geniculate nucleus for the flashing checkerboard. The ventral striatum ROI used two 8 mm spheres centred at MNI coordinates (x = ±8, y = 10, z = −12) taken from a previous fMRI study of viewing appetizing vs. bland food pictures (Beaver et al., 2006). The lateral geniculate nucleus ROI used two 10 mm spheres centred at MNI coordinates (x = ±22, y = −24, z = −5) taken from a previous fMRI study viewing a flashing checkerboard (Schneider et al., 2004). The other ROIs used masks generated from the WFU Pickatlas (version 2.4) using the automated anatomical labelling atlas, using only voxels with y > 0 to define the anterior insula, the medial section of the middle orbital frontal cortex for the medial OFC, and the inferior orbital frontal cortex for the lateral OFC (Tzourio-Mazoyer et al., 2002; Maldjian et al., 2003, 2004).
Coordinates of peak voxel activation within each ROI were determined at the group level for fasted and fed visits. The coordinates of peak voxel activation within each ROI for the high-calorie vs. low-calorie foods contrast (or flashing checkerboard vs. fixation cross) at the group level for the fasted visit were then used to extract data separately on the magnitude of activation [beta value blood oxygen level-dependent (BOLD) contrast] at the fasted and fed visits for individual subjects for the high-calorie vs. object, low-calorie vs. object, high-calorie vs. low-calorie and object vs. blurred picture (or flashing checkerboard vs. fixation cross) contrasts. Beta values were extracted separately for each hemisphere, and also combined to give average bilateral activation.
Statistics
Comparison between groups was performed using non-directional paired Student’s t-tests (fasted vs. fed). A two-way repeated-measures anova with post-hoc Student–Newman–Keuls test was performed to look at the interaction of breakfast consumption (fasted vs. fed) and food picture category (high-calorie vs. low-calorie) on BOLD contrasts in each ROI and appeal ratings. Equal variance between groups was confirmed using the Levene Median Test with a threshold P = 0.01. Linear regression analysis was used to examine the relationship (Pearson correlation coefficient r) between the effect of breakfast consumption on brain activation in each ROI and the effect of breakfast consumption on appeal bias for high-calorie foods. The dependent factor was the individual average bilateral BOLD contrast values for high-calorie vs. low-calorie food contrasts in the fed state subtracted from values obtained in the fasted state. The independent factor was the individual appeal rating difference between high-calorie and low-calorie foods in the fed state subtracted from the rating difference obtained in the fasted state. Statistical analsysis was performed using SigmaStat 2.03 (SPSS, San Rafael, CA, USA). All data are presented as mean ± standard error of mean (SEM). Significance was taken as P < 0.05.
Results
Appetite visual analogue scales
VAS ratings confirmed subjects were more hungry (t19 = 20.80, P < 0.001), would find eating more pleasant (t19 = 24.38, P < 0.001), desired more food (t19 = 18.88, P < 0.001) and were less full (t19 = 14.52, P < 0.001) when fasted than after eating breakfast (supporting Table S1).
ROI analysis
Confirming that fasting selectively enhances activation to high-calorie foods in brain regions implicated in reward, there was a significant interaction between breakfast consumption (fasted vs. fed) and food picture category (high-calorie vs. low-calorie) on activation of our ROIs bilaterally (Fig. 1, Tables 1 and 2; interaction between effects of nutritional state and food category: L ventral striatum F1,19 = 5.04, P = 0.037; R ventral striatum F1,19 = 7.24, P = 0.014; bilateral ventral striatum F1,19 = 7.67, P = 0.001; L amygdala F1,19 = 8.39, P = 0.009; R amygdala F1,19 = 7.35, P = 0.014; bilateral amygdala F1,19 = 12.32, P = 0.002; L insula F1,19 = 9.00, P = 0.007; R insula F1,19 = 12.27, P = 0.002; bilateral insula F1,19 = 17.64, P < 0.001; L medial OFC F1,19 = 6.49, P = 0.020; R medial OFC F1,19 = 6.72, P = 0.018; bilateral medial OFC F1,19 = 8.96, P = 0.007; L lateral OFC F1,19 = 7.62, P = 0.012; R lateral OFC F1,19 = 5.27, P = 0.033; bilateral lateral OFC F1,19 = 12.11, P = 0.003).
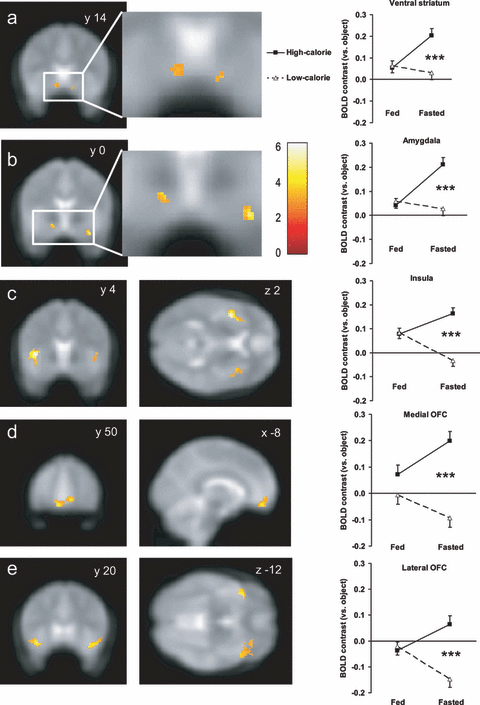
Fasting biases brain reward systems towards high-calorie foods. Left: increased group activation for viewing high-calorie compared with low-calorie foods when fasted in a priori regions of interest (ROI): (a) ventral striatum, (b) amygdala, (c) anterior insula, (d) medial OFC and (e) lateral OFC. Colour bar indicates T values. Activations are thresholded at P < 0.005 uncorrected, minimum cluster size 5 voxels, overlaid onto the average EPI scan for all subjects. Coordinates are given in standard MNI space. By contrast, no voxels were activated above this threshold after eating breakfast when fed (statistical parametric maps not shown). Coordinates of peak activated voxels in each ROI are given in Table 1. Right: magnitude of average bilateral activation (BOLD contrast relative to objects) in peak activated voxel within each ROI for high-calorie (black square, solid line) and low-calorie (white triangle, dotted line) foods after eating breakfast (fed) and when skipping breakfast (fasted). Magnitude of activation in ROIs for each hemisphere separately is given in Table 2. Data shown as mean ± SEM from two-way repeated-measures anova, n = 20 per group. ***P < 0.001 high-calorie vs. low-calorie foods. Coordinates of peak activated voxels in each ROI are given in Table 1.
| ROI and L/R hemisphere | Fed | Fasted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Voxels (n) | Z-value | x | y | z | Voxels (n) | Z-value | |
| Ventral striatum | ||||||||||
| L | – | – | – | – | n.s. | −12 | 10 | −12 | 55 | 3.02*,a |
| R | – | – | – | – | n.s. | 14 | 14 | −14 | 9 | 3.03*,b |
| Amygdala | ||||||||||
| L | – | – | – | – | n.s. | −18 | 0 | −12 | 22 | 3.31**,b |
| R | – | – | – | – | n.s. | 34 | 2 | −24 | 29 | 4.47**,d |
| Insula | ||||||||||
| L | – | – | – | – | n.s. | −40 | 4 | 2 | 655 | 4.45**,c |
| R | – | – | – | – | n.s. | 38 | 16 | −14 | 38 | 3.80**,b |
| Medial OFC | ||||||||||
| L | – | – | – | – | n.s. | −8 | 50 | −12 | 144 | 3.56**,b |
| R | – | – | – | – | n.s. | 12 | 46 | −6 | 215 | 3.52**,b |
| Lateral OFC | ||||||||||
| L | – | – | – | – | n.s. | −38 | 20 | −12 | 133 | 3.91**,b |
| R | – | – | – | – | n.s. | 30 | 34 | −16 | 403 | 3.90**,b |
- Activation within regions of interest (ROI) at second-level group analysis for high-calorie greater than low-calorie food picture contrast in fed and fasted state (n = 20 per group). Results represent coordinates of peak statistical voxel (x, y, z in MNI space), number of voxels within cluster (2 × 2 × 2 mm), and Z statistic using statistical threshold P < 0.005, minimum 5 voxel cluster size. *P = 0.001, **P < 0.001, uncorrected. aP = 0.05, bP < 0.05, cP < 0.005, dP < 0.001, family wise error small volume correction. n.s. = non-significant.
| ROI and L/R hemisphere | Fed | Fasted | ||
|---|---|---|---|---|
| High-calorie | Low-calorie | High-calorie | Low-calorie | |
| Appeal rating | −0.26 ± 0.27 | −0.03 ± 0.22 | 1.72 ± 0.23a,*** | 1.48 ± 0.23*** |
| Ventral striatum | ||||
| L | 0.044 ± 0.046 | 0.085 ± 0.057 | 0.206 ± 0.060b | 0.056 ± 0.048 |
| R | 0.064 ± 0.054 | 0.043 ± 0.055 | 0.201 ± 0.057c | 0.007 ± 0.050 |
| Amygdala | ||||
| L | 0.004 ± 0.047 | 0.045 ± 0.045 | 0.323 ± 0.075b,** | 0.129 ± 0.047 |
| R | 0.078 ± 0.038 | 0.071 ± 0.041 | 0.100 ± 0.060c | −0.073 ± 0.069 |
| Insula | ||||
| L | 0.066 ± 0.053 | 0.044 ± 0.044 | 0.197 ± 0.050c | −0.032 ± 0.058 |
| R | 0.093 ± 0.054 | 0.121 ± 0.053 | 0.128 ± 0.071c | −0.034 ± 0.070* |
| Medial OFC | ||||
| L | 0.038 ± 0.111 | −0.078 ± 0.114 | 0.245 ± 0.098c | −0.131 ± 0.110 |
| R | 0.107 ± 0.060 | 0.067 ± 0.053 | 0.153 ± 0.045c | −0.055 ± 0.051 |
| Lateral OFC | ||||
| L | −0.088 ± 0.104 | −0.045 ± 0.105 | 0.044 ± 0.079b | −0.239 ± 0.090 |
| R | 0.014 ± 0.060 | 0.002 ± 0.063 | 0.087 ± 0.065c | −0.055 ± 0.067 |
- Appeal rating difference (compared with object pictures) and magnitude of BOLD activation at peak voxel within regions of interest (ROI) depending on breakfast consumption (fasted vs. fed) and food picture (high-calorie vs. low-calorie). Coordinates of peak voxel determined from high-calorie greater than low-calorie foods contrast when fasted (Table 1). Two-way repeated-measures anova using nutritional state and food picture category as factors, and appeal rating difference or β values extracted at peak voxel for individual subject contrast estimates (vs. objects) for high-calorie and low-calorie foods as data (n = 20 per group). Post-hoc comparison aP < 0.05, bP < 0.01, cP < 0.001, for high-calorie vs. low-calorie foods within fed or fasted. *P < 0.05, **P < 0.01, ***P < 0.001, for fed vs. fasted within low-calorie or high-calorie.
When fasted, there was significantly greater activation to high-calorie than to low-calorie foods in the ventral striatum, amygdala, anterior insula, and medial and lateral OFC (Fig. 1, Tables 1 and 2; post-hoc multiple comparisons: L ventral striatum q19 = 3.92, P = 0.009; R ventral striatum q19 = 5.73, P < 0.001; bilateral ventral striatum q19 = 5.85, P < 0.001; L amygdala q19 = 5.12, P = 0.001; R amygdala q19 = 5.91, P < 0.001; bilateral amygdala q19 = 6.91, P < 0.001; L insula q19 = 7.23, P < 0.001; R insula q19 = 5.47, P < 0.001; bilateral insula q19 = 8.00, P < 0.001; L medial OFC q19 = 6.50, P < 0.001; R medial OFC q19 = 5.68, P < 0.001; bilateral medial OFC q19 = 6.89, P < 0.001; L lateral OFC q19 = 4.82, P < 0.001; R lateral OFC q19 = 5.31, P < 0.001; bilateral lateral OFC q19 = 6.36, P < 0.001). There were no voxels within any of the ROIs whose activation for the high-calorie vs. low-calorie food contrast when fasted showed a significant correlation with BMI surviving small volume correction when including BMI as a covariate in the general linear model.
By contrast, when fed, there were no significant differences in activation to high-calorie foods than to low-calorie foods within any of these ROIs (Fig. 1, Tables 1 and 2; post-hoc multiple comparisons: L ventral striatum q19 = 1.07, P = 0.46; R ventral striatum q19 = 0.62, P = 0.66; bilateral ventral striatum q19 = 0.34, P = 0.81; L amygdala q19 = 1.09, P = 0.45; R amygdala q19 = 0.23, P = 0.87; bilateral amygdala q19 = 0.65, P = 0.65; L insula q19 = 0.69, P = 0.63; R insula q19 = 0.95, P = 0.50; bilateral insula q19 = 0.13, P = 0.93; L medial OFC q19 = 2.00, P = 0.17; R medial OFC q19 = 1.08, P = 0.45; bilateral medial OFC q19 = 1.83, P = 0.20; L lateral OFC q19 = 0.74, P = 0.61; R lateral OFC q19 = 0.43, P = 0.77; bilateral lateral OFC q19 = 0.48, P = 0.74).
Food appeal rating
Both high-calorie and low-calorie foods were rated as more appealing when fasted than when fed (Table 3; high-calorie t19 = 7.17, P < 0.001; low-calorie t19 = 5.58, P < 0.001), but this was not seen for object or blurred pictures (Table 3; object t19 = 2.00, P = 0.06; blurred t19 = 0.02, P = 0.98).
| Picture appeal rating | Fed | Fasted |
|---|---|---|
| Absolute rating† | ||
| High-calorie foods | 2.15 ± 0.19 | 3.98 ± 0.13*** |
| Low-calorie foods | 2.39 ± 0.16 | 3.74 ± 0.15*** |
| Household objects | 2.41 ± 0.14 | 2.26 ± 0.16 |
| Blurred | 1.91 ± 0.18 | 1.91 ± 0.19 |
| Chocolate high-calorie foods | 2.29 ± 0.22 | 3.98 ± 0.18*** |
| Sweet non-chocolate high-calorie foods | 2.27 ± 0.20 | 3.94 ± 0.15*** |
| Savoury high-calorie foods | 2.00 ± 0.18 | 3.99 ± 0.13*** |
| Difference in rating between‡ | ||
| High-calorie foods & objects | −0.26 ± 0.27 | 1.72 ± 0.23*** |
| Low-calorie foods & objects | −0.03 ± 0.22 | 1.48 ± 0.23*** |
| Chocolate high-calorie foods & objects | −0.12 ± 0.28 | 1.72 ± 0.26*** |
| Sweet non-chocolate high-calorie foods & objects | −0.14 ± 0.28 | 1.68 ± 0.23*** |
| Savoury high-calorie foods & objects | −0.41 ± 0.26 | 1.73 ± 0.23*** |
| High-calorie & low-calorie foods | −0.23 ± 0.11 | 0.24 ± 0.12*** |
| Chocolate high-calorie & low-calorie foods | −0.10 ± 0.15 | 0.25 ± 0.19** |
| Sweet non-chocolate high-calorie & low-calorie foods | −0.12 ± 0.14 | 0.20 ± 0.11* |
| Savoury high-calorie & low-calorie foods | −0.39 ± 0.10 | 0.25 ± 0.12*** |
| Objects & blurred | 0.51 ± 0.21 | 0.35 ± 0.19 |
- Data shown are mean ± SEM, n = 20 per group. †1 = ‘not at all’, 5 = ‘a lot’. ‡First category minus second category. *P < 0.01, **P < 0.005, ***P < 0.001 fed vs. fasted (paired Student’s t-test).
There was a significant interaction between breakfast consumption (fasted vs. fed) and food picture category (high-calorie vs. low-calorie) on food appeal (Tables 2 and 3, interaction between effects of nutritional state and food category: F1,19 = 36.84, P < 0.001, for both absolute appeal rating and appeal rating relative to objects). The appeal of high-calorie foods was significantly greater than that of low-calorie foods when fasted (post-hoc multiple comparison: q19 = 2.93, P = 0.049), and when fed there was a trend for the appeal of low-calorie foods to be greater than high-calorie foods (post-hoc multiple comparison: q19 = 2.80, P = 0.06) (Table 2).
A significant interaction between breakfast consumption and food picture category on food appeal was seen for each sub-type of high-calorie food compared with low-calorie foods (Table 3, interaction between effects of nutritional state and food category: chocolate F1,19 = 11.53, P = 0.003; sweet non-chocolate F1,19 = 8.65, P = 0.008; savoury F1,19 = 44.18, P < 0.001, for both absolute appeal rating and appeal rating relative to objects).
A significantly greater appeal bias towards high-calorie over low-calorie foods was seen when fasted compared with when fed (Fig. 2, Table 3, t19 = 6.07, P < 0.001). This was seen for each type of high-calorie food used (Table 3): chocolate (t19 = 3.40, P = 0.003), sweet non-chocolate (t19 = 2.94, P = 0.008) and savoury (t19 = 6.65, P < 0.001).
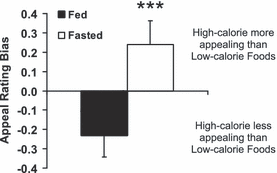
Fasting increases the appeal bias towards high-calorie over low-calorie foods. Difference between appeal ratings (scored out of 5) for high-calorie and low-calorie foods when fed (solid bar) and fasted (white bar) breakfast. Data shown as mean ± SEM, n = 20 per group. ***P < 0.001 fed vs. fasted.
To examine the relationship between the effect of breakfast consumption on brain reward systems and food appeal, we next looked for correlations between ROI activation and subjective appeal scores in individual subjects. The increase in appeal rating bias for high-calorie over low-calorie foods with fasting was positively correlated with the change in activation in both the medial OFC (r18 = 0.49, P = 0.029), and lateral OFC (r18 = 0.48, P = 0.034) for the high-calorie vs. low-calorie food contrast with fasting (Fig. 3).
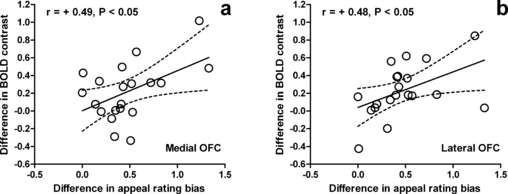
Effect of fasting on OFC activation correlates with change in appeal bias for high-calorie foods. Effect of fasting (individual difference between fasted and fed) on BOLD activation at peak activated voxel for high-calorie vs. low-calorie foods contrast in the (a) medial OFC and (b) lateral OFC correlates with the effect of fasting (individual difference between fasted and fed) on appeal rating bias for high-calorie over low-calorie foods. r represents Pearson correlation coefficient. Dashed lines represent 95% confidence interval of solid regression line. Coordinates of peak activated voxels in each ROI are given in Table 1.
Whole-brain analysis
Analysis of BOLD signal elsewhere revealed a number of additional brain regions known to interact with these reward systems, including the hippocampus, anterior cingulate cortex and dorsolateral prefrontal cortex, which were preferentially activated when fasted and viewing high-calorie vs. low-calorie foods (supporting Tables S4 and S5), consistent with previous studies (Killgore et al., 2003; Cardinal & Everitt, 2004; Hinton et al., 2004; Wilson et al., 2004; Uher et al., 2006; Fuhrer et al., 2008; Stoeckel et al., 2008).
Control activation
There was no significant effect of breakfast consumption on the reaction time to rate the appeal of any of the picture categories (supporting Table S1), suggesting the absence of any effect on attention to the picture viewing task (high-calorie foods t19 = 0.20, P = 0.85; low-calorie foods t19 = 0.42, P = 0.68; objects t19 = 1.36, P = 0.19; blurred t19 = 0.94, P = 0.36).
In order to rule out the possibility of any non-specific effect of breakfast consumption or visits on BOLD signal we examined the effect on brain activation during other non-food related tasks. Breakfast consumption (comparison of fed vs. fasted) had no significant effect on the activation in any ROI while viewing household objects compared with blurred pictures (supporting Fig. S1a; L ventral striatum t19 = 0.23, P = 0.82; R ventral striatum t19 = 1.16, P = 0.26; bilateral ventral striatum t19 = 0.86, P = 0.40; L amygdala t19 = 1.05, P = 0.31; R amygdala t19 = 1.16, P = 0.26; bilateral amygdala t19 = 0.32, P = 0.75; L insula t19 = 1.51, P = 0.15; R insula t19 = 0.39, P = 0.70; bilateral insula t19 = 1.02, P = 0.32; L medial OFC t19 = 0.13, P = 0.90; R medial OFC t19 = 1.41, P = 0.17; bilateral medial OFC t19 = 0.51, P = 0.62; L lateral OFC t19 = 0.39, P = 0.70; R lateral OFC t19 = 1.65, P = 0.12; bilateral lateral OFC t19 = 0.22, P = 0.83).
Furthermore, breakfast consumption had no significant effect on activation in the occipital cortex or lateral geniculate nucleus while viewing a 4-Hz flashing checkerboard (supporting Fig. S1b–d, supporting Table S3; L lingual gyrus t19 = 1.76, P = 0.10; R lingual gyrus t19 = 0.28, P = 0.78; bilateral lingual gyrus t19 = 0.76, P = 0.46; L calcarine sulcus t19 = 1.82, P = 0.08; R calcarine sulcus t19 = 1.03, P = 0.32; bilateral calcarine sulcus t19 = 1.67, P = 0.11; L lateral geniculate nucleus t19 = 0.21, P = 0.84; R lateral geniculate nucleus t19 = 0.07, P = 0.94; bilateral lateral geniculate nucleus t19 = 0.09, P = 0.93).
By contrast, fasting significantly increased the magnitude of activation within each ROI for the high-calorie food vs. low-calorie food contrast (supporting Table S2, paired t-test fasted vs. fed: L ventral striatum t19 = 2.25, P = 0.037; R ventral striatum t19 = 2.69, P = 0.014; bilateral ventral striatum t19 = 2.77, P = 0.012; L amygdala t19 = 2.90, P = 0.009; R amygdala t19 = 2.71, P = 0.014; bilateral amygdala t19 = 3.51, P = 0.002; L insula t19 = 3.00, P = 0.007; R insula t19 = 3.50, P = 0.002; bilateral insula t19 = 4.20, P < 0.001; L medial OFC t19 = 2.55, P = 0.020; R medial OFC t19 = 2.59, P = 0.018; bilateral medial OFC t19 = 2.99, P = 0.007; L lateral OFC t19 = 2.76, P = 0.012; R lateral OFC t19 = 2.30, P = 0.033; bilateral lateral OFC t19 = 3.48, P = 0.003).
Discussion
It is recognized that nutritional state impacts on food reward. Acute fasting or more chronic negative energy balance and weight loss increases the appeal and pleasantness of food (Cabanac, 1971; Uher et al., 2006; Stoeckel et al., 2007; Cameron et al., 2008), but differential effects on different foods have not always been examined in detail.
Our results demonstrate that skipping breakfast enhances the engagement of brain reward systems by high-calorie foods, with activation bias for high-calorie over low-calories foods seen bilaterally within the ventral striatum, amygdala, anterior insula, and medial and lateral OFC when fasted but not after eating breakfast. Furthermore, the behavioural data showed that subjective food appeal was only biased towards high-calorie foods when fasted. This appeal bias was seen for both sweet (chocolate or non-chocolate) and savoury high-calorie foods. The effect of breakfast consumption on activation within the medial and lateral OFC was directly correlated with the effects on food appeal.
These results are consistent with previous studies using human functional neuroimaging of appetite to investigate the separate effects of food (vs. non-food) stimuli, different foods or nutritional state on activation of corticolimbic regions. In normal weight subjects in a fasted hungry state, visual food vs. non-food stimuli is reported to produce greater activation in regions including the amygdala, insula and OFC (Gordon et al., 2000; LaBar et al., 2001; Hinton et al., 2004; Wang et al., 2004; Holsen et al., 2005; Simmons et al., 2005; St Onge et al., 2005; Porubska et al., 2006; Fuhrer et al., 2008). These areas are also implicated in the neural response to smell and taste (Gottfried et al., 2002; Delparigi et al., 2005; O’Doherty, 2007; Small et al., 2007).
Furthermore previous studies have shown that activity is greater in the ventral striatum, OFC and amygdala when viewing appetizing compared with bland or disgusting foods, which is correlated with individual differences in reward sensitivity (Beaver et al., 2006), is greater in the insula, medial and dorsolateral prefrontal cortex when viewing high-calorie vs. low-calorie foods (Gordon et al., 2000; Killgore et al., 2003), and is greater in the amygdala and OFC when choosing highly preferred foods from a menu (Hinton et al., 2004). Activity is also greater in the medial OFC when viewing foods self-reported as preferentially craved, such as chocolate (Rolls & McCabe, 2007), while activity in the medial and lateral OFC changes during taste or smell of a food repeatedly consumed to a state of aversion (‘sensory-specific satiety’) (O’Doherty et al., 2000; Small et al., 2001; Kringelbach et al., 2003). Furthermore, obese subjects are reported to have greater activation in the ventral striatum, amygdala, insula, and medial and lateral OFC when viewing high-calorie vs. low calorie foods, as compared with non-obese subjects (Stoeckel et al., 2008).
Brain activity at rest in the insula and OFC also increased under conditions of prolonged fasting (Del Parigi et al., 2002). Acute fasting also increased activity in the ventral striatum, amygdala, insula and medial OFC in response to food vs. non-food stimuli compared with when fed (LaBar et al., 2001; Hinton et al., 2004; Holsen et al., 2005; Uher et al., 2006; Farooqi et al., 2007; Fuhrer et al., 2008). The subjective rating of hunger when fasted for at least 5 h has been shown to correlate with activation in the insula to food pictures (Porubska et al., 2006). Activity in the amgydala and OFC has also been associated with enhanced memory of food stimuli when fasted (Morris & Dolan, 2001).
Our study extends these previous findings to show that fasting preferentially increases the appeal of high-calorie over low-calorie food visual stimuli and the associated activity in the ventral striatum, anterior insula, amydala, and medial and lateral OFC. The examination of activity during control tasks (viewing object vs. blurred pictures or a flashing checkerboard) suggests that these effects are unlikely to be due to any non-specific effect of nutritional state on BOLD signal during fMRI (Noseworthy et al., 2003). Our discovery of an interaction between activity in corticolimbic regions with food type and nutritional state is in agreement with a previous H215O PET study, which showed that fasting (vs. post-prandial state) preferentially activates the amygdala and medial OFC when choosing high-incentive over low-incentive foods written on a menu (Hinton et al., 2004). Furthermore, our finding that the change in appeal bias towards high-calorie foods when switching from the fed to fasted state is correlated with the change in the activation of the medial and lateral OFC suggests a role for the OFC in the representation of food reward value. This is in agreement with earlier studies demonstrating that the decrease in pleasure when tasting a repeatedly consumed single food during sensory-specific satiety is correlated with reduced activation in the medial or lateral OFC (Small et al., 2001; Kringelbach, 2004).
Fasting increased the appeal of both high-calorie and low-calorie foods (both absolute and relative to objects), and increased activation of brain reward systems to high-calorie foods (relative to object pictures). However, activation of brain reward systems to low-calorie foods (relative to object pictures) did not increase and often appeared to decrease with fasting (without any change in activation to the object pictures themselves). This difference is obviously influenced by the selection of peak voxels within each ROI based on the high-calorie vs. low-calorie food contrast. Comparison of quantitative versus qualitative differences in BOLD activation compared with appeal rating scores may, however, have limitations due to their inherent methodological differences. Nevertheless, this does suggest that activation in these ROIs represents not just perceptual coding of the appeal of food pictures but also other factors and cognitive processes, which may include imagined or remembered taste and smell, emotional and autonomic responses, reward expectancy, motivation, restraint and craving.
We did not find any bias in brain reward systems towards high-calorie foods when non-obese subjects were studied after eating breakfast in our study. This may be due to subjects having been instructed to eat until they were fully satiated. It remains to be seen how the presence or absence of a reward bias for high-calorie foods after eating a meal is altered by meal size and macronutrient composition in non-obese subjects, or by chronic negative energy balance such as weight loss (Rosenbaum et al., 2008). Furthermore, there may be differential effects on food reward systems in the post-prandial state in obesity. Obese subjects may have decreased ability to inhibit food intake and reduced impulse control to environmental food cues (Myslobodsky, 2003), reduced satiety (le Roux et al., 2006), and show disturbances in dopamine pathways using PET, similar to that in other addictive behaviours (Volkow & Wise, 2005). Obese and post-obese subjects have been found to have altered activity at rest in corticolimbic regions after eating a meal (Delparigi et al., 2004; Le et al., 2006, 2007), while obese subjects have exaggerated activation of brain reward systems when viewing high-calorie vs. low-calorie foods (Rothemund et al., 2007; Stoeckel et al., 2008). None of our subjects was obese and only one was overweight on BMI criteria, which precludes assessment of the effect of obesity on reward system activation in this study. Nevertheless, there was no significant effect seen of BMI on activation for the high-calorie vs. low-calorie food contrast when fasted in any of the ROIs. This can be addressed directly in future studies by comparing non-obese and obese subjects.
Due to the mixed-block fMRI picture paradigm our study was not designed to distinguish whether different categories of high-calorie foods produced differential activation in reward systems. Similarly, although there was a suggestion that sweet high-calorie foods tended to be of greater appeal than savoury high-calorie foods when fed, the study was not designed or powered to examine this in detail. Future studies can address this specifically using an event-related fMRI design or blocks separating sweet and savoury high-calorie foods with sufficient numbers of different pictures.
Physiological parameters that change with food intake may be responsible for our observations of alterations in brain reward bias towards high-calorie foods. The neurochemical systems mediating food reward include dopaminergic, endocannabinoid, serotonergic, opioid and orexin pathways (Cardinal & Everitt, 2004; Kringelbach, 2004; Volkow & Wise, 2005; Palmiter, 2007; Berthoud & Morrison, 2008). The fat-derived anorexigenic hormone leptin inhibits dopaminergic neurons projecting to the ventral striatum in rodents (Cota et al., 2006). In obese humans with congenital leptin-deficiency, leptin treatment reduces hunger, ‘liking’ of food pictures, and fMRI activation to food images in the ventral striatum and insula cortex, while leptin administration to non-leptin-deficient obese subjects undergoing caloric restriction and weight reduction reduces insula activation to food stimuli (Baicy et al., 2007; Farooqi et al., 2007; Rosenbaum et al., 2008). However, as changes in plasma leptin are not expected within a few hours of eating breakfast, other physiological changes seem likely to explain the findings from our study (Korbonits et al., 1997; Hinton et al., 2004).
A specific role for increased plasma levels of the stomach-derived orexigenic hormone ghrelin when fasted is suggested by observations from rodent studies that ghrelin can stimulate midbrain dopamine neurons projecting to the ventral striatum (Cummings et al., 2007). In humans, ghrelin administration appears to increase both hunger and activation to food pictures in the amygdala, OFC and anterior insula (Malik et al., 2008). However, it remains unknown whether ghrelin may have a selective effect on high-calorie foods such as that observed in the present study.
In humans, gastric distension and increased plasma levels of anorexigenic gut hormones, such as peptide YY and insulin, after eating a meal can alter resting activity in brain reward systems, including the ventral striatum, amygdala, insula and lateral OFC (Anthony et al., 2006; Batterham et al., 2007; Wang et al., 2008). Future studies will need to address the potential role of specific physiological changes and neurotransmitter systems in mediating the reward shift to high-calorie foods with fasting.
Our results are also of interest in the context of the known associations between breakfast habits and obesity risk. In tackling obesity, much emphasis has been placed on controlling weight through caloric restriction, but this has poor long-term success (Dansinger et al., 2007). Skipping breakfast has been linked in many cross-sectional studies with obesity and greater consumption of high-calorie, especially high-fat, foods later in the day in children, adolescents and adults (Rampersaud et al., 2005; Niemeier et al., 2006; van der Heijden et al., 2007; Dubois et al., 2009; Timlin et al., 2008). Although there can be difficulty in clarifying the direction of this association, longitudinal studies have suggested a causative link between skipping breakfast and subsequent weight gain (Rampersaud et al., 2005; Niemeier et al., 2006; van der Heijden et al., 2007; Timlin et al., 2008). Skipping meals is also associated with increased obesity and more frequent binging in binge-eating disorder (Masheb & Grilo, 2006).
Although factors such as reduced physical activity may play a role, the results from our neuroimaging study suggest a potential brain reward mechanism influencing food preference by which skipping breakfast may lead to weight gain. Variable effects of skipping breakfast on weight gain between individuals may depend on variations in the degree to which reward bias and actual consumption of energy-dense foods overrides the calories not consumed by missing meals. However, any bias towards consumption of high-calorie foods will impair the ability of missing meals to produce weight loss, a technique commonly employed by obese and even normal weight individuals trying to lose weight (Serdula et al., 1993; Yeomans et al., 2004). Indeed, skipping breakfast leads to increased impulsive snacking and fat intake in obese individuals in a caloric restriction weight-reduction programme (Schlundt et al., 1992). Similar processes may underlie the observation that food restriction increases the rewarding properties of drugs of misuse in both animals and humans (Palmiter, 2007). Although there may be difficulties extrapolating neuroimaging results to the behavioural level, and the effects of chronic dietary habits may be different to acute changes, people preferentially eat foods that they find appealing and the majority of our subjects ate breakfast regularly (Stoeckel et al., 2007). Our finding that fasting by skipping breakfast biases brain reward systems towards high-calorie foods would therefore support the advice for regular consumption of a healthy breakfast as part of dietary prevention and treatment of obesity (Rampersaud et al., 2005).
In conclusion, we have shown that fasting by skipping breakfast selectively increases activation to pictures of high-calorie over low-calorie foods in the ventral striatum, amygdala, anterior insula, and medial and lateral OFC (compared with after eating breakfast). We have shown further that fasting enhances the subjective appeal of high-calorie more than low-calorie foods, and that the change in appeal bias towards high-calorie foods is positively correlated with medial and lateral OFC activation. These results demonstrate an interaction between homeostatic and hedonic aspects of feeding behaviour, with fasting biasing brain reward systems towards palatable, energy-dense foods.
Acknowledgements
We thanks the UK Medical Research Council, European Union NuSISCO (Marie-Curie Fellowship to C.G.P.) and NIHR Biomedical Research Centre Funding for financial support; Helen Marshall, Lada Krasnosselskaia, Rita Nunes, David Larkman and Jo Hajnal for MRI physics support; and Alex Dresner, Rebecca Rhodes, Paul Grasby, Alle Meije Wink, David Sharp and Jane Warren for advice and assistance with fMRI.
Abbreviations
-
- BMI
-
- body mass index
-
- BOLD
-
- blood oxygen level-dependent
-
- fMRI
-
- functional magnetic resonance imaging
-
- OFC
-
- orbitofrontal cortex
-
- PET
-
- positron emission tomography
-
- ROI
-
- regions of interest
-
- VAS
-
- visual analogue scale




