Minimally manipulated oligodendrocyte precursor cells retain exclusive commitment to the oligodendrocyte lineage following transplantation into intact and injured hippocampus
Abstract
Oligodendrocyte precursor cells (OPCs) are widely regarded as the best characterized cell population in the mammalian CNS and until recently were believed to be a lineage-restricted precursor terminally differentiating to postmitotic oligodendrocytes. Recent evidence has suggested that OPCs may have in vitro and in vivo neuronal potential. In this report we examine the differentiation potential of cortical OPC populations following transplantation into the neurogenic environment of the intact neonatal and adult hippocampus. Donor OPCs were minimally manipulated and not subjected to long-term ex vivo manipulation such as expansion or treatment with mitogens. Minimally manipulated OPCs did not exhibit any intrinsic neuronal potential in vitro prior to transplantation. Following transplantation of GFP-OPCs into intact neonatal and adult hippocampus, cells were able to survive and integrate for at least 14 weeks but did not exhibit neuronal differentiation. Induction of a focal neurotoxic lesion also did not result in neuronal differentiation of graft-derived OPCs. These findings show that unselected and unmanipulated populations of cortical OPCs remain as precursor cells, commit to the oligodendrocyte lineage and fail to respond to the extrinsic cues of a neurogenic or injured environment.
Introduction
Oligodendrocyte precursor cells (OPCs) are dispersed throughout the CNS and represent the best characterized cell in the glial lineage; they terminally differentiate into mature oligodendrocytes in the absence of extrinsic cues (Temple & Raff, 1985; Barres et al., 1994). Recent studies, however, raise the prospect that OPCs have a wider phenotypic potential than previously assumed and thus may qualify as a neural stem cell (Belachew et al., 2003; Gaughwin et al., 2006). This is of potential clinical interest given that OPCs are numerous and widely distributed, and have a capacity to proliferate following injury (Levine et al., 2001; Dawson et al., 2003).
OPCs have been observed in vitro to be capable of ‘respecification’ to become multipotent cells and subsequently produce neuronal-committed cells following exposure to extrinsic cues (Kondo & Raff, 2000; Belachew et al., 2003; Aguirre et al., 2004). However, the precise mechanism underlying neuronal differentiation is uncertain, and the contribution and influence of epidermal growth factor (EGF)- and/or fibroblast growth factor (FGF)-responsive precursors or prolonged culture remain unclear (Dawson et al., 2000). Recent insights suggest that in vitro neuronal potential is dependent on bone morphogenetic protein (BMP)-mediated signalling and reactivation of the Sox2 gene (Kondo & Raff, 2004). The relevance of this in vitro finding to in vivo studies claiming intrinsic neuronal potential of OPC-like cells is uncertain. Comparing studies of OPC behaviour is compounded by the range of markers used to identify OPCs, which include chondroitin sulphate proteoglycan neuron/glial antigen 2 (NG2) and platelet-derived growth factor receptor (PDGFR)α expression. There is some evidence for regional heterogeneity of OPCs, with for example evidence for oligodendrocyte generation independent of PDGFRα signalling (Spassky et al., 1998, 2001; Gensert & Goldman, 2001). NG2 expression throughout the white and grey matter is widely used and often equated to OPC identification (Levine et al., 2001; Horner et al., 2002). Furthermore, expression of the surface proteoglycan NG2 is not exclusively restricted to the OPC. NG2 expression has been observed on macrophages, endothelial cells, polydendrocytes and astrocytes following injury (Bu et al., 2001; Jones et al., 2002; Fukushi et al., 2004; Nishiyama, 2007). New neurons are generated from two regions in the postnatal brain, the subventricular zone and the hippocampus, with granule cells being generated from precursor populations residing in the subgranular zone of the dentate gyrus. Furthermore, there is evidence to suggest a role for hippocampal-derived astrocyte signals, regulated by Wnt signalling, in the induction of neuronal fate (Wagner et al., 1999; Song et al., 2002; Lie et al., 2005). Against this background we have recently shown that in vitro soluble hippocampal astrocyte-derived signals resulted in limited neuronal differentiation of in vivo expanded OPC populations (Gaughwin et al., 2006). However, the influence of extended in vitro culture propagation along with unknown signals contained within B104 expansion media is unknown and limits extrapolation of these findings to endogenous OPCs or other studies using defined conditions.
In this study we have examined the neuronal potential of neonatal cortical-derived green fluorescent protein (GFP)-OPCs that were not subjected to any long-term in vitro propagation or prior treatment with mitogens or defined signals following transplantation into the neonatal [postnatal day (P)0] and adult rat hippocampus. We report that minimally manipulated GFP-OPCs were capable of integration into the neurogenic niche of the hippocampus and survived for at least 14 weeks. Transplanted GFP-OPCs, however, did not differentiate into neurons but remained as immature precursors. Furthermore, to examine the response of grafted OPCs to injury, focal lesions were induced in the CA1 region. No evidence of neurogenesis from surviving GFP-OPCs was observed.
Materials and methods
Tissue dissection
All cell culture reagents were obtained from Invitrogen/Gibco (UK) unless otherwise specified. Experimental animals in this study were used under the United Kingdom Animals (Scientific Procedures) Act 1986. GFP neonatal rats (P0–2; gift from Dr Okabe) were killed with a lethal injection of Euthatal (sodium pentobaribital, 0.05 mL). Following removal of meninges, cortex was isolated and manually minced before enzymatic digestion with 0.1% trypsin in EDTA, followed by 0.001% DNase (Sigma, UK) and centrifugation at 230 g for 5 min. The resulting cell pellet was resuspended in triturating solution (containing 0.05% w/v Trypsin Inhibitor, 1% w/v bovine serum albumin and 0.002% w/v DNAse in Hanks' balanced salt solution) and titurated using a flamed glass pipette to obtain a single-cell suspension.
Mixed glial preparation
To obtain OPC populations for transplantation, neonatal cerebral cortex suspensions were seeded onto flasks [poly-d-lysine (PDL)-coated, ∼ 15 × 106 cells seeded per T75 flask] and maintained in serum buffer [Dulbecco's modified Eagle's medium (DMEM) supplemented with 1% penicillin–streptomycin–fungizone (PSF) and 10% foetal calf serum (FCS), v/v] for 7 days with complete media changes after 24 h, 3 days and 6 days, as previously described (McCarthy and De Vellis, 1980; Wright et al., 1997).
Conditioned media
Hippocampal astrocyte-derived condition media (HACM) was derived from wild-type neonatal rat (P0–2) hippocampal glial monolayers (2.5 × 106 cells per 25 cm2 flask) grown in serum buffer for 7 days under culture conditions. Proliferating cells (top-dwelling microglia and oligodendrocyte precursors) were eliminated by treating cultures with cytosine arabinoside (20 µm) in serum buffer for 72 h (Song et al., 2002). Cultures were shaken and washed twice, and maintained for 24 h in conditioning buffer [serum-free DMEM supplemented with 1% penicillin–streptomycin–fungizone and 1% N2 supplement (v/v)]. After a further two washes the medium was replaced with fresh conditioning buffer; culturing proceeded for 24 h and was followed by filtering (0.45 µm) to remove debris and immediate freezing.
Precursor cultures
To assess the response of OPCs to undefined extracellular cues from HACM, OPCs were seeded (1.5 × 106 per flask) into PDL-coated 25-cm2 culture flasks in culture buffer [serum-free DMEM supplemented with: penicillin–streptomycin–fungizone, 1%; N2 supplement, 1% (both v/v); N-acetyl cysteine, 60 µg/mL; and forskolin, 5 mm] supplemented with HACM (50% v/v). Medium was changed every 48 h and cell samples characterized at 7 days.
Transplantation of OPCs into rat hippocampus
One day prior to transplantation experiments, GFP mixed-glial preparations (7 days) were shaken for 1 h to remove top-dwelling microglia. DMEM with 1% PSF and 10% FCS was then added and the mixed glial preparations returned to the incubator for at least 1 h. Flasks were then shaken overnight (16–18 h) to remove the OPCs ready for transplantation. OPCs were maintained as a single-cell suspension at 7.5 × 104 cells/µL in HBSS on ice.
The care and treatment of the animals was in accordance with United Kingdom Animals (Scientific Procedures) Act 1986. For the neonatal experiments, Sprague–Dawley P0 neonatal animals were anaesthetized by placing them on ice. Animals were placed into a plasticine mould to prevent movement during the injection. Coordinates were taken from bregma: AP −1.3 and ML −1.5 mm (Englund et al., 2002). GFP-OPCs were then drawn into a Hamilton syringe and 1 µL injected into the hippocampus at a depth of DV −1.7 mm over 2 min. The syringe was left in situ for a further 5 min before being withdrawn slowly. The pup was then placed on a heated blanket before being returned to the mother.
For the adult experiments, Sprague–Dawley adult rats were anaesthetized using isoflurane. Rats were stabilized in a stereotaxic frame. The skull was exposed and bregma was isolated. Using a small drill, a hole was made in the skull at the following coordinates from bregma: AP −3.6 and ML −2.3 mm. Using a siliconized glass micropipette, 1 µL of OPCs were delivered at a depth of DV −3.0 mm over 2min. The micropipette was left in situ for a further 5 min and then withdrawn slowly (Fricker et al., 1999). The muscle and skin layers were then sutured using 4–0 suture. The animal was placed in a heated recovery cage before being returned to the home cage and fed water and dry food ad libitum.
Lesion
Two lesion paradigms were used. In the first, animals received a transplant (7.5 × 104 cells) of GFP-OPCs at P0 to examine the role of integrated GFP-OPCs, which are known to survive well following transplantation. The animals were maintained in home cages and fed water and dry food ad libitum for 10 weeks. Animals were then anaesthetized using isoflurane and stabilized in a stereotaxic frame. The skull was exposed to reveal bregma and a hole was drilled in the skull at the following coordinates from bregma: AP −3.8, ML −2.5 mm, −2.5 mm (Kimonides et al., 1998). Using a micropipette, 0.5 µL of a solution of NMDA (25 mm) was infused over 5 min. The needle was left in situ for a further 5 min and slowly removed. The muscle and skin layers were then sutured using 4–0 suture. The animal was placed in a heated recovery cage before being returned to the home cage and fed water and dry food ad libitum.
In the second experiment, minimally manipulated GFP-OPCS (7.5 × 104 cells) were transplanted into intact young adult (8 weeks) hippocampus at the following coordinates from bregma: AP −3.6, ML −2.3 mm. Using a siliconized glass micropipette, 1 µL of OPCs were delivered at a depth of DV −3.0 mm over 2 min. The micropipette was left in situ for a further 5 min and then withdrawn slowly (Fricker et al., 1999). One week later animals were subjected to the same NMDA (25 mm) lesion as in the previous experimental model (Kimonides et al., 1998).
Immunocytochemistry
Paired samples of dissociated GFP-OPCs were plated onto PDL (1 µg/mL)- and laminin (10 µg/mL)-coated borosilicate coverslips (∼ 5 × 104 cells per coverslip) corresponding to the samples used for transplantation. Twenty-four hours following plating, cells were fixed using 4% paraformaldehyde in 0.1 m PBS (pH 7.4). The following antibodies were used: anti-A2B5 (a monoclonal antibody to an epitope on oligodendrocyte progenitors; 1 : 5 supernatant, mouse IgM; Eisenbarth et al., 1979); anti-O4 (a monoclonal antibody to oligodendrocyte cell surfaces; 1 : 5 supernatant, mouse IgM; Sommer & Schachner, 1981); antigalactocerebroside (1 : 5 supernatant, mouse IgG3; Ranscht et al., 1982); anti-NG2 (1 : 250, rabbit and goat, kind gift from W. Stallcup); antimicrotubule-associated protein 2 (1 : 500; Sigma, clone-AP-20); Tuj1 (1 : 500; Sigma, β-III tubulin); and antiglial fibrillary acidic protein (GFAP; 1 : 4000, rabbit; DAKO, Denmark). Cell nuclei were visualized with Bis-benzimide stain (Hoechst 33323; Sigma). Coverslips were mounted on glass slides using Flurosave (Calbiochem) and viewed under a Leitz DMRD fluorescent microscope (Leica Microsystems, Germany) with appropriate filters for cell identification and counting. For each coverslip, five consecutive random fields were counted using a grid with all cells counted. At least four coverslips for each immunostain were analysed. The number of GFP+ colabelled cells is expressed as mean ± SEM for each experiment (n = 4).
Immunohistochemistry
Neonatal and adult animal groups were killed at 2- and 8-week time points, while NMDA-lesioned animals were killed 1 and 4 weeks after lesioning using an overdose of sodium pentobarbital (80 mg/kg, i.p.) and perfused transcardially with 0.1 m PBS followed by 4% paraformaldehyde in 0.1 m PBS (pH 7.4). The brains were then removed and postfixed for 4 h before being immersed in 30% sucrose solution. Immunohistochemistry was carried out using standard procedures as previously described (Burnstein et al., 2004). Fixed brains were sectioned coronally at 40 µm using a freezing microtome (Leica). A one-in-six series of sections was processed for each stain unless otherwise stated. Tissue was washed in Tris-buffered saline (TBS) and subsequently blocked in TBS with 0.2% Triton X-100 and 5% normal donkey serum for 2 h prior to overnight incubation at 4 °C with primary antibody. Tissue sections were subsequently washed, and appropriate secondary antibodies (1 : 500; Molecular Probes) were applied. Finally, the sections were washed ×3 for 5 min in TBS before being mounted on gelatinized slides and coverslipped using Flurosave (Calbiochem). The following antibodies were used: anti-GFP (1 : 500, rabbit and goat; Molecular Probes); neuronal nuclei (NeuN; 1 : 400, mouse; Chemicon); doublecortin (DCX; 1 : 500, goat; Santa Cruz Biotechnology, USA); GFAP (1 : 500; mouse CY3-conjugated; Sigma); NG2 (1 : 200; kind gift from W. Stallcup); Olig2 (1 : 500, rabbit; Chemicon); MBP (1 : 50, rat; AbD Serotec, UK); and PDGFα receptor (1 : 1000, rat; R & D Systems).
For confocal analysis, stacks of images were captured on a Leica TCS-NT-UV confocal laser scanning microscope using a 63 × 1.25 NA Leica lens. Typical stacks were composed of 10–20 optical sections of 1 µm thickness taken at 0.8-µm intervals. Sequential acquisitions were performed in the different channels in order to avoid any misinterpretation of the results due to signal cross-talk. For in vivo quantification, one in six sections from each animal were stained for anti-GFP. Transplanted GFP+ cells were identified, counted and at the same time assigned to one of six locations (hippocampus, corpus callosum, cingulum, cortex, ventricle and other) to establish the distribution of the transplanted cells. Estimated numbers were then averaged for the number of animals at each time point.
Results
OPC isolation and characterization
In order to examine the in vitro potential of cortical OPCs, paired aliquots of freshly isolated GFP-OPCs derived from standard neonatal mixed glial preparations (McCarthy & De Vellis, 1980) were first characterized. Following plating for 24 h, the majority of cells expressed the antigens A2B5 (87.5 ± 7.9%) and NG2 (96.0 ± 2.63%), consistent with an OPC phenotype (Fig. 1A and B). GFAP+ cells made up < 1% of the population (0.65 ± 0.51%). The remainder of the cells were identified as differentiating oligodendrocyte lineage cells, expressing O4 (5.70 ± 2.90%, Fig. 1C). No neuronal differentiation was observed at the time of transplantation. All subsequent experiments used the highly enriched OPC population and, critically, cells were not subjected to any in vitro propagation or ‘priming’ treatment steps prior to transplantation.
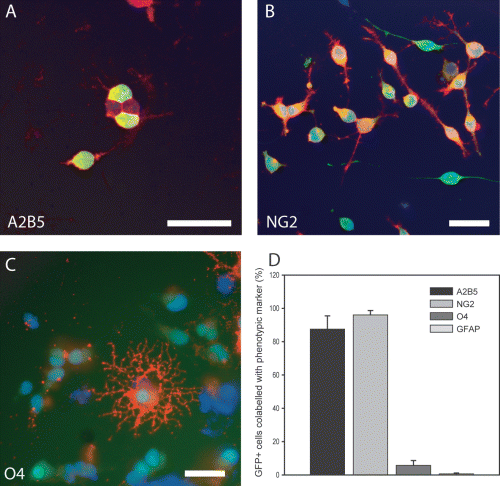
In vitro characterization of cortical-derived GFP cells following fresh isolation from standard mixed glial preparations. Paired samples of isolated GFP cells were analysed 24 h post-plating. Representative micrographs show cortical-derived GFP cells immunostained for (A) A2B5, (B) NG2 and (C) O4. (D) Quantification of phenotypic characterization showing that GFP cells predominantly expressed markers of oligodendrocyte precursor cells. Negligible numbers of astrocyte cells were identified (D) and no neuronal cells were identified in the cultures. Scale bar, 50 µm.
Hippocampal astrocyte-derived signals induced neuronal differentiation
Recent evidence from our own lab and others has observed neuronal differentiation of OPC cultures following extended culture and manipulation (Belachew et al., 2003; Gaughwin et al., 2006). Soluble hippocampal signals have also been shown to promote neurogenesis (Lie et al., 2005). In order to examine whether GFP-OPCs had in vitro neuronal potential we cultured GFP-OPCs in the presence of HACM (Gaughwin et al., 2006). GFP-OPCs exposed to HACM were able to form some immature neurons over 7 days (7.1 ± 1.2%; Fig. 2A and B, Tuj1 staining) compared to the control sample (0.9 ± 1.3%) not exposed to the conditioned media.
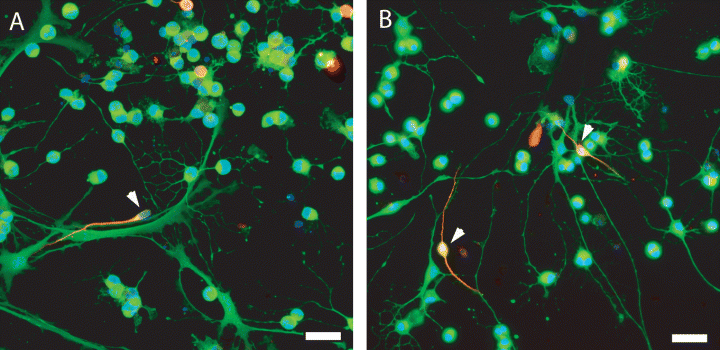
In vitro, GFP-OPCs were exposed to hippocampal astrocyte-derived conditioned media for 7 days. (A and B) GFP-OPCs exposed to the HACM were able to form an increasing number of immature neurons over 7 days (7.1 ± 1.2%) compared to control samples (0.9 ± 1.2%) not exposed to the conditioned media (representation of HACM-treated OPC cultures; arrowheads identify Tuj1–GFP+ OPCs). Immature and mature oligodendrocytes gradually declined over the same time period. Scale bar, 50 µm.
Graft-derived GFP cells survived and integrated following transplantation into the intact hippocampus of neonatal and adult rats
The hippocampus is a neurogenic niche and hippocampal-derived signals have been shown to instruct neuronal fate (Song et al., 2002; Gaughwin et al., 2006). To examine whether endogenous hippocampal-derived signals were sufficient to instruct a neuronal fate we transplanted GFP-OPCs into the hippocampus (below the level of the CA1 region) of neonate and adult rats. Animals were killed at 2 and 8 weeks following transplantation (Fig. 3A–C). For in vivo counts, one in six sections stained for anti-GFP and positive cells were identified on a fluorescence microscope (Leica Microsystems, Germany). GFP+ cells from animals from each time point were then counted and their location recorded. A substantial number of engrafted GFP+ cells were observed at both time points post-transplantation in both the neonate and adults animals (Fig. 3B and C, respectively). GFP+ cells were primarily identified within the hippocampus, including CA1–3 and dentate gyrus (60–90%) at both 2 and 8 weeks (Fig. 3D–G). GFP+ cells were also identified in other regions of the brain including the corpus callosum, cingulum and cortex (Fig. 3D–G). A small percentage of engrafted GFP cells appeared to have migrated rostrally and caudally, particularly into the ependymal wall of ventricles in the neonatally transplanted animals (2.69 ± 1.40%; Fig. 3D and E).
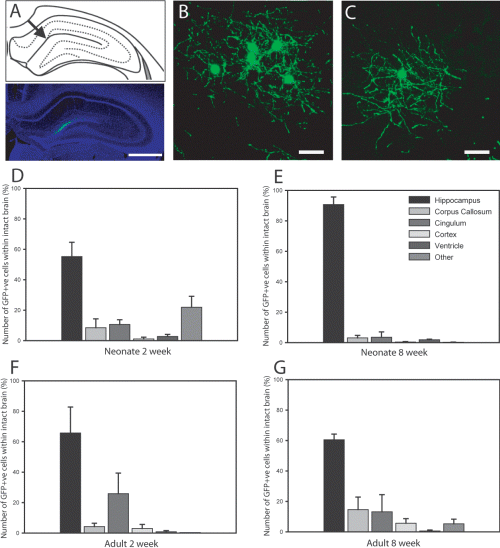
Graft-derived GFP+ cells survived and integrated following transplantation below the CA1 region of the hippocampus of neonatal and adult. (A) Schematic and low-power representation of the location of the majority of the transplanted GFP cells. (B and C) High-power images from the hilus layer of the hippocampus show that transplanted GFP+ cells survived in both the (B) neonatal and (C) adult intact brain for at least 14 weeks (representative examples taken 8 weeks after grafting). Cells were also identified in CA1 directly above the dentate gyrus and CA3 regions. GFP cells within intact brains were counted under a fluorescence microscope. (D–G) Graphs represent the location of transplanted GFP cells within the neonate and adult at both 2 and 8 weeks post-transplantation. (D and E) In the neonate host, graft-derived cells were predominantly identified within the hippocampus with some cells (< 2%) observed to have migrated to the ventricles. (F and G) Similarly grafted cells in the adult animals were mainly located within the hippocampus, with low numbers identified in other brain regions. Scale bars, 1 mm (A), 50 µm (B and C).
Postnatal unmanipulated OPCs maintained an immature oligodendrocyte phenotype and did not acquire a neuronal fate following transplantation into the intact adult and neonate hippocampus
Following engraftment and integration into the neonate and adult hippocampus, the phenotypic potential of the donor population was examined at 2 and 8 weeks. The overwhelming majority of GFP+ cells expressed the surface proteoglycan NG2 (Fig. 4A–C). Co-expression of NG2 and GFP was confirmed by confocal microscopy (Fig. 4C). Transplanted GFP+ cells were identified in close proximity to endogenous myelin but were not found to colocalize under confocal microscopy (Fig. 4D–F). GFP+ cells at both time points were negative for the astrocyte marker GFAP (Fig. 4G–I) and the neuronal markers DCX and NeuN (NeuN, Fig. 4J–L). These findings indicate that GFP cells survived and integrated but failed to acquire neuronal antigens in response to the in vivo hippocampal environment.
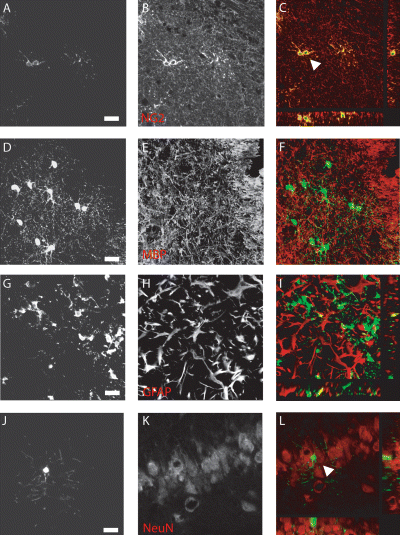
Transplanted GFP cells maintained an immature oligodendrocyte phenotype and did not acquire a neuronal fate in the intact neonate or adult hippocampus. Following engraftment, immunohistochemistry was performed to identify the phenotype of the GFP-positive cells. (Immunographs A–C) The majority of GFP-cells expressed the surface proteoglycan NG2 as shown by confocal microscopy (image taken at CA1). (D–F) GFP cells were able to integrate and were often found amongst myelin-forming cells but were not found to colocalize (MBP; image taken from beneath the corpus callosum). (G–I) Similarly engrafted GFP+ cells were found amongst GFAP+ astrocytes but were not found to express GFAP themselves (image from the dentate gyrus region). (J–L) GFP+ cells in both the neonate and adult were negative for the immature neuronal marker DCX and more mature NeuN (NeuN; cells identified in the dentate gyrus). Representative images taken at 8 weeks after grafting into neonatal animals. Scale bars, 50 µm.
OPCs did not respond to focal neurotoxic lesion by neuronal differentiation
NMDA lesions were performed to examine whether the integrated GFP-OPCs could be influenced by the lesioned hippocampus. Focal NMDA lesions were induced at 1 and 10 weeks after transplantation of GFP-OPCs in adult and neonatal host animals, respectively. Lesioned animals had a characteristic void of neuronal nuclei in the hippocampus (Fig. 5A). In both the neonatal and adult host, low numbers of GFP+ cells were identified within the hippocampus, substantially less than in the intact brains (21.3 ± 0.6 and 24.7 ± 2.7%, respectively; Fig. 5B). Surviving transplanted GFP+ cells had the morphological characteristics of oligodendrocyte lineage cells (Fig. 5C and D). Phenotypic analysis confirmed that the graft-derived cells remained as OPCs, expressing the surface proteoglycan NG2 and PDGFRα (Fig. 6A–C and D–F, respectively). There was a lack of colocalization with DCX, NeuN and GFAP although the GFP+ cells were in close proximity to the endogenous cells expressing these markers (Fig. 6G–I, MBP; Fig. 6J–L, NeuN).
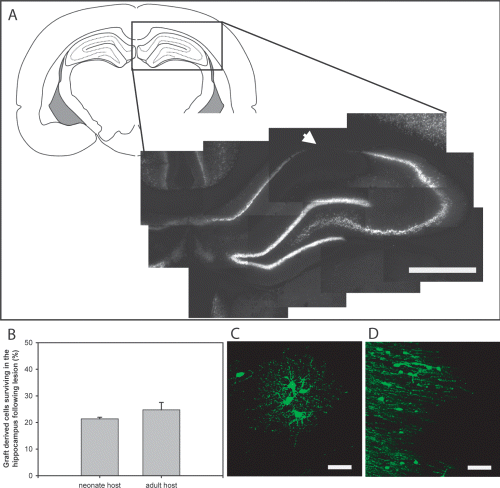
Response of transplanted GFP-OPCs to a focal neurotoxic lesion. One and 10 weeks following engraftment of GFP-OPCs into the neonate and young adult hippocampus, animals were subjected to an excitotoxic lesion using NMDA. (A) This lesion resulted in a typical, distinct neuronal loss in the CA1 region. (B) Low numbers of GFP+ cells were identified within the hippocampus following NMDA lesion. (C and D) Surviving GFP+ cells morphologically appeared oligodendrocyte-like; representative examples from (C) neonate host and (D) adult host. Scale bars, 1 mm (A), 50 µm (C and D).
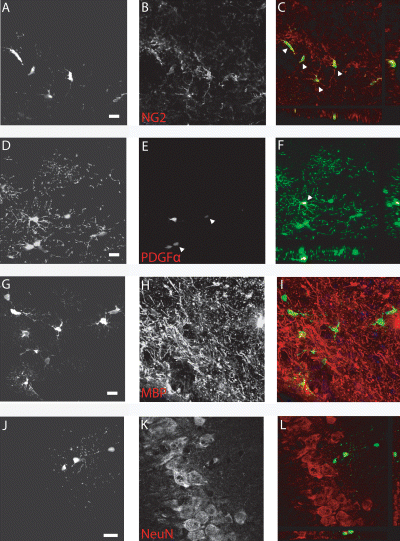
Phenotypic analysis confirmed that surviving GFP+ transplanted cells retained an immature oligodendrocyte phenotype, using a range of immunohistochemical markers. (A–F) Surface proteoglycan NG2 and PDGFRα expression was observed on the GFP+ cells (arrowheads in A–C and D–F, respectively; example taken from CA2 region just beyond the lesion). (G–L) A lack of colocalization with DCX, NeuN, GFAP and MBP was observed in both the neonate and young adult transplanted animals [examples of (G–I) NeuN and (J–L) MBP]. Scale bar, 50 µm.
Discussion
The possibility that an abundant, widely dispersed and cycling cell population within the CNS has stem cell-like potential has generated considerable interest. We report that unselected and minimally manipulated postnatal OPCs did not generate neurons following transplantation into the neurogenic environment of the intact and injured hippocampus despite evidence of robust survival up to 14 weeks after transplantation.
Two primary lines of evidence, in vitro and in vivo, support the idea of OPCs having neuronal potential. In vitro studies of propagated OPCs have demonstrated neuronal potential following complex treatments with growth factors and/or defined BMP signalling (Kondo & Raff, 2000; Kondo & Raff, 2004). In addition we have previously reported that undefined soluble hippocampal astrocyte-derived signals induce neuronal differentiation of an A2B5-selected and -expanded population (Gaughwin et al., 2006). The molecular identity of hippocampal-derived soluble signals is also of great interest with, for example, the Wnt pathway being shown to play an important role; in particular, Wnt3 over-expression is capable of increasing neurogenesis from progenitors both in vitro and in vivo (Lie et al., 2005). In vivo evidence of neuronal potential of OPCs is largely derived from studies of NG2+/2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP)-GFP+ cells (Belachew et al., 2003; Aguirre et al., 2004; Chittajallu et al., 2004). Importantly, these studies show electrophysiological behaviour compatible with a neuronal identity. However, the specificity of NG2 to the OPC is challenged by the unequivocal demonstration of NG2 on non-OPC populations (Eisenbarth et al., 1979; Aguirre et al., 2004; Nishiyama, 2007). Furthermore, in these studies the NG2+ population also express EGF receptors, which are not typically expressed by OPCs (Leutz & Schachner, 1982).
In this study we sought to examine the behaviour of OPCs in the hippocampal neurogenic niche. We limited in vitro culturing by using OPCs freshly isolated from mixed glial preparations, without any further culture or exposure to defined mitogens such as PDGF or undefined signals. Significantly, the transplanted population was highly enriched in oligodendrocyte lineage cells, predominantly OPCs (> 90%), without any evidence of in vitro constitutive neuronal potential.
In the intact brain a large number of cortically derived GFP-OPCs survived and displayed limited migratory capability. Furthermore, the cells remained as OPCs and did not exhibit any neuronal or astroglial differentiation even at 14 weeks following grafting. We have previously shown very limited immature neuronal differentiation by B104 conditioned media-expanded and A2B5-selected OPC populations following transplantation into the uninjured adult hippocampus (Gaughwin et al., 2006). Interestingly, clonal analysis has demonstrated that not all OPCs are capable of in vitro neuronal differentiation, suggesting heterogeneity (Gaughwin et al., 2006). It appears likely that extended culture and/or selection may impose a distinct ‘competence’ on OPCs to respond to extrinsic defined signals (e.g. BMP) or undefined (hippocampal) cues (Kondo & Raff, 2000; Belachew et al., 2003; Gaughwin et al., 2006). The absence of prior ‘conditioning’ may explain why unselected, nonexpanded transplanted populations used in this study were unable to commit to a neuronal population upon exposure to physiological and injury neurogenic cues in vivo. In contrast, in the current study we have observed the in vitro neuronal potential of GFP-OPCs following exposure to soluble factors derived from HACM. This observation is consistent with earlier studies (Kondo & Raff, 2000; Belachew et al., 2003; Gaughwin et al., 2006). It is of interest that minimally manipulated OPCs show a differential response to undefined in vitro hippocampal cues compared to in vivo hippocampal signals. These differences are likely to represent distinct inductive and inhibitory signals in these two paradigms. A recent study has identified Wnt3, a known positive regulator of neurogenesis, in HACM (Lie et al., 2005). The adult neurogenic niche is a complex environment containing numerous positive and negative regulators of neurogenesis such as Notch, Eph/ephrins and vascular endothelial growth factor (Alvarez-Buylla & Lim, 2004; Brederlau et al., 2004).
The issue of OPC heterogeneity is well recognized although the extent to which this results in functional differences is unclear. For example, functional heterogeneity has been disclosed with grey matter NG2+ cells displaying different ion channel profiles from those of NG2+ cells in the white matter (Chittajallu et al., 2004). More recently a study by Bouslama-Oueghlani et al. (2005) identified two distinct populations of NG2+ cells within the mouse cerebellum (Bouslama-Oueghlani et al., 2005). Our studies were restricted to cortically-derived OPCs. Studies of OPCs derived from different regions including cortex (Gaughwin et al., 2006), optic nerve (Kondo & Raff, 2000) and hippocampus and SVZ (Belachew et al., 2003; Aguirre et al., 2004) have all shown some in vivo neuronal differentiation. The extent to which regional identity of the OPC may confer distinct patterns of neuronal potential remains unclear. In vivo studies focusing on intrinsic neuronal potential provide some evidence of NG2 populations derived from the SVZ and hippocampus giving rise to neuronal populations (Belachew et al., 2003; Aguirre et al., 2004; Chittajallu et al., 2004). Whether this NG2 population represents an OPC or perhaps a more immature multipotent cell is unclear. However, a recent study examining NG2-null mutants and hippocampal neurogenesis observed that the deletion of NG2 does not influence neurogenesis in the hippocampus of adult, nor does it affect behavioural tasks (Thallmair et al., 2006). This study suggests that the NG2 progenitor population does not influence hippocampal neurogenesis.
In the present study we also examined the influence of the injured environment on the phenotypic potential of grafted OPCs. Neurogenesis has been observed following a number of injury models including global ischemia, seizures, excitotoxic lesions and photolytic lesions (Cameron et al., 1995; Bengzon et al., 1997; Magavi et al., 2000; Jin et al., 2001; Collin et al., 2005). Furthermore, injury has been shown to induce neural differentiation in regions of the adult CNS that do not normally undergo neurogenesis (Magavi et al., 2000). We used a focal NMDA lesion to create a discrete area of neuronal loss within the hippocampus at both at 1 and 10 weeks after grafting into neonatal and young adult hosts. However, 1 and 4 weeks post-lesion (up to 14 weeks post-transplantation), no evidence of GFP-derived neurons was observed. In addition, there was a substantial loss of GFP-OPCs in the vicinity of the injury. The precise cause of the loss of OPCs is unclear; however, NMDA receptors have recently been identified on OPCs (Karadottir et al., 2005; Salter & Fern, 2005). Future studies should thus explore the influence of alternative forms of injury including inflammation, cortical stab lesion and photolytic lesions on grafted GFP-OPCs (Magavi et al., 2000; Hampton et al., 2004). A recent study examining the regional potential of discrete areas within the hippocampus identified the anterior pole of the dentate gyrus as quiescent during adulthood (Melvin et al., 2007). Furthermore, neurogenesis in this same region was subsequently observed when the animals were placed in an enriched environment, adding to previous studies that have shown increased endogenous neurogenesis following enrichment (van Praag et al., 1999, 2005; Olson et al., 2006). These findings suggest that, in addition to transplantation into the hippocampus, studies should begin to evaluate enrichment and stimulation of the hippocampus (separate from injury) to examine the neurogenic potential of engrafted OPCs.
In the present study, minimally manipulated neonatal cortex-derived GFP-OPCs that were not propagated or exposed to defined signals were capable of survival and integration into the hippocampus of neonatal and adult rats for at least 14 weeks. No evidence of neural differentiation was observed in the intact or injured hippocampus. This study provides a platform for examining the potential of integrated GFP-OPCs and whether they can be influence by further stimulation or cues.
Acknowledgements
We thank Professor W. Stallcup for his generous gift of NG2 antibodies. We thank Dr Okabe for the GFP rats. D.J.W. is supported by a BBSRC research grant.
Abbreviations
-
- BMP
-
- bone morphogenetic protein
-
- DCX
-
- doublecortin
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- EGF
-
- epidermal growth factor
-
- FGF
-
- fibroblast growth factor
-
- GFAP
-
- glial fibrillary acidic protein
-
- GFP
-
- green fluorescent protein
-
- HACM
-
- hippocampal astrocyte-derived conditioned media
-
- NeuN
-
- neuronal nuclei
-
- NG2
-
- chondroitin sulphate proteoglycan neuron/glial antigen 2
-
- OPC
-
- oligodendrocyte precursor cell
-
- P
-
- postnatal day
-
- PDGF
-
- platelet-derived growth factor