Spatio-temporal dynamics of oscillatory network activity in the neonatal mouse cerebral cortex
Abstract
We used a 60-channel microelectrode array to study in thick (600–1000 µm) somatosensory cortical slices from postnatal day (P)0–P3 mice the spatio-temporal properties of early network oscillations. We recorded local non-propagating as well as large-scale propagating spontaneous oscillatory activity. Both types of activity patterns could never be observed in neocortical slices of conventional thickness (400 µm). Local non-propagating spontaneous oscillations with an average peak frequency of 15.6 Hz, duration of 1.7 s and maximal amplitude of 66.8 µV were highly synchronized in a network of ∼200 µm in diameter. Spontaneous oscillations of lower frequency (10.4 Hz), longer duration (23.8 s) and larger amplitude (142.9 µV) propagated with 0.11 mm/s in the horizontal direction over at least 1 mm. These propagating oscillations were also synchronized in a columnar manner, but these waves synchronized the activity in a larger neuronal network of 300–400 µm in diameter. Both types of spontaneous network activity could be blocked by the gap junction antagonist carbenoxolone. Electrical stimulation of the subplate (SP) or bath application of the cholinergic agonist carbachol also elicited propagating network oscillations, emphasizing the role of the SP and the cholinergic system in the generation of early cortical network oscillations. Our data demonstrate that a sufficiently large network in thick neocortical slice preparations is capable of generating spontaneous and evoked network oscillations, which are highly synchronized via gap junctions in 200–400-µm-wide columns. These via synchronized oscillations coupled networks may represent a self-organized functional template for the activity-dependent formation of neocortical modules during the earliest stages of development.
Introduction
Synchronized network oscillations have been observed during early stages of development in a variety of neuronal structures, such as the spinal cord (for review, see Spitzer, 2002), retina (for review, see Torborg & Feller, 2005), hippocampus (for review, see Le Van et al., 2005) and cerebral cortex (for review, see Khazipov & Luhmann, 2006; for a comprehensive review, see Moody & Bosma, 2005). These transient oscillations cause a prominent increase in the intracellular calcium concentration, which subsequently regulates gene expression (for review, see Fields et al., 2005). During embryonic and perinatal development, specific activity patterns regulate specific genes that control neuronal differentiation and neurotransmitter specification (for review, see Spitzer et al., 2004), modulate proliferation (Weissman et al., 2004), growth and remodelling of dendrites (for review, see Lohmann & Wong, 2005; Redmond & Ghosh, 2005), neuronal migration (for review, see Komuro & Kumada, 2005), refinement of axonal projections (for review, see Firth et al., 2005) and apoptosis (for review, see Hara & Snyder, 2007).
A number of in vitro and recent in vivo studies have described the patterns of synchronized network oscillations in the cerebral cortex of newborn rodents. Spontaneous (Garaschuk et al., 2000; Corlew et al., 2004) as well as neurotransmitter-evoked (Flint et al., 1999; Peinado, 2000; Calderon et al., 2005) calcium waves propagate over large areas across the immature cerebral cortex. Calcium imaging studies also revealed the columnar arrangement of coactive, gap junction-coupled neurons in so-called neuronal domains of 100–200 µm in diameter (Yuste et al., 1995; Kandler & Katz, 1998). This columnar arrangement could be recently confirmed with multiple extracellullar recordings in the intact cerebral cortex of the newborn rat (Kilb & Luhmann, 2003) and mouse (Dupont et al., 2006). In this intact in vitro preparation, activation of muscarinic acetylcholine or metabotropic glutamate receptors (Wagner & Luhmann, 2006) elicits a transient network oscillation, which reflects the highly synchronized activity of gap junction-coupled neurons organized in a column of ∼200 µm in diameter. Recent in vivo recordings in the somatosensory cortex (Khazipov et al., 2004; Minlebaev et al., 2007) and visual cortex (Hanganu et al., 2006) of newborn rats have demonstrated spontaneous and periphery-driven, spatially confined spindle burst oscillations. However, the spatio-temporal properties of large-scale network oscillations are difficult to study with a limited number of recording electrodes in in vivo and intact in vitro preparations, because the exact positions of all recording electrodes is often not exactly known. Here we use a 60-channel microelectrode array (MEA) with exact interelectrode distances of 100 or 200 µm to investigate in newborn mouse [postnatal day (P)0–P3] somatosensory cortical slices of 1 mm thickness the spatio-temporal properties of spontaneous, carbachol-induced and electrical stimulus-induced synchronized network oscillations.
Materials and methods
Tissue preparation and MEA recordings
All experiments were conducted in accordance with the national and European (86/609/EEC) laws for the use of animals in research, and were approved by the University of Mainz Ethical Committee. Acute 400–1000-µm-thick coronal or sagittal slices including the primary somatosensory cortex were prepared from neonatal C57BL6 mice (P0–P3; day of birth = P0) as described previously (Luhmann et al., 2000). In brief, newborn mice were anaesthetized by hypothermia and decapitated. The brain was rapidly removed and transferred to oxygenated (95% O2/5% CO2), ice-cold (2–5 °C) artificial cerebrospinal fluid (ACSF) of the following composition (in mm): NaCl, 124; KCl, 5; CaCl2, 1.6; MgSO4, 1; NaHCO3, 26; NaH2PO4, 1.25; glucose, 10 (pH 7.4). Slices were cut in oxygenated, ice-cold ACSF with a vibroslicer (TPI, St Louis, MO, USA) and transferred to an incubation chamber containing ACSF at 32–33 °C. After an incubation period of 1–2 h, slices were transferred to a 3D 60-channel MEA (interelectrode spacing 100 or 200 µm, electrode resistance 250–450 kOhm and 600–900 kOhm, respectively; Ayanda Biosystems, Lausanne, Switzerland) on a MEA 1060-INV-BC interface [Multi Channel Systems (MCS), Reutlingen, Germany], which was mounted on an inverted microscope (Optika microscopes, Bergamo, Italy) equipped with a digital camera (Nikon Coolpix 4500, Tokyo, Japan). Slices were perfused with ACSF at a rate of 2–3 mL/min held constant at 32 °C by a temperature control unit (TCO2 and TH01, MCS).
Field potentials were recorded simultaneously at all 60 electrode sites with a sampling rate of 1 kHz and 1 Hz high-pass filter using the MEA_RACK software (MCS). For bipolar electrical stimulation of the slice, two neighbouring planar microelectrodes were selected as stimulation sites, and current pulses were applied via an isolator unit (STG 2004, MCS; 10–80 µA, 10 pulses of 200 µs duration at 50 Hz). Oscillations were elicited by electrical stimulation at intervals of at least 15 min.
Data analysis
Data were imported to a custom-written program in Matlab version 6.5 (Mathworks, Natick, MA, USA), with datastrm.m and nextdata.m (MC_Rack, MCS). Field potentials recorded in all 60 channels were plotted, and oscillatory network activity was analysed in its duration (interval between onset and end of one complete oscillatory cycle) and amplitude (voltage difference between the maximal positive and negative peak). Time–frequency plots were calculated by transforming field potentials using Morlet continuous wavelet (cwt.m, Matlab), smoothed (smooth.m, Matlab) and normalized to values between 0 and 1 (Torrence & Compo, 1998; Wagner & Luhmann, 2006). Fast Fourier transformation spectra were calculated and oscillations were analysed in their maximal power. Cross-correlograms and coherence analyses for oscillatory activity between channels were calculated by the use of Matlab, and strengths were quantified by measuring the maximal cross-correlation coefficient and maximal coherence coefficient, respectively.
Drugs
The cholinergic agonist carbamylcholine chloride (carbachol, 30–100 µm, Sigma-Aldrich) was prepared in distilled water as stock solution (100 mm), stored at 4 °C and applied for 30–60 s via the bathing solution. The gap junction blocker carbenoxolone (100 µm, Sigma-Aldrich) was prepared freshly in ACSF.
Histology
After the electrophysiological experiments, slices were carefully removed from the MEA and fixed for at least 24 h in 4% paraformaldehyde. Slices were sectioned with a vibratome at 70 µm thickness and Nissl stained. Digital photographs of the cortical slices on the MEA were used to align photographs of the Nissl-stained sections to the 60 recording sites (Fig. 1A). This procedure allowed the layer-specific identification of each electrode.
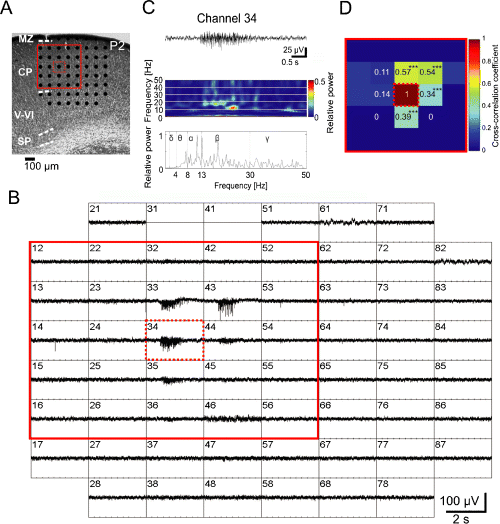
Local, non-propagating spontaneous network oscillations in the newborn mouse somatosensory cortex. (A) Photograph of Nissl-stained coronal slice of the somatosensory cortex from a P2 mouse. Marginal zone (MZ), cortical plate (CP), layers V–VI, subplate (SP) and position of the 60-channel MEA are illustrated. (B) MEA recordings from the 1000-µm-thick slice shown in (A). Spontaneous oscillatory activity can be observed at five recording positions in the CP. Channel numbers 31 and 41 were not properly functioning, and were turned off. The spacing between electrodes is 100 µm (see scale bar in A). (C) Extracellular recording at electrode number 34 (dotted red square in A and B) shown at higher resolution. The panels below show corresponding wavelet analysis and Fourier spectrum. (D) Cross-correlation analysis of the 25 channels marked in (A) and (B) by a red square. Colour code and numbers give the cross-correlation coefficient of each channel in comparison to channel number 34 in the middle position. Asterisks in (D) indicate significantly higher cross-correlation values (***P ≤ 0.001) from comparisons to channel 34 (Z-test). Note the high cross-correlation between recordings separated from central channel 34 by maximal 100 µm.
Statistics
Statistical analyses were performed with SigmaPlot 2001 (SPSS, Chicago, IL, USA). Values throughout this report are given as mean ± SEM. For statistical comparisons, a paired samples t-test was performed. The significance of the cross-correlation and coherence values in 1, 2 were determined by the use of the Z-test and marked by asterisks (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001).
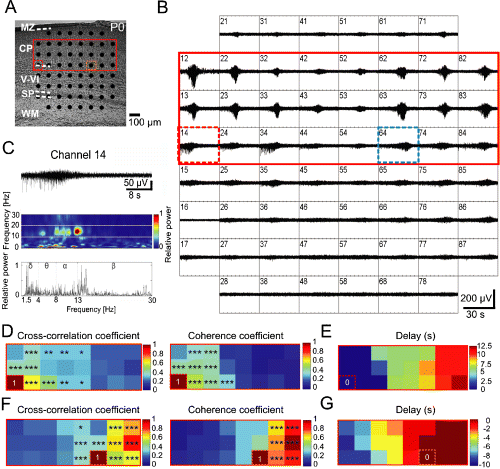
Propagating spontaneous network activity in P0 mouse parietal cortex. (A) Photograph of Nissl-stained coronal slice with cortical layers and position of MEA. (B) MEA recordings from the 1000-µm-thick slice shown in (A). Spontaneous oscillatory activity propagated from medial recording sites (channel numbers 12–15) to lateral electrodes (numbers 82–86). The spacing between electrodes is 100 µm (see scale bar in A). (C) Recording at electrode number 14 (dotted red rectangle in A and B) shown at higher resolution with corresponding wavelet analysis and Fourier spectrum. (D) Colour-coded plots of the cross-correlation and coherence coefficients for the 24 channels marked in (A) and (B) by a red rectangle. All data were obtained in correlation to channel 14 (lower left corner). (E) Delay of oscillatory activity onset compared with channel 14 in colour-coded plot for the same 24 channels as in (D). (F) Colour-coded plots of the cross-correlation and coherence coefficients for the same 24 channels as in (D) and (E), but in correlation to channel 64 (dotted blue rectangle in B). (G) Delay of oscillatory activity onset compared with channel 64. Asterisks in (D) and (F) indicate significantly higher cross-correlation (left) and coherence (right) values (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001) from comparisons to channels 14 (D) and 64 (F), respectively (Z-test). CP, cortical plate; MZ, marginal zone; SP, subplate; WM, white matter.
Results
Spatio-temporal properties of spontaneous oscillatory network activity
The spatio-temporal properties of spontaneous network oscillations in newborn (P0–P3) mouse parietal cortex were studied with 60-channel MEA chips in acutely prepared slices. We never observed large-scale network oscillations in slice preparations of conventional thickness (400 µm, n = 13 slices). Synchronized oscillatory network activity could be only observed in slice preparations of at least 600 µm thickness or in intact cortices (Dupont et al., 2006). In 28 out of 123 parietal cortical slices (22.8%) with a thickness between 600 and 1000 µm we could record in 5 mm extracellular potassium prominent spontaneous field potential oscillations (Fig. 1). The incidence of network oscillations correlated with the thickness of the slices. Whereas only 11 out of 71 slices (15.5%) with a thickness of 600–800 µm revealed spontaneous network oscillations, 16 out of 51 slices (31.4%) with a thickness of 1000 µm showed prominent synchronized activity. These data indicate that a neuronal network of sufficient 3D size is required to generate this type of early neocortical activity. The incidence of large-scale network activity also depended on the extracellular potassium concentration. Whereas only one out of 17 (5.9%) 1000-µm-thick slices analysed in 3 mm revealed robust synchronized network oscillations, six out of 13 slices (46%) with the same thickness recorded in 8 mm potassium showed network oscillations.
All subsequent data were obtained from parietal cortical slices of 1000 µm thickness and were recorded in 5 mm extracellular potassium. Under these experimental conditions, spontaneous network oscillations could be recorded at an average interval of 15.4 ± 3.2 min (n = 31 oscillations in 11 slices) over observation periods of 2–3 h. Two different patterns of spontaneous activity, locally non-propagating (Fig. 1) and propagating (Fig. 2), could be observed. Local non-propagating spontaneous oscillations could be observed in nine out of 16 slices (56%). These spontaneous oscillations were spatially restricted to a neuronal network of approximately 200 µm in diameter (Fig. 1A and B). Wavelet and Fast Fourier analyses demonstrated a predominance of alpha and beta frequencies in the oscillatory field potential responses (Fig. 1C). The local non-propagating network oscillations revealed an average peak frequency of 15.6 ± 2.7 Hz, duration of 1.7 ± 0.3 s and maximal amplitude of 66.8 ± 13.1 µV (n = 9). The clusters of synchronized activity with high cross-correlation (> 0.5, Fig. 1D) and coherence coefficients (> 0.6) were generally localized in the cortical plate (CP), but oscillatory field potentials recorded in lower cortical layers and in the subplate (SP) were synchronized in a columnar arrangement (Dupont et al., 2006).
The second type of spontaneous network oscillations could be observed in 14 out of 16 slices (87.5%). In these slices the activity propagated over at least 1 mm in the medio-lateral (n = 11) or latero-medial (n = 3) direction. The propagating speed was significantly (P < 0.05) higher in the vertical direction (2.4 ± 1.33 mm/s) than in the horizontal direction (0.11 ± 0.01 mm/s). When compared with the local non-propagating spontaneous activity, the propagating oscillations had a significantly lower peak frequency (10.4 ± 0.8 Hz, P < 0.05), longer duration (23.8 ± 4.9 s, P < 0.01) and larger amplitude (142.9 ± 18.4 µV, P < 0.01; n = 14, Fig. 2C). Propagating network oscillations were also synchronized in a columnar manner, but as revealed by cross-correlation and coherence analyses these spontaneous oscillations synchronized the activity in a larger neuronal network of 300–400 µm in diameter due to the propagation of the activity in horizontal direction (Fig. 2D and F). Seven out of the 16 slices tested in detail (44%) showed local non-propagating as well as propagating spontaneous oscillations.
Spontaneous network oscillations depend on gap junctional coupling
In order to study the role of gap junctions in the generation and propagation of spontaneous synchronized activity, the broad spectrum gap junction blocker carbenoxolone was bath applied at a concentration of 100 µm to neocortical slices showing spontaneous network oscillations. In four out of four slices tested (all from P0–P1 mice), carbenoxolone completely blocked the occurrence of spontaneous local as well as propagating synchronized network activity for observation periods of up to 1.5 h (Fig. 3). Spontaneous activity partially recovered following carbenoxolone washout for 1–2 h (Fig. 3C), demonstrating that gap junctions play an important role in the generation of synchronized oscillations in the newborn rodent cerebral cortex. Interestingly, spontaneous non-synchronized action potentials could be recorded in the presence of carbenoxolone (Fig. 3B), demonstrating that spontaneous action potential firing was preserved but that carbenoxolone blocked the local synchronization of neuronal activity.
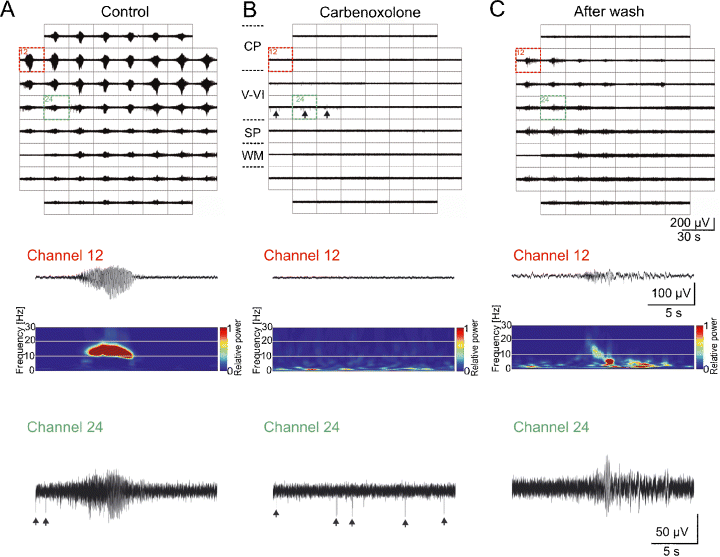
Spontaneous network oscillations require gap junctional coupling. MEA recordings of spontaneous propagating network oscillations from a P1 mouse neocortical slice under control conditions (A), after addition of 100 µm carbenoxolone (B), and after 95 min washout of carbenoxolone (C). The middle panels show representative recordings obtained from channel 12 and corresponding wavelet analysis. During the 88-min application of carbenoxolone, no spontaneous network oscillations could be recorded. However, non-synchronized spontaneous action potentials could be observed in carbenoxolone (arrows in MEA recordings in upper panel and in channel 24 recording in lower panel). CP, cortical plate; SP, subplate; WM, white matter.
Electrical stimulation of the SP elicits oscillatory network activity
We have previously reported that the SP plays an important role in the generation of cholinergic network oscillations in the intact neocortex in vitro preparation of the newborn mouse (Dupont et al., 2006). Because the MEA chip allows a more detailed analysis of this SP-driven activity, we used a bipolar electrical stimulation protocol to activate the SP and to record the cortical network activity with a 200-µm MEA chip (Fig. 4A). In all investigated slices (n = 6), electrical stimulation of the SP elicited propagating oscillatory responses with an average peak frequency of 18 ± 1.5 Hz, duration of 0.6 ± 0.1 s and amplitude of 190.4 ± 41 µV (Fig. 4B and C). These electrically evoked oscillations differ significantly in their duration and amplitude (both P ≤ 0.05) from the spontaneous non-propagating activity and also in their peak frequency (P ≤ 0.001) and duration (P ≤ 0.05) from the spontaneous propagating oscillations.
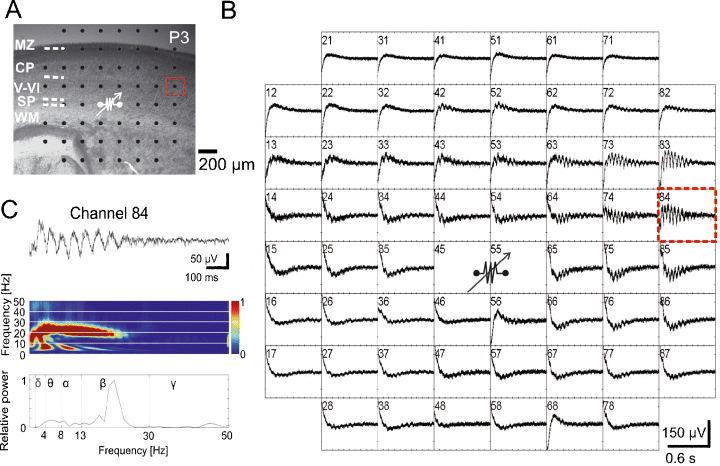
Local electrical stimulation of the subplate (SP) elicits propagating network oscillations. (A) Photograph of P3 mouse Nissl-stained coronal slice with cortical layers and position of MEA. (B) MEA recordings from the neocortical slice shown in (A), with the site of bipolar stimulation in the SP (80 µA, 200 µs duration, 50 Hz, 10 times). The spacing between the electrodes is 200 µm (see scale bar in A). (C) Recording at electrode number 84 (dotted red rectangle in A) shown at higher resolution with corresponding wavelet analysis and Fourier spectrum. CP, cortical plate; MZ, marginal zone; WM, white matter.
Cholinergic network oscillations
The spatio-temporal properties of cholinergic network oscillations in the newborn mouse parietal cortex were studied with MEA chips by bath application of the cholinergic agonist carbachol (30–100 µm). Whereas carbachol application to the intact cerebral cortex in vitro preparation very reliably elicited oscillatory network oscillations activity in the newborn mouse (Dupont et al., 2006) and rat (Kilb & Luhmann, 2003), carbachol application to thick neocortical slices evoked the characteristic network response in only 36 out of 81 slices (44%; Fig. 5). These oscillations showed an average frequency of 12.9 ± 0.7 Hz, duration of 9.3 ± 1.4 s and maximal amplitude of 161.0 ± 14.0 µV (n = 35). These data further support our hypothesis that an intact large neuronal network is required for the generation of network oscillations in the newborn rodent cerebral cortex.

Carbachol-induced propagating network oscillations in the newborn mouse somatosensory cortex. (A) Photograph of a P3 mouse Nissl-stained coronal slice with cortical layers and position of MEA. (B) MEA recordings from the slice shown in (A) with carbachol (100 µm)-induced oscillatory network activity. The spacing between the electrodes is 200 µm (see scale bar in A). (C) Recording at electrode number 22 (dotted red rectangle in A) shown at higher resolution with corresponding wavelet analysis and Fourier spectrum. CP, cortical plate; MZ, marginal zone; SP, subplate; WM, white matter.
Discussion
Using the 60-channel MEA chip we characterize with a high-temporal resolution the functional properties of oscillatory network activity in the newborn mouse somatosensory cortex in vitro. Our data clearly demonstrate that the probability of spontaneous network oscillations correlates with the thickness of the neocortical slices, strongly supporting the hypothesis that an intact neuronal network of sufficient dimension is required to generate this type of inherent activity. Wu and colleagues already showed that spontaneous hippocampal network rhythms do not consistently occur in conventional ∼400-µm-thick slices with a limited network connectivity, but rather require an intact intrinsic circuitry that can only be maintained in slices with a thickness of ∼1 mm (Wu et al., 2005a,b).
The spontaneous, carbachol- and stimulation-induced network oscillations described in the present study are in many aspects similar to those reported previously in the intact cerebral cortex in vitro preparation (Dupont et al., 2006), and the in vivo somatosensory or visual cortex of the newborn rat (Khazipov et al., 2004; Hanganu et al., 2006; Minlebaev et al., 2007). Spatially confined spindle-shaped field potential oscillations in the immature rat somatosensory and visual cortex show under in vivo conditions a similar frequency (10–20 Hz) and comparable duration as the local non-propagating spontaneous oscillations observed in the present study. However, in vivo spindle-shaped bursts occurred much more frequently in slightly older animals (2.5–8 per min; Khazipov et al., 2004; Hanganu et al., 2006; Minlebaev et al., 2007). This difference can be explained most likely by the fact that a large number of spindle bursts in vivo are triggered by the periphery (Khazipov et al., 2004; Hanganu et al., 2006), and that the rate of this spontaneous oscillatory activity increases significantly during the first postnatal days (Hanganu et al., 2006).
Propagating and non-propagating oscillatory activity
The use of the 60-channel MEA chip that covered a cortical area of 0.7 × 0.7 mm2 (100 µm interelectrode distance) or 1.4 × 1.4 mm2 (200 µm interelectrode distance) allowed us to differentiate between propagating and non-propagating synchronized network oscillations. Local non-propagating spontaneous oscillations with a high coherence could be recorded in a neocortical network of about 200 µm in diameter. Yuste et al. (1992) described so-called neuronal domains with similar spatial dimensions in neocortical slices from newborn rats by the use of fura-2 calcium imaging. Yuste et al. (1992) further demonstrated that the spontaneously coactive neurons within each neuronal domain are coupled by gap junctions. Our results are in good agreement with this previous report, and our electrophysiological recordings further demonstrate that neurons within these domains oscillate synchronously in the alpha/beta frequency range. It is tempting to speculate that these local oscillatory networks in the CP may represent an early functional template, a cortical ‘precolumn’, for the subsequent development of a cortical columnar network (Fig. 1), e.g. a barrel in layer IV of the somatosensory cortex of rodents (Woolsey & Van der Loos, 1970). In the mouse, barrels can be histologically identified with Nissl and cytochrome oxidase staining not before P5 (Lee et al., 2005). However, our MEA recordings indicate that spontaneously active neurons form a functional neuronal network of ∼200 µm in diameter already around birth. The question which mechanisms determine or influence the formation of this early neocortical network is currently not completely solved, but the ‘radial unit hypothesis’ certainly represents a good model (for review, see Rakic, 1988). Noctor et al. (2001) demonstrated that neocortical neurons migrate along clonally related radial glia, and suggested that the lineage relationship between neurons and proliferative radial glia may underlie the radial organization of the neocortex. It may well be that clonally related neurons in the developing cerebral cortex are initially coupled via gap junctions, which would also allow the exchange of biochemical signals (i.e. inositol trisphosphate; Kandler & Katz, 1998) and consolidation of connectivity patterns within an early neuronal ensemble. A number of recent studies demonstrated the presence of columnar, patterned, spontaneous activity in the immature cerebral cortex before the onset of the critical period, indicating that a columnar architecture develops earlier and more rapidly than was previously assumed (for review, see Katz & Crowley, 2002).
Not only are neuroblasts physiologically coupled by gap junctions into columnar clusters (LoTurco & Kriegstein, 1991), but also early generated SP neurons (Dupont et al., 2006). Especially, SP neurons are well suited to be activated during the earliest stages of cortical development by a thalamic input (Friauf et al., 1990; Hanganu et al., 2002; Higashi et al., 2002; Molnár et al., 2003) or by ascending neuromodulatory systems, such as the cholinergic projection (Calarco & Robertson, 1995; Mechawar & Descarries, 2001; I. L. Hanganu, A. Okabe, V. Lessman and H. J. Luhmann, unpublished results). During the early stages of corticogenesis, the SP functions as a reliable relay station between the various corticopetal inputs and the developing CP (for review, see Kostovic & Jovanov-Milosevic, 2006). The SP is required for the generation of cholinergic network oscillations in the neonatal mouse cerebral cortex (Dupont et al., 2006), and ablation of the SP at early stages prevents the formation of cortical columns (Kanold et al., 2003). The present study demonstrates that electrical stimulation of the SP elicits oscillatory network activity that resembles in many aspects the spontaneous oscillations (Fig. 5). This observation indicates that stimulation of the SP activates an intrinsic neocortical circuit, which is capable of generating this type of early synchronized activity.
Besides local non-propagating oscillations, a second type of spontaneous network activity could be observed in the large majority of the thick slice preparations. This activity propagated in the medio-lateral or latero-medial direction over at least 1 mm and differed from the non-propagating oscillations in frequency, duration and amplitude. However, propagating spontaneous oscillations were also synchronized in a columnar manner, although in a larger network (Fig. 2), suggesting that similar mechanisms mediate the functional coupling of local neuronal clusters in a propagating wave of activity. Spontaneous oscillations propagated in the horizontal direction with an average speed of 110 µm/s, very similar to the muscarine-induced travelling calcium waves described by Peinado (2000) in newborn rat neocortical slices (50–300 µm/s). The significantly faster propagation speed in the vertical direction (2.4 ± mm/s) compared with the horizontal direction (0.11 mm/s) may result from a higher columnar coupling efficiency. Interestingly, a faster intracortical spread of activity in the vertical direction could also be observed by the use of optical recording techniques in adult neocortical slices (Albowitz et al., 1990). Calcium imaging studies (Yuste et al., 1992; Kandler & Katz, 1998) as well as dye coupling experiments (LoTurco & Kriegstein, 1991; Dupont et al., 2006) in neocortical slices from neonatal rodents have previously documented a columnar organization of electrically coupled neurons, suggesting a more efficient coupling in the vertical direction.
Why should the neonatal cerebral cortex oscillate?
Synchronized oscillatory activity represents a common functional property of many immature neuronal networks (see Introduction, and Moody & Bosma, 2005). In the cerebral cortex, early network oscillations can be either generated spontaneously (Garaschuk et al., 2000; Opitz et al., 2002; McCabe et al., 2006; Minlebaev et al., 2007; present study), driven by the periphery (Khazipov et al., 2004; Hanganu et al., 2006; Minlebaev et al., 2007) or elicited by ascending neuromodulatory inputs (Hanganu et al., 2007). Rapid oscillatory activity in the alpha/beta frequency range has not only been observed in the cerebral cortex of newborn animals, but also in the neocortex of premature human neonates of 29–31 weeks post-conceptional age (Vanhatalo et al., 2005; Milh et al., 2007; for review, see Khazipov & Luhmann, 2006). Synchronized network oscillations in the alpha/beta frequency range may reflect an intrinsic capacity of the immature cerebral cortex to generate early functional networks. Perturbations of this early activity, like disruption of the spontaneous input from the retina before the onset of vision, results in a structural and functional disorganization of the columnar architecture and in imprecise maps in the primary visual cortex (Cang et al., 2005; Huberman et al., 2006). Activity patterns during this so-called ‘precritical period’ would enable the formation of early networks and maps, which are subsequently fine-tuned during the critical period by experience-dependent mechanisms (for review, see Feller & Scanziani, 2005).
The mature cortex generates a variety of oscillatory activities that play a central role in different physiological and pathophysiological processes (for comprehensive review, see Buzsáki, 2006). The adult cerebral cortex reveals a rhythmic pattern of activity approximately once every 3–5 s, which is generated by intracortical recurrent excitation and regulated by synaptic inhibition (so-called ‘UP’ or depolarized state; Sanchez-Vives & McCormick, 2000). This intrinsic activity pattern can be observed in the mature cortex in vivo (for review, see Destexhe et al., 2003), as well as in neocortical slices when the bath solution resembles the in vivo situation in its ionic composition (e.g. 1 mm Ca2+, 1 mm Mg2+, 3.5 mm K+Sanchez-Vives & McCormick, 2000). However, these recurrent depolarizations occur less frequently in neocortical slices from immature animals (Tseng & O'Donnell, 2005), especially when the slices are relatively thin (300 µm).
The functional role of oscillatory network activity in the pre- and neonatal cortex is currently not clear. It has been suggested that certain temporal patterns of electrical activity and intracellular calcium signalling may play specific roles in the activity-dependent regulation of gene expression, and that specific genes are regulated by specific patterns of activity (for review, see Fields et al., 2005; Torborg & Feller, 2005). Two recent reports have demonstrated that the periodic spontaneous activity pattern of retinal ganglion cells is correlated with activation of the cAMP pathway, which is needed for the ordering of the retinotopic map (Dunn et al., 2006; Nicol et al., 2007). Retinal ganglion cells must fire high-frequency bursts with a slow periodicity on a timescale of seconds to minutes to activate the intracellular biochemical cascade. Repetitive burst discharges also represent the ideal stimulus pattern for the secretion of brain-derived neurotrophic factor (for review, see Lessmann et al., 2003), which plays a central role as survival factor and in the activity-dependent formation of early neuronal networks (for review, see Huang & Reichardt, 2001). Early oscillatory activity may not only control progressive processes as network formation, but also regressive processes, such as the activity-dependent regulation of programmed cell death in the developing cerebral cortex (Heck et al., 2007). It remains to be elucidated which electrical activity patterns and which molecular mechanisms regulate the formation of neuronal networks during early stages of corticogenesis.
Acknowledgements
This study was supported by a grant of the Deutsche Forschungsgemeinschaft to H.J.L. (DFG Lu375/4). J.J.S. is a member of the Neuroscience Graduate School at the University of Mainz.
Abbreviations
-
- ACSF
-
- artificial cerebrospinal fluid
-
- CP
-
- cortical plate
-
- MEA
-
- microelectrode array
-
- P
-
- postnatal day
-
- SP
-
- subplate.