Characterization of the vulnerability to repeated stress in Fischer 344 rats: possible involvement of microRNA-mediated down-regulation of the glucocorticoid receptor
Abstract
In the present study, we established and characterized an animal model of vulnerability to repeated stress. We found that control Sprague–Dawley (SD) rats showed a gradual decrease in the HPA axis response following 14 days of repeated restraint stress, whereas Fischer 344 (F344) rats did not show such HPA axis habituation. Similar habituation was observed in the expression of c-fos mRNA, corticotropin-releasing hormone hnRNA, and phospho-CREB and phospho-ERK proteins in the hypothalamic paraventricular nucleus (PVN) of SD rats, but not in the F344 rats. In addition, repeatedly restrained F344 rats exhibited decreased cell proliferation in the dentate gyrus of the hippocampus and increased anxiety-related behaviours, while repeatedly restrained SD rats exhibited a selective enhancement of hippocampal cell proliferation in the ventral area. Moreover, we found a lower expression of glucocorticoid receptor (GR) protein, but not mRNA, in the PVN of F344 rats compared to SD rats. We also identified that microRNA (miR)-18a inhibited translation of GR mRNA in cultured neuronal cells and that increased expression of miR-18a in the PVN was observed in F344 rats compared with SD rats. These strain differences in GR protein levels were not found in the hippocampus and prefrontal cortex, and the expression of miR-18a was much lower in these brain regions than in the PVN. Our results suggest that F344 rats could be a useful animal model for studying vulnerability to repeated stress, and that miR-18a-mediated down-regulation of GR translation may be an important factor to be considered in susceptibility to stress-related disorders.
Introduction
The hypothalamic–pituitary–adrenal (HPA) axis controls the production and release of adrenal glucocorticoids in response to stress and daily circadian rhythm. Dysregulation of the HPA axis is known to be associated with vulnerability to a number of psychiatric diseases including major depression, anxiety disorders and post-traumatic stress disorder (de Kloet et al., 2005; Seckl & Holmes, 2007).
Several lines of evidence have indicated that chronically stressed animals often exhibit suppressed or decreased HPA responses upon re-exposure to the same, or a homotypic, stressor. This decrement, termed habituation, has been observed with various stress paradigms, including restraint (Melia et al., 1994; Dhabhar et al., 1997; Ma et al., 1999; Cole et al., 2000; Viau & Sawchenko, 2002; Girotti et al., 2006), cold (Bhatnagar & Meaney, 1995) and immobilization (Garcia et al., 2000). This plasticity in the regulation of HPA activity as a consequence of repeated stress is thought to protect the organism from the potentially damaging effects of hypercorticosteroidism (Armario et al., 2004). Habituation is thought to be partly regulated by corticosterone-mediated negative feedback, a regulatory mechanism that restores the stress-stimulated HPA axis to basal levels via activation of glucocorticoid type I (mineralocorticoid receptor; MR) and/or type II (glucocorticoid receptor; GR; Dallman et al., 1987; Cole et al., 2000; Jaferi & Bhatnagar, 2006). However, there is very little understanding of the mechanisms responsible for stress habituation.
Different strains of mouse or rat have different neuroendocrine, neurogenic, physical or behavioural phenotypes that are heritable and stable (Dhabhar et al., 1995, 1997; Kempermann et al., 1997; Fernandes et al., 2004; Hovatta et al., 2005). In particular, Fischer 344 (F344) rats have been widely used in the study of HPA axis function (Kosten & Ambrosio, 2002). F344 rats are known to consistently present an exaggerated acute stress-induced corticosterone secretion relative to Sprague–Dawley (SD) and Lewis strains (Dhabhar et al., 1995, 1997). F344 rats have also been reported to exhibit no habituation of HPA axis activity during restraint stress episodes over a period of 10 days (Dhabhar et al., 1997). These observations suggest that F344 rats are a stress-hyperresponsive strain and may have a vulnerability to repeated restraint stress (RRS). However, little is known about the biochemical, physiological and behavioural effects of repeated stress in F344 rats. Therefore, in the first experiment of the present study, we characterized the neuroendocrine and biochemical responses to RRS in F344 and control SD rats. In the second experiment, hypothesizing the aberrant expression of GR and/or MR in the HPA dishabituation in F344 rats, we measured the expression of these mRNAs and proteins in several brain regions. In the third experiment, we focused on a class of small noncoding transcripts called microRNAs (miRNAs), which promote translational repression or mRNA degradation, to investigate the molecular mechanism underlying the aberrant GR translation in F344 rats. Finally, we measured behaviour and hippocampal cell proliferation as a consequence of dishabituation to RRS in F344 rats.
Materials and methods
Animals
Male SD and Fischer 344 (F344) rats (Japan SLC Inc., Hamamatsu, Japan) were housed three per cage in clear polycarbonate cages with wood chip bedding at 24 °C in a humidity-controlled room on a 12-h light–dark cycle (light on at 08.00, off at 20.00 h) and maintained for 1 week before experimental use (9 weeks old at stress onset). Food and water were continuously available except during stress sessions and behavioural tests. All experimental procedures were performed according to the Guidelines for Animal Care and Use at Yamaguchi University School of Medicine and in accordance with the Japanese Neuroscience Society. Experimental protocols were approved by the Committee on the Ethics of Animal Experiments at Yamaguchi University School of Medicine.
Stress procedures
Rats were weighed and individually subjected to restraint stress by placing them into wire mesh restrainers secured at the head and tail ends with clips as previously reported (Watanabe et al., 1992). Non-restrained rats were weighed and then returned to their home cage.
General experimental procedures
Each strain of rats was divided into nonrestrained, single- and repeatedly (3-, 7- or 14-session) restrained groups. Single-restraint animals were left in their home cage until the test day. Repeatedly restrained rats were placed into restrainers for 2 h (10.00–12.00 h) for 2, 6 or 13 consecutive days prior to the test day. On the test day, rats from singly- and repeatedly restrained groups were killed by decapitation at 30 min after the initiation of restraint. Non-restrained control rats were rapidly removed from their cages and decapitated. Trunk blood was collected in heparinized tubes and plasma was separated by centrifugation and stored at −80 °C for corticosterone determination. Adrenal glands were removed and their weights were calculated as a percentage of body weight. To determine the expression levels of stress-related molecules, including c-fos mRNA, corticotropin-releasing hormone (CRH) heteronuclear RNA (hnRNA), cyclic AMP response-element binding protein (CREB), phosphorylated CREB (pCREB), extracellular signal-regulated kinase (ERK)1/2 and phophorylated ERK (pERK)1/2, the hypothalamic tissue containing the paraventricular nucleus (PVN) was dissected according to the technique of Palkovits (1973). The tissues were frozen in liquid nitrogen, and then stored at −80 °C until use. For in situ hybridization, brains were rapidly removed and frozen with prechilled 2-methylbutane with dry ice and then stored at −80 °C until slice preparation. To evaluate the levels of GR and MR mRNAs and proteins, and miRNA-18a (miR-18a), nonrestrained and 14-session repeatedly restrained animals (24 h after the final stress session) were deeply anaesthetized and the PVN region, hippocampus and prefrontal cortex were dissected, frozen in liquid nitrogen and then stored at −80 °C until use.
Corticosterone assay
Corticosterone concentration was determined using a commercial enzyme immunoassay kit (Assay designs, Ann Arbor, MI, USA). The sensitivity of this assay is 26.99 pg/mL and the intra- and interassay coefficients of variation were 6.6 and 7.8%, respectively.
RNA isolation and cDNA synthesis
Total RNA from dissected tissues or cells was extracted by using the mirVana miRNA isolation kit (Ambion, Austin, TX, USA) and treated with DNase (DNA-free kit, Ambion). One microgram of total RNA was used for cDNA synthesis by QuantiTect Reverse Transcription kit (Qiagen, Chatsworth, CA, USA). The primer mix of this kit contains oligo-dT and random primers to ensure cDNA synthesis from all regions of RNA transcripts. The cDNA was stored at −80 °C until use.
Quantitative real-time polymerase chain reaction (qRT-PCR) and reverse transcription-PCR
qRT-PCR was performed in an Applied Biosystems 7300 Fast Real-Time PCR System with SYBR green PCR master mix (Applied Biosystems, Foster City, CA, USA) according to the manufacturer's protocol. PCR conditions were 15 min at 95 °C, 45 cycles of 15 s at 95 °C and 30 s at 60 °C. Amplification of the single PCR product was confirmed by monitoring the dissociation curve and electrophoresis on 1.2% agarose gels stained with ethidium bromide. Amplification curves were visually inspected to set a suitable baseline range and threshold level. The relative quantification method was employed for quantification of target molecules according to the manufacturer's protocol, in which the ratio between the amount of target molecule and a reference molecule within the same sample was calculated. All measurements were performed in triplicate. Levels of GAPDH mRNA and U6 snRNA were used to normalize the relative expression levels of target mRNA or miRNA, respectively. Reverse transcription-PCR was performed using Platinum Taq DNA polymerase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. PCR conditions were 1 min at 95 °C, 30 cycles of 30 s at 95 °C, 30 s at 60 °C and 30 s at 72 °C. Amplification of the single PCR product was visualized by electrophoresis on 1.5% agarose gels stained with ethidium bromide. The PCR primers used were as follows (5′ to 3′): c-fos forward, GAGGGAGCTGACAGATACGC; c-fos reverse, GGCTGCCAAAATAAACTCCA; CRH hnRNA forward, GGCAGGAATGGAGACAGAGA; CRH hnRNA reverse, TAAGCTATTCGCCCGCTCTA; GR forward, GTCCATGGGGCTGTATATGG; GR reverse, TCCAGAAGCCGAAAGTCTGT; MR forward, TCTTTGGAGGAGGTCAGAGC; MR reverse, AAAATGGACTCCACGTTTGTG; pre-miR-18a forward, TGCGTGCTTTTTGTTCTAAGG; pre-miR-18a reverse, TGCCAGAAGGAGCACTTAGG; pre-miR-124a forward, TCTCTCTCCGTGTTCACAGC; pre-miR-124a reverse, ACCGCGTGCCTTAATTGTAT; GR-3′-UTR site A forward, AGGTTGTGCAAATTAACAGTCC; GR-3′-UTR site A reverse, CCACAGTTTACCCAGCAGGT; GR-3′-UTR site B forward, CCTGTGAATTTCTTCACTGTTGA; GR-3′-UTR site B reverse, TTTGGCCACCTTGAATAGAAA; GAPDH forward, TGCCACTCAGAAGACTGTGG; GAPDH reverse, TTCAGCTCTGGGATGACCTT; U6 snRNA forward, TGCTTCGGCAGCACATATAC; U6 snRNA reverse, AGGGGCCATGCTAATCTTCT.
Northern blotting
For miRNA Northern blotting, 10 µg of total RNA was separated on a 15% denaturing polyacrylamide gel. The total RNA was transferred onto Hybond N+ membranes (GE Healthcare Bio-Sciences, Piscataway, NJ, USA), UV-crosslinked, baked for 60 min at 80 °C and hybridized using ULTRAhyb-Oligo buffer (Ambion) according to the manufacturer's protocol. Oligonucleotides complementary to mature miRNAs and 32P-end-labelled with T4 kinase (Promega, Madison, WI, USA), were used as probes. Probe sequences were as follows: miR-18a, 5′-CTATCTGCACTAGATGCACCTTA-3′; U6 snRNA, 5′-GAATTTGCGTGTCATCCTTGCGCAGGGGCCATGCTAA-3′. Levels of ribosomal RNA were visualized on gels stained with ethidium bromide. A U6 snRNA probe was applied to normalize the relative miRNA expression levels. Densitometric analysis was performed by FLA2000 (Fujifilm, Tokyo, Japan).
In situ hybridization
In situ hybridization for c-fos mRNA was performed as previously described (Watanabe et al., 1994; Kato et al., 1996). In brief, 16-µm-thick sections were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 min, acetylated in 0.25% acetic anhydride in 0.1 m triethanolamine–HCl (pH 8.0), rinsed in 2 × SSC and allowed to air-dry. The hybridization mixture containing 35S-labelled c-fos riboprobes was applied to slides and the sections were incubated at 52 °C overnight in a humidified chamber. Following hybridization, the sections were rinsed in 2 × SSC at room temperature, treated with Rnase A for 30 min at room temperature and subsequently washed in 0.2 × SSC for 1 h at 52 °C. After air-drying, the slides were exposed to Kodak X-OMAT film for 1 week at room temperature. Film images of the brain sections were captured by an image analysis system (Neuroscience Inc., Tokyo, Japan).
Protein extraction and Western blotting
Western blotting was performed as previously described with minor modifications (Funato et al., 2006). In brief, 20 or 60 µg of proteins were separated on 7% or 12% Tris–glycine gels and transblotted onto polyvinylidene difluoride membranes (GE Healthcare Bio-Sciences). The membranes were incubated with antibodies directed against: pERK1/2 (1 : 400); ERK1/2 (1 : 1000); pCREB (1 : 500); CREB (1 : 1000; Cell Signalling, Beverly, MA, USA); histone H3 (1 : 1000); phosphorylated histone H3 (p-H3, 1 : 500; Upstate Cell Signalling Solutions, Beverly, MA, USA); GR (M-20, 1 : 1000); MR (C-19, 1 : 500) or β-actin (1 : 2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with an appropriate horseradish peroxidase-conjugated secondary antibody, the blots were developed with an ECL-Plus detection kit (GE Healthcare Bio-Sciences). Densitometric analysis was performed by Inquiry software (Neuroscience Inc.).
Bromodeoxyuridine (BrdU) immunohistochemistry
BrdU administration
BrdU (Sigma, St Louis, MO, USA) was prepared in 0.9% saline to a dilution of 20 mg/mL BrdU and 0.007 m NaOH. On the final stress session, repeatedly restrained and nonrestrained rats were administered BrdU (100 mg/kg) intraperitoneally (i.p.) twice after the termination of restraint stress. A total of 200 mg/kg BrdU was given via two i.p. injections with a 6-h interval (12.00 and 18.00 h).
Perfusion and slice preparation
Twenty-four hours after the first BrdU injection (on the 15th day), rats were deeply anaesthetized with Nembutal and perfused transcardially with saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (PB; pH 7.4). Their brains were removed, postfixed in the same fixative for 24 h and then cryoprotected in 30% sucrose in 0.1 m PB for 3 days at 4 °C. Brains were frozen in 2-methylbutane prechilled with dry ice and then stored at −80 °C until use. Forty-micrometer-thick coronal sections throughout the entire dentate gyrus of the hippocampus were cut on a freezing microtome and collected in six-well plates containing cryoprotectant (25% ethylene glycol and 25% glycerol in 0.05 m PB, pH 7.4) and then stored at −20 °C until use.
BrdU immunohistochemistry
The free-floating sections were incubated in 50% formamide in 2 × SSC at 65 °C for 2 h followed by PBS washes. The sections were then incubated in 2 m HCl for 30 min and in 0.1 m boric acid solution (pH 8.5) for 10 min. After the PBS washes, the sections were pretreated with 0.6% hydrogen peroxide in PBS for 30 min, washed with PBS and incubated for 1 h at room temperature with a blocking solution composed of 0.3% TritonX-100 and 5% normal goat serum in PBS. Sections were then incubated with the primary antibody to BrdU (1 : 400; Chemicon, Temecula, CA, USA) in the blocking solution for 48 h at 4 °C. After rinsing in PBS, the sections were incubated in biotinylated horse antimouse secondary antibody (Vectastain Elite ABC kit, Vector Laboratory, Burlingame, CA, USA) for 2 h at room temperature. Following PBS washes, the sections were incubated in avidin–biotin complex for 1 h at room temperature. Finally, the sections were developed with a solution of 0.03% DAB (Sigma) and 0.03% hydrogen peroxide in Tris-buffered saline and were mounted onto slides, counterstained with cresyl violet, dehydrated, cleared and covered with a coverslip under Permount (Fisher Scientific, Pittsburgh, PA, USA).
Quantification of BrdU-labelled cells in the dentate gyrus
The number of BrdU-labelled cells on every tenth bilateral section throughout the whole dentate gyrus and dorsal (bregma −2.80 to −4.00 mm; Paxinos & Watson, 1998) and ventral hippocampus (bregma −5.20 to −6.80 mm) were counted using a light microscope (Nikon, Tokyo, Japan). The number of BrdU-labelled cells was multiplied by 10 to estimate the total number of BrdU-labelled cells throughout the target regions. To correct the overestimation linked to counting the same nucleus on two adjacent sections, the following formula (Abercrombie, 1946) was applied: N = n[t/(t + d)], where N corresponds to the `true' number, t is the section thickness, d is the nucleus diameter and n is the estimated number.
Behavioural procedures
Twenty-four hours after the final stress session (on the 15th day), rats were subjected to the following behavioural experiments. All experiments were performed in a blind fashion.
Social interaction test
Each subject was placed in a measuring cage and allowed to stay for 120 min. A male conspecific juvenile was then introduced into the cage and the amount of time spent in social interaction (e.g. grooming, licking, sniffing, crawling over or under) of the testing animal was recorded during a 3-min session.
Novelty-suppressed feeding test
Subjects were weighed (body weight A) and singly housed after the termination of the final restraint stress session on the 14th day, and food pellets were removed from their cages. Water remained available ad libitum. Twenty-four hours after food removal, rats were weighed (body weight B) and transferred to a clean holding cage in the testing room. The testing apparatus consisted of a circular arena (60 cm in diameter). A piece of rat chow was placed in the centre of the arena. Each subject was placed in the testing area and the time to the first feeding episode was recorded for 5 min. After the termination of the test, the animal was returned to the home cage with food pellets and the amount of food consumed was measured for 4 h. The percentage loss of body weight was estimated as: [(body weight B)/(body weight A)] × 100.
Generation of DNA constructs
The human GR-3′-UTR (Miesfeld et al., 1986) containing two putative target sites of miR-18a (see Fig. 5A) was amplified from human cDNA (SH-SY5Y cells) using the following primers: GR-3′-UTR forward, 5′-AGGTTGTGCAAATTAACAGTCC-3′; GR-3′-UTR reverse, 5′-TTTGGCCACCTTGAATAGAAA-3′. PCR products were cloned into the pGL3 control vector (Promega), downstream of the luciferase coding sequence. To express the miR-18a gene in SH-SY5Y cells, the genomic sequence containing the pre-miR-18a gene sequence plus 50 base pairs flanking each side were amplified from human DNA (SH-SY5Y cells) using the following primers: miR-18a forward, 5′-TCGGGAAGCCAAGTTGGGGT-3′; miR-18a reverse, 5′-CTATTAAAACACCTATATAC-3′, and then inserted into pcDNA3 (Invitrogen).
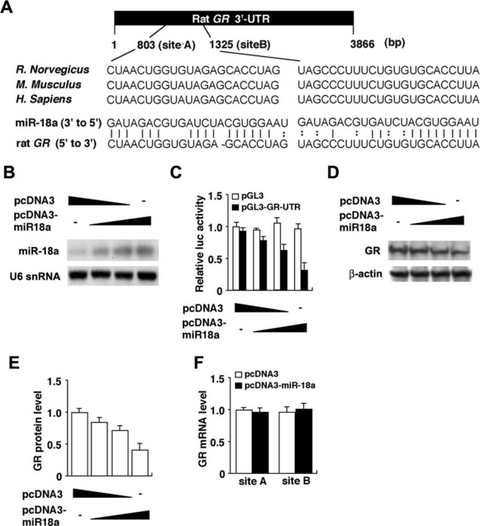
MiR-18a inhibited GR translation in human neuroblastoma SH-SY5Y cells. (A) Schematic diagram of the putative miR-18a-binding sites within the GR-3′-UTR is shown. Two target sites of miR-18a (starting positions at 803 and 1325 bp in rat GR-3′-UTR) are found within the human, mouse and rat GR-3′-UTR. The sequence of miR-18a is conserved among human, mouse and rat. (B) Northern blot analysis of mature miR-18a levels in the pcDNA3-miR18a transfected cells. The mature miR-18a was confirmed in SH-SY5Y cells that were transfected with this expression vector in a dose-dependent manner. (C) Relative luciferase activity of the GR-3′-UTR reporter gene (closed bar) or control pGL3 gene (open bar) in the absence or presence of the miR-18a expression vector in SH-SY5Y cells. (D) Western blot analysis of endogenously expressed GR protein in the SH-SY5Y cells transfected with miR-18a expression vector. (E) Quantitative analysis of GR protein levels from experiment D. (F) Levels of endogenously expressed GR mRNA including site A or site B in the absence (open bar) or presence (closed bar) of the miR-18a expression vector in SH-SY5Y cells.
Cell culture, transfection and assay
Cell culture experiments were performed as previously described with minor modifications (Uchida et al., 2006). Human neuroblastoma SH-SY5Y cells were maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum, penicillin (100 U/mL), streptomycin (100 µg/mL) and insulin (60 ng/mL) at 37 °C in 5% CO2. For the reporter assay, SH-SY5Y cells were transiently cotransfected in 24-well plates by using the Lipofectamine and PLUS reagent (Invitrogen) with the pGL3 or pGL3-GR-3′-UTR vector (0.2 µg/well) together with the miR-18a expression vector (0.2, 0.4 or 0.8 µg/well) and/or empty vector (pcDNA3). The pCMV-β-galactosidase vector (0.2 µg/well) was also cotransfected as a control for transfection efficiency. Thirty hours after the transfection, luciferase and β-galactosidase activity were measured by using a Luciferase assay system (Promega) and β-galactosidase assay system (Promega), respectively. Luciferase activity was normalized to β-galactosidase activity. All reporter assays were performed in triplicate in three independent experiments. To evaluate the endogenous GR expression levels, SH-SY5Y cells were transiently transfected in six-well plates using the Lipofectamine and PLUS reagent (Invitrogen) with the miR-18a expression vector (1, 2 or 4 µg/well) and/or empty vector (pcDNA3). Forty-eight hours after the transfection, protein and total RNA were isolated from whole-cell extracts, and Western blot analysis, Northern blotting and qRT-PCR were performed as described above.
Statistical analysis
Data are presented as the mean ± SEM. The data on body weight gain were analysed using anova for repeated measures. Grouped data obtained from 2, 3 were analysed by multifactor repeated-measures anova with strain (SD and F344) as a main factor and session number (NRS, acute, 3rd, 7th and 14th). Grouped data obtained from 6-8, 4 were analysed by two-way anova with strain (SD and F344) and stress (nonrestraint and repeated restraint). Significant effects or interactions obtained from anova were further analysed by post hoc comparisons using Bonferroni's correction. In all cases, P-values < 0.05 were considered statistically significant.
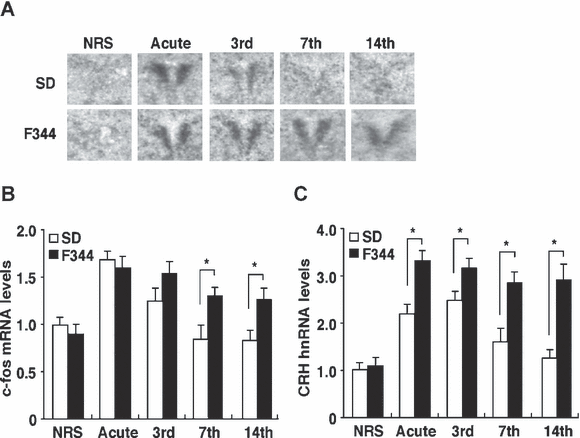
Changes in c-fos mRNA and CRH hnRNA expression in the PVN of SD and F344 rats following acute and repeated restraint stress exposure. (A) Sample autoradiograms obtained from in situ hybridization experiments for c-fos mRNA expression 30 min after the initiation of restraint stress in the PVN of nonrestrained (NRS), acutely and repeatedly 3-, 7- and 14-session restrained SD (open bar) and F344 (closed bar) rats. (B) Quantitative analyses of c-fos mRNA levels in the PVN obtained from qRT-PCR (n = 6 for all groups). (C) CRH hnRNA levels 30 min after the initiation of restraint stress in the PVN of NRS, acutely and repeatedly 3-, 7- and 14-session restrained SD and F344 rats (n = 6 for all groups) were quantified by qRT-PCR. Data are presented as mean ± SEM. *P < 0.05 vs. SD rats in the corresponding stress session.
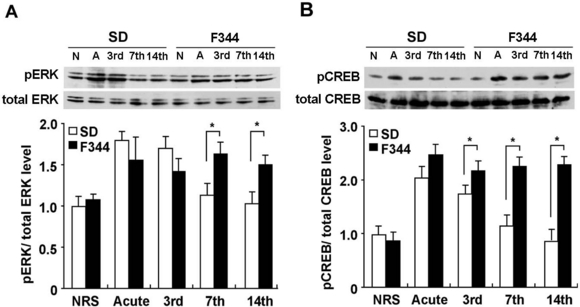
Effects of acute and repeated restraint stress on the expression of pCREB and pERK in the PVN. (A and B) Western blot analysis of pERK1/2, total ERK1/2, pCREB and total CREB levels 30 min after the initiation of restraint stress in the PVN of acutely and repeatedly 3-, 7- and 14-session restrained SD (open bar) and F344 (closed bar) rats as well as nonrestrained rats (N). The histograms show a quantitative analysis of pERK1/2 and pCREB levels (n = 8 for all groups). Data are presented as mean ± SEM. *P < 0.05 vs. SD rats in the corresponding stress session.
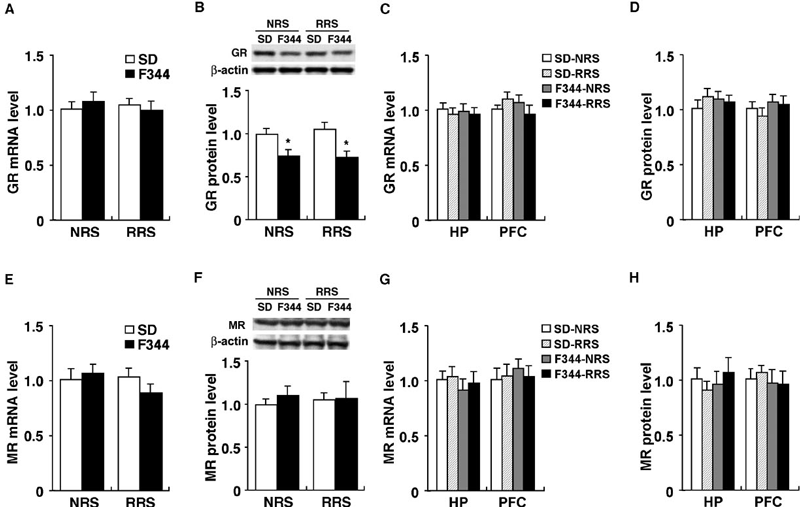
Expression analyses of GR and MR mRNAs and proteins. Expression of (A and C) GR mRNA, (B and D) GR protein, (E and G) MR mRNA and (F and H) MR protein in (A, B, E and F) the PVN, (C, D, G and H) the hippocampus (HP) and (C, D, G and H) the prefrontal cortex (PFC) of nonrestrained (NRS) and 14-day-restrained (RRS) SD and F344 rats were quantified by qRT-PCR and Western blot analysis, respectively; n = 6 for all groups. Data are presented as mean ± SEM. *P < 0.05 vs. SD rats in the corresponding stress session.
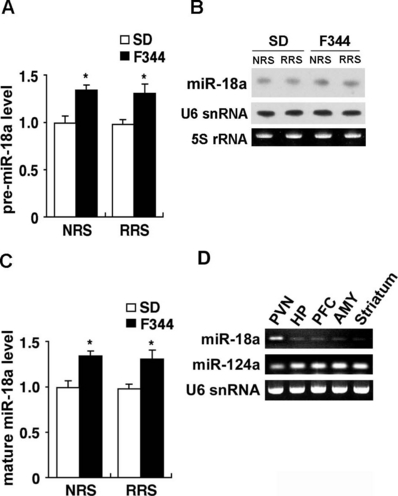
Increased expression of miR-18a in the PVN of F344 rats. (A) Levels of pre-miR-18a in the PVN of nonrestrained (NRS) and 14-day restrained (RRS) SD (open bar) and F344 (closed bar) rats were quantified by qRT-PCR (n = 6 for all groups). (B) Northern blot analysis showing the levels of mature miR-18a in the PVN of NRS and RRS animals. U6 snRNA was used as an internal control. 5S rRNA was visualized by staining the gel with ethidium bromide. (C) Levels of mature miR-18a in the PVN of NRS and RRS animals were quantified by qRT-PCR (n = 6 for all groups). (D) Reverse transcription–PCR for pre-miR-18a, pre-miR-124a and U6 snRNA were performed on cDNA from the indicated brain regions of adult SD rat (HP, hippocampus; PFC, prefrontal cortex; AMY, amygdala). Data are presented as mean ± SEM. *P < 0.05 vs. SD rats in the corresponding stress session.
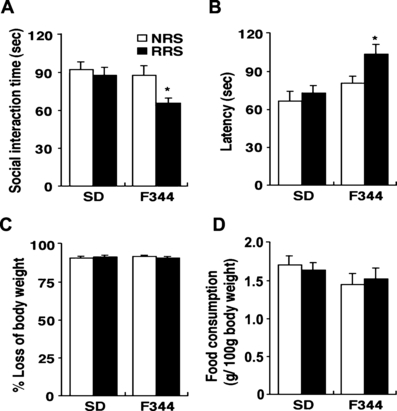
Effects of repeated restraint stress on anxiety-related behaviour. Non-restrained (NRS; open bar) or 14-day restrained (RRS; closed bar) animals were subjected to a social interaction test and a novelty-suppressed feeding test (n = 10 for all groups). (A) Social interaction time in the social interaction test and (B) latency to begin eating, (C) percentage loss of body weight and (D) food consumption in the novelty-suppressed feeding test were measured. Data are presented as mean ± SEM. *P < 0.05 vs. nonrestrained controls in the corresponding strain.
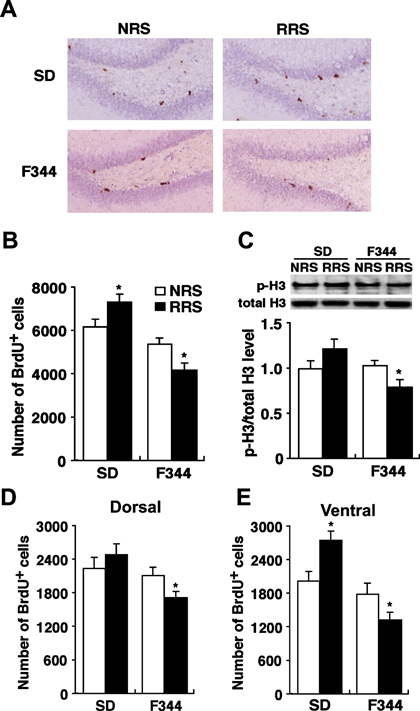
Effects of repeated exposure to restraint stress on cell proliferation in the entire dentate gyrus and dorsal and ventral areas. (A) Microscopic images of BrdU immunohistochemistry in the dentate gyrus sections from nonrestrained and 14-day-restrained SD and F344 rats. (B) Quantitative analysis of the number of BrdU-positive cells in the entire dentate gyrus of nonrestrained (NRS; open bar) and 14-day-restrained (RRS; closed bar) SD and F344 rats (n = 6 for all groups). (C) Levels of p-H3, an endogenous marker of cell proliferation, were quantified by Western blot analysis (n = 6 for all groups). (D and E) Quantitative analysis of the number of BrdU-positive cells in (D) the dorsal and (E) ventral hippocampus (n = 6 for all groups). Data are presented as mean ± SEM. *P < 0.05 vs. nonrestrained controls in the corresponding strain.
Results
Neuroendocrine response to restraint stress in F344 rats
To examine the neuroendocrine effects of acute and repeated restraint stress, we measured changes in daily body weights, adrenal weights and plasma corticosterone levels. Changes in body weight for subjects, across 6 days of prestress and during the 14-day stress period, are shown in Fig. 1A. In the nonrestrained group, there was no significant effect on body weight gain between the SD and F344 rats (F1,200 = 1.06, P > 0.05). In contrast, there was a significant effect of strain upon body weight gain in the repeatedly restrained group (F1,200 = 7.72, P < 0.05) between the SD and F344 rats. Body weight gain of restrained F344 rats was significantly less than that of nonrestrained F344 rats and that of restrained SD rats after the 4th restraint stress presentation. Adrenal weights of rats from the 3-, 7- and 14-session are shown in Fig. 1B. There were significant effects of strain (F1,30 = 30.29, P < 0.01), session number (F3,30 = 9.00, P < 0.01) and the combination of these two factors (F3,30 = 5.06, P < 0.01). F344 rats from the 7th and 14th restraint stress sessions exhibited a significantly increased adrenal weight compared with restrained SD and nonrestrained F344 rats.
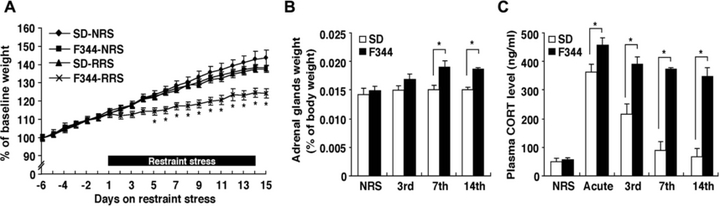
Effects of restraint stress on body weight gain, adrenal gland weight and plasma corticosterone (CORT) levels in SD and F344 rats. (A) Body weight gain is shown as a percentage of the initial body weight (n = 6 for all groups). (B) Weight of adrenal glands is shown as a percentage of body weight at the end of the 3-, 7- and 14-session and nonstressed condition (NRS; n = 6 for all groups). (C) Plasma CORT levels in NRS rats and rats exposed to acute, 3-, 7- and 14-session 30 min after the initiation of restraint stress (n = 6 for all groups). Data are presented as mean ± SEM. *P < 0.05 vs. SD rats in the corresponding stress session.
To examine the HPA axis responses to stress, plasma corticosterone levels were determined 30 min after the initiation of restraint stress (Fig. 1C). There were significant effects of strain (F1,40 = 87.58, P < 0.01), session number (F4,40 = 34.03, P < 0.01) and the combination of these two factors (F4,40 = 10.15, P < 0.01). Prior to the application of stress, there was no significant difference between SD and F344 rats for plasma corticosterone levels. Acute stress markedly increased plasma corticosterone levels in both SD and F344 rats, but F344 rats showed a significantly greater response than SD rats. SD rats showed a gradual but complete suppression of plasma corticosterone levels after the 3rd through to the 14th restraint stress session. Plasma corticosterone levels of SD rats from the 3rd restraint session were not as high as those in acutely restrained rats, but were significantly higher than those in the nonrestrained and 7th and 14th restraint groups, which were not significantly different from each other. In contrast, plasma corticosterone levels of F344 rats remained high throughout the 14-day restrained episodes.
Effects of acute and repeated restraint stress on c-fos mRNA and CRH hnRNA expression in the PVN
The PVN is a region that plays a crucial role in the regulation of the HPA axis during stress and has been reported to represent the functional plasticity to RRS (Ma et al., 1999; Girotti et al., 2006; Kwon et al., 2006; Romeo et al., 2006). To examine the levels of neuronal activity of the PVN during restraint stress, we measured the expression levels of c-fos mRNA as a marker for neuronal activation. Figure 2A shows representative autoradiograms obtained from in situ hybridization experiments for c-fos mRNA expression 30 min after the initiation of restraint stress in the PVN of SD and F344 rats. The acute stress markedly increased the c-fos mRNA expression in both SD and F344 rats. After the 7th restraint episode, however, SD rats showed a low or nondetectable c-fos mRNA signal whereas F344 rats continued to show high levels of c-fos mRNA expression after the 7th restraint episode. qRT-PCR (Fig. 2B) also revealed that c-fos mRNA induction decreased gradually after the 3rd restraint stress session and had completely disappeared by the 14th in SD rats. In contrast, F344 rats did not show such a decrease during any of the restraint episodes. There were significant effects of strain (F1,40 = 24.66, P < 0.01), session number (F4,40 = 15.32, P < 0.01) and the combination of these two factors (F4,40 = 12.56, P < 0.01).
Previous reports have indicated that an induction of c-fos in the PVN following restraint stress was localized in CRH neurons (Dayas et al., 1999). We measured the expression levels of CRH hnRNA 30 min after the initiation of restraint stress in the PVN, as a direct measure of activity within this cell population (Fig. 2C). There were significant effects of strain (F1,40 = 72.96, P < 0.01), session number (F4,40 = 34.03, P < 0.01) and the combination of these two factors (F4,40 = 9.93, P < 0.01). The acute stress markedly increased CRH hnRNA expression in both SD and F344 rats, but F344 rats showed a greater response than SD rats. The expression levels of CRH hnRNA of the acute and 3rd restraint session SD rats was significantly higher than that of nonrestrained rats, but the expression level of CRH hnRNA was completely suppressed after the 7th restraint stress session, whereas F344 rats did not show such changes.
Effects of acute and repeated restraint stress on CREB and ERK activation in the PVN
It has been reported that ERK activation is associated with the CREB, and the ERK–CREB pathway regulates c-fos expression (Ginty et al., 1994; Xia et al., 1996; Impey et al., 1998). To examine whether levels of ERK and CREB activation in the PVN would be altered in SD and F344 rats by an acute or repeated restraint stress, we measured pERK1/2 and pCREB levels 30 min after the initiation of restraint stress (Fig. 3). There were significant effects of strain (F1,40 = 17.10, P < 0.01), session number (F4,40 = 7.05, P < 0.01) and the combination of these two factors (F4,40 = 6.93, P < 0.01) in the levels of pERK. The levels of pCREB also showed similar effects of strain (F1,40 = 29.35, P < 0.01), session number (F4,40 = 19.49, P < 0.01) and the combination of these two factors (F4,40 = 6.01, P < 0.01). The acute stress markedly increased pERK (Fig. 3A) and pCREB (Fig. 3B) levels in both SD and F344 rats, with no significant differences between these rats. The levels of pERK and pCREB were completely suppressed by the 7th session in SD rats, whereas F344 rats showed significantly greater pERK and pCREB levels than SD rats during the 7th and 14th restraint episodes.
Expression levels of GR and MR mRNAs and proteins
GR is well known to be involved in HPA regulation and the adaptation to stress (Holsboer, 2000; Pariante & Miller, 2001; de Kloet et al., 2005). In addition, stress habituation is thought to be partly regulated by corticosterone-mediated negative feedback, a regulatory mechanism that restores the stress-stimulated HPA axis to basal levels via activation of GR and/or MR (Dallman et al., 1987; Cole et al., 2000; Jaferi & Bhatnagar, 2006). To examine whether there is any difference in the expression levels of GR and MR in the PVN, hippocampus or prefrontal cortex between nonrestrained and 14-session repeatedly restrained F344 and SD rats, we performed qRT-PCR and Western blot analysis (Fig. 4). In the PVN, qRT-PCR (Fig. 4A) revealed no significant differences in the expression of GR mRNA among all groups (F3,20 = 1.32, P > 0.05). However, Western blot analysis (Fig. 4B) revealed that GR protein (∼ 94 kDa) levels of F344 rats were significantly lower than those of SD rats in nonstressed conditions (F3,20 = 4.41, P < 0.05) as well as in the 14th repeated stress condition (P < 0.05). In addition, the 54 kDa form of GR protein was detected in our experiment, and its significantly lower expression was also observed in F344 rats compared with SD rats (data not shown). In the hippocampus and prefrontal cortex, there was no significant difference in the expression of GR mRNA (hippocampus, F3,20 = 0.57, P > 0.05; prefrontal cortex, F3,20 = 0.67, P > 0.05) and protein (hippocampus, F3,20 = 0.25, P > 0.05; prefrontal cortex, F3,20 = 0.53, P > 0.05) among all groups (Fig. 4C and D). These results suggest that decreased expression of GR protein is specific for the PVN of F344 rats and that this decrease is post-transcriptionally regulated.
There was no significant difference in the expression of MR mRNA (F3,20 = 1.91, P > 0.05; Fig. 4E), or of MR protein (F3,20 = 0.34, P > 0.05; Fig. 4F), in the PVN among all groups. In the hippocampus and prefrontal cortex, there was no significant difference in the expression of MR mRNAs (hippocampus, F3,20 = 0.19, P > 0.05; prefrontal cortex, F3,20 = 0.77, P > 0.05) and protein (hippocampus, F3,20= 0.61, P > 0.05; prefrontal cortex, F3,20 = 0.37, P > 0.05) among all groups (Fig. 4G and H).
MiR-18a inhibited translation of GR mRNA
A class of small, noncoding transcripts of ∼ 21 nucleotides called miRNAs silence gene expression by binding to the 3′-UTR of target mRNAs and promote translational repression or mRNA degradation (Bartel, 2004; Kosik, 2006). Therefore, the observed discrepancy between GR mRNA and protein levels in SD and F344 rats (Fig. 4) might be mediated by a translation-inhibiting effect of a GR-related miRNA that is differentially expressed between strains. To analyse the molecular mechanism in which miRNAs are involved in GR expression, we searched for candidate miRNAs targeting for GR mRNA using miRBase (Griffiths-Jones et al., 2006) and TargetScan (Lewis et al., 2003) databases. Figure 5A shows the sequences of rat miR-18a and its putative target site of GR-3′-UTR in human, rat and mouse. Importantly, the putative target sequences of miR-18a on the GR-3′-UTR are well conserved among human, rat and mouse, and the miR-18a sequence is completely conserved among these three species.
To investigate this potential interaction experimentally, human GR-3′-UTR containing two of the predicted miR-18a target sites were placed into the 3′-UTR of a luciferase reporter plasmid. Then, we introduced the luciferase expression vector under a constitutively active promoter, with or without the miR-18a expression vector (pcDNA3-miR-18a), into human neuroblastoma SH-SY5Y cells and measured the levels of luciferase activity to determine the effects of miR-18a on luciferase translation. The increased expression of miR-18a was confirmed in the SH-SY5Y cells that were transfected with the pcDNA3-miR-18a vector (Fig. 5B). The relative luciferase activity was markedly suppressed after miR-18a cotransfection in a dose-dependent manner (Fig. 5C). To more directly test the validity of putative targets, we examined whether miR-18a could repress endogenous GR protein expression. Western blot analysis revealed a decrease in GR protein levels in miR-18a-transfected cells in a dose-dependent manner (Fig. 5D and E). Thus, miR-18a has the capacity to reduce the expression of GR protein. In addition, we observed no effects on GR mRNA levels in miR-18a-transfected cells (Fig. 5F), indicating that miR-18a inhibits translation of GR mRNA without mRNA degradation.
Increased expression of miR-18 in F344 rats
We examined the expression of pre- and mature miR-18a in the PVN of SD and F344 rats (Fig. 6). qRT-PCR (Fig. 6A) revealed that pre-miR-18a expression was significantly increased in F344 rats compared with SD rats in nonstressed conditions (P < 0.05) as well as in the 14th repeated stress condition (P < 0.05). In addition, Northern blot analysis (Fig. 6B and C) revealed a significantly increased expression of mature miR-18a in F344 rats compared with SD rats in nonstressed conditions (P < 0.05) as well as in the 14th repeated stress condition (P < 0.05). These results suggest that decreased expression of GR in the PVN of F344 rats may be due, at least in part, to the increased miR-18a expression.
To further characterize the expression of miR-18a, we used reverse transcription-PCR analysis of RNAs isolated from selected brain regions of adult SD rats, some of which are known to be involved in HPA regulation. These studies showed that, compared to the PVN, pre-miR-18a was expressed at much lower levels in the hippocampus, prefrontal cortex, amygdala and striatum, whereas pre-miR-124a was enriched in these brain regions (Fig. 6D). In addition, we could not detect mature miR-18a expression in the hippocampus and prefrontal cortex using Northern blot analysis (data not shown). Moreover, as shown in Fig. 4, the expression levels of GR protein in the hippocampus and prefrontal cortex were not significantly different between SD and F344 rats, in contrast to the PVN. Taken together, the data suggest that the regulation of GR translation by miR-18a may be specific to the PVN.
Increased anxiety-related behaviour in F344 rats after repeated restraint stress
To examine whether there was difference in the anxiety-related behaviour between SD and F344 rats by the 14th RRS session, we performed the social interaction test and the novelty-suppressed feeding test at 24 h after the final stress session. The social interaction time provides an index of anxiety and depression-like behaviour, with more anxious rats spending less time in social interaction (File & Seth, 2003). Repeatedly restrained F344 rats exhibited significantly shorter social interaction times than the other groups (F3,34 = 3.38, P < 0.05; Fig. 7A).
In the novelty-suppressed feeding test, food-deprived animals were placed in a situation that provokes conflict between the drive to eat a food pellet placed in the centre of a brightly lit open field and the fear of this brightly lit open space (Santarelli et al., 2003; Heurteaux et al., 2006). The latency to begin eating has also been used as an index of anxiety and/or depression-like behaviour because classical anxiolytic drugs and antidepressants decrease it. Repeatedly restrained F344 rats exhibited significantly longer latency to begin eating than other groups (F3,36 = 8.51, P < 0.05; Fig. 7B), with no differences in feeding activity in the home cage (F3,36 = 1.49, P > 0.05; Fig. 7D) or in weight loss (F3,36 = 2.06, P > 0.05; Fig. 7C) induced by food deprivation.
Effects of cell proliferation in the dentate gyrus of the hippocampus after repeated restraint stress
It is well known that stress and glucocorticoids affect cell proliferation and/or neurogenesis in the dentate gyrus of the hippocampus (Fuchs & Gould, 2000; Sapolsky, 2004; Duman & Monteggia, 2006; Mirescu & Gould, 2006). To examine whether the levels of cell proliferation would be altered after the 14th restraint session, we performed BrdU immunohistochemistry to quantify the number of proliferating cells in the entire dentate gyrus of the hippocampus of repeatedly restrained rats (Fig. 8A and B). Prior to the application of stress, there were no significant differences in the number of BrdU-positive cells between SD and F344 rats (P > 0.05). Repeatedly restrained F344 rats exhibited significantly fewer BrdU-positive cells than nonrestrained F344 rats (P < 0.01). In contrast, repeatedly restrained SD rats exhibited significantly higher BrdU-positive cells than nonrestrained SD rats (P < 0.05). We also examined the expression of phosphorylated histone H3 (p-H3), an endogenous marker of cell proliferation, in whole hippocampus by Western blot analysis (Fig. 8C). There was significantly lower expression of p-H3 in repeatedly restrained F344 rats than in nonrestrained F344 rats (P < 0.05). Repeatedly restrained SD rats tended to exhibit an increased expression of p-H3 compared with nonrestrained SD rats, but this was not statistically significant (P = 0.07).
To further characterize the effects of RRS on cell proliferation in the hippocampus we quantified the number of BrdU-positive cells in the dorsal and ventral hippocampus, as recent reports have suggested distinct roles in the regulation of memory, anxiety, HPA axis and the actions of antidepressants between these two areas (Jayatissa et al., 2006; Sahay & Hen, 2007). Repeatedly restrained F344 rats exhibited significantly fewer BrdU-positive cells than nonrestrained F344 rats in both the dorsal (Fig. 8D, P < 0.01) and ventral (Fig. 8E, P < 0.01) hippocampus. In contrast, repeatedly restrained SD rats exhibited a significantly higher number of BrdU-positive cells than nonrestrained SD rats in the ventral hippocampus (Fig. 8E, P < 0.01), whereas there was no significant difference in the dorsal hippocampus (Fig. 8D; P > 0.05).
Discussion
In this study, we characterized differences in biochemical, neuroendocrine, neurogenic and behavioural phenotypes between SD and F344 rats during and after RRS exposure. Our major finding is that F344 rats exhibited no habituation to RRS, suggesting that comparing F344 and SD rats can provide useful information regarding mechanisms of susceptibility to stress effects upon brain function and behaviour, such as those associated with HPA axis function, anxiety and mood disorders. Moreover, we report data suggesting that the increased expression of miR-18a in the PVN of F344 rats may affect HPA axis regulation through an inhibition of GR translation.
HPA axis habituation to repeated restraint stress
We found that SD rats showed a habituation of plasma corticosterone, c-fos mRNA, CRH hnRNA, pERK and pCREB levels during repeated restraint presentation, whereas F344 rats did not show such habituation. Previous reports have indicated that ERK activation is associated with the CREB and ERK–CREB pathway that regulates c-fos expression (Ginty et al., 1994; Xia et al., 1996; Impey et al., 1998). Induction of c-fos in the PVN with restraint is localized in CRH neurons (Dayas et al., 1999). In addition, transcriptional regulation of the CRH gene in the PVN involves CREB-mediated mechanisms (Seasholtz et al., 1988; Yamamori et al., 2004). Together with these findings, the suppression of ERK–CREB signalling in the CRH neurons of the PVN might be required for the HPA axis habituation by RRS.
In general, MR is thought to be involved in the appraisal process and the onset of the stress response while GR is associated with the regulation of HPA negative feedback, which terminates the stress reactions (de Kloet et al., 2005). It has been reported that the expression of habituation is completely dependent on corticosteroid negative feedback (Cole et al., 2000), suggesting that GR is an important molecule for the expression of habituation. Actually, our data showed that GR protein level in the PVN of F344 rats was significantly lower than that of SD rats, whereas MR mRNA and protein levels were unaltered. In addition, it has been reported that GR expression levels in the parvocellular division of the PVN of the hypothalamus of repeatedly stressed rats were significantly correlated with the degree of habituation (Helmreich et al., 1997). However, a previous study has reported that treatment with a GR antagonist alone in repeatedly restrained rats did not prevent HPA habituation, although MR antagonist treatment did prevent it (Cole et al., 2000). Another study has reported that the habituation of c-fos expression occurred in adrenalectomized rats (Melia et al., 1994), suggesting that habituation occurs independently of the negative feedback of glucocorticoids. Thus, the role of GR and MR in the expression of HPA habituation remains unclear and other molecule(s) are undoubtedly required for the HPA habituation. Further studies will be necessary to solve these issues.
Role of miR-18a-mediated down-regulation of GR translation in the HPA system
Our data indicate that strain differences in GR protein levels occur in the PVN but not in the hippocampus or the prefrontal cortex, which have been implicated in the negative feedback regulation of the HPA axis (Diorio et al., 1993; Radley et al., 2006). We report that GR mRNA levels are not different between rat strains in the PVN, but GR protein levels are lower in F344 rats and miR-18a levels are higher in F344 rats. As discussed above, the role of GR in HPA habituation is unclear, but it is thought that GR can repress stress-induced responses such as neuropeptide synthesis (e.g. CRH and vasopressin) and thereby terminate ongoing stress reactions (de Kloet et al., 2005). Indeed, our data showed higher plasma corticosterone and CRH hnRNA levels in acutely restrained F344 rats than in SD rats. In addition, a previous study indicated that F344 rats display an incomplete shut-off of the corticosterone response to acute stress compared with SD rats (Dhabhar et al., 1997). Moreover, GR-heterozygous mice, whose expression level of GR is 50% lower than wild-type mice, showed an increased stress-induced corticosterone level while GR-overexpressing mice had a lower HPA axis response to stress (Ridder et al., 2005). Thus, because miR-18a is able to inhibit translation of GR, we suggest that miR-18a-mediated down-regulation of GR translation in the PVN may be involved in the regulation of the HPA axis response to stress. Moreover, it is possible that the enhanced expression of miR-18a could be a vulnerability factor for the development of stress-related disorders.
A previous study has reported that miR-18a expression was gradually decreased during brain development in mouse (Miska et al., 2004). Thus, miR-18a probably contributes to normal brain development. It remains an interesting possibility that increased expression of miR-18a in adult F344 rats may be programmed during the early postnatal period and miR-18a-mediated down-regulation of GR may lead to aberrant brain development which, in turn, characterizes an exaggerated HPA axis response to stress.
F344 rats as an animal model for vulnerability to repeated-restraint stress
F344 rats showed increased anxiety-related behaviours (social interaction time and novelty-suppressed feeding) and decreased hippocampal cell proliferation as a result of the 14 days of restraint stress, while SD rats showed stress resistance under the same experimental conditions. However, it should be noted that, with more severe and longer RRS such as the 21 days of restraint stress (6 h/day), SD rats do show an increase in anxiety behaviour (Wood et al., 2004) and fear conditioning (Conrad et al., 1999), and a decrease in hippocampal neurogenesis (Pham et al., 2003). Thus F344 rats are thought to be more vulnerable to RRS than SD rats.
It has been reported that the reduced social interaction time and increased latency to feed in the novelty-suppressed feeding test was observed in an animal model for depression (Berton et al., 2006; Chen et al., 2006; Tsankova et al., 2006; Yirmiya et al., 2006). Importantly, chronic treatment with antidepressants, but not single treatment, stimulated these behaviours (Santarelli et al., 2003; Berton et al., 2006; Heurteaux et al., 2006; Tsankova et al., 2006; Yirmiya et al., 2006). Thus, these behaviours may be associated with not only an anxiety phenotype but also a depression-like phenotype.
Increased hippocampal cell proliferation is a novel indicator of stress habituation
There is a wealth of data demonstrating that various types of stressor affect hippocampal cell proliferation and neurogenesis, and it has been proposed that stress-induced reductions in neurogenesis contribute to the pathophysiology of anxiety disorders and mood disorders (Fuchs & Gould, 2000; Sapolsky, 2004; Duman & Monteggia, 2006; Mirescu & Gould, 2006). Actually, it has been reported that stress-induced animal models for depression, such as chronic social defeated stress or chronic unpredictable stress, show reduced hippocampal cell proliferation (Banasr et al., 2007; Czeh et al., 2007). Also, our study indicated that the repeatedly restrained F344 rats showed lower cell proliferation in both dorsal and ventral regions, suggesting that F344 rats showed a maladaptive response to RRS. In contrast, SD rats, habituated to RRS, showed the selective enhancement of cell proliferation in the ventral hippocampus of repeatedly restrained SD rats, suggesting that the increased hippocampal cell proliferation could be one of the indicators of stress habituation.
Conclusion
Our present study provides a useful animal model for understanding the adaptation to repeated stress and the susceptibility to anxiety and mood disorders. Furthermore, our data suggest that miR-18a-mediated regulation of GR translation in the PVN might be an important mechanism for regulating the stress response, and that the repeated exposure to corticosterone in response to stress through the dysregulation of GR translation could result in the aberrant behaviours and hippocampal cell proliferation.
Acknowledgements
The authors thank Drs K. Shinoda, S. Nakamura, T. Fujioka, K. Yamaguchi and Y. Mizukami for helpful discussion. This study was supported in part by a Grant-in-Aid for Scientific Research from Japanese Ministry of Education, Culture, Sports, Science and Technology and a grant for Research on Psychiatric and Neurological Diseases and Mental Health from the Japanese Ministry of Health, Labor and Welfare.
Abbreviations
-
- BrdU
-
- bromodeoxyuridine
-
- CREB
-
- cyclic AMP response-element binding protein
-
- CRH
-
- corticotropin-releasing hormone
-
- ERK
-
- extracellular signal-regulated kinase
-
- F344
-
- Fischer 344
-
- GR
-
- glucocorticoid receptor
-
- hnRNA
-
- heteronuclear RNA
-
- HPA
-
- hypothalamic–pituitary–adrenal
-
- miR-18a
-
- miRNA-18a
-
- miRNA
-
- microRNA
-
- MR
-
- mineralocorticoid receptor
-
- pCREB
-
- phosphorylated CREB
-
- pERK
-
- phophorylated ERK
-
- p-H3
-
- phosphorylated histone H3
-
- PVN
-
- paraventricular nucleus of the hypothalamus
-
- qRT-PCR
-
- quantitative real-time polymerase chain reaction
-
- RRS
-
- repeated restraint stress
-
- SD
-
- Sprague–Dawley.