Modulation of a spinal locomotor network by metabotropic glutamate receptors
Abstract
We have explored the potential involvement of the three main classes of metabotropic glutamate receptor in the modulation of a spinal locomotor network using tadpoles of the anuran amphibian Xenopus laevis. Selective activation of group I receptors in Xenopus embryos and young larvae using the general group I agonist DHPG [(S)-3,5-dihyroxyphenylglycine] significantly increased the frequency of swimming and the number of spontaneously occurring swimming episodes, as monitored by extracellular recordings from ventral roots. Group I receptor activation was without significant effect on the duration or amplitude of motor bursts, the duration of swimming episodes, or the head-to-tail delay in the propagation of swimming activity. Activation of either group II or group III receptors, however, following bath applications of the specific agonists APDC [(2R,4R)-aminopyrrolidine-2,4-dicarboxylic acid] and L-AP4 (l-2-amino-4-phosphonobutanoate), respectively, produced a net inhibitory effect on many of the parameters of fictive swimming at both developmental stages, including a reduction in swimming frequency and episode duration, along with a significant reduction in motor burst amplitude and duration in larval animals only. Applications of selective antagonists provide evidence for activation of all three groups during swimming. The group II and III antagonists EGLU (1-ethyl-2-benzimidazolinone) and MAP4 [(S)-2-amino-2-methyl-4-phosphonobutanoate], respectively, increased, while group I antagonists, CPCCOEt and MPEP, decreased swim frequency. Our findings thus provide evidence for the presence and endogenous activation of three classes of metabotropic glutamate receptor which may function as an intrinsic modulatory control system during fictive swimming in Xenopus tadpoles.
Introduction
Tadpoles of the South African clawed toad, Xenopus laevis, are an extremely attractive and well-established model system in which to study the operation and development of neural networks underlying locomotion (for reviews see Roberts et al., 1998; Sillar et al., 1998). At the time of hatching, Xenopus tadpoles possess a relatively simple nervous system which comprises only eight classes of differentiated spinal neuron (Roberts & Clarke, 1982) and produces a distinctive swimming pattern that quickly matures soon after hatching (Sillar et al., 1991). The rhythmic motor pattern underlying swimming can be initiated in intact immobilized animals by sensory stimulation and studied easily using electrophysiological recording methods. Once initiated, this fictive motor rhythm is self-sustaining without the need for exogenously applied pharmacological agents, providing this model system with an important advantage over those in which NMDA and/or 5-hydroxytryptamine (serotonin, 5-HT) must be added to the bathing solution to trigger activity (e.g. lamprey, neonatal rat).
The central pattern generator (CPG) for swimming in Xenopus tadpoles can be considered as two locomotor half-centres on the left and right sides of the spinal cord. The synaptic drive underlying swimming results from a combination of descending ipsilateral excitation together with reciprocal mid-cycle inhibition which couples the two half-centres in antiphase (Roberts, 1989). The excitation originates from three known sources: glutamatergic interneurons (Dale & Roberts, 1984), cholinergic connections from motorneurons (Perrins & Roberts, 1995a, b) and electrotonic coupling between motorneurons (Perrins & Roberts, 1995a). Of primary interest in this study is the excitatory amino acid neurotransmitter glutamate which, when released from the glutamatergic premotor interneurons onto the motor network, generates a significant proportion, about 30%, of the total excitatory drive during swimming (Dale & Roberts, 1984, 1985; Perrins & Roberts, 1995a; Zhao & Roberts, 1998; Li et al., 2001).
Although it is established that the actions of glutamate at ionotropic glutamate receptors contributes to the tonic and phasic excitatory synaptic components underlying the drive for swimming (Dale & Roberts, 1985), the metabotropic glutamate receptors (mGluRs) have yet to be characterized in Xenopus and therefore the role of these metabotropic receptors forms the focus of this study. mGluR-mediated modulation of locomotor pattern generation has been documented in only a few other vertebrates [e.g. lamprey (for a review see El Manira et al., 2002), mouse (O'Neill et al., 2003) and neonatal rat (Marchetti et al., 2003; Taccola et al., 2003, 2004a, b)], whilst other studies have examined the effects of mGluR activation on isolated spinal neurons [e.g. turtle (Delgado-Lezama et al., 1997; Russo et al., 1997; Svirskis & Hounsgaard, 1998), tortoise (Kozhanov et al., 2001) and Rana pipiens (Holohean et al., 1999)]. However, this is the first time that mGluR-mediated effects on the motor network have been shown in hatchling Xenopus tadpoles. It was of considerable interest therefore to establish whether the effects on a self-sustaining CPG output were similar to those described in other model systems, and to assess the extent to which mGluRs are endogenously activated during locomotion.
Materials and methods
Animals
All experiments were performed on embryonic (stage 37/38) and larval (stage 42) Xenopus laevis tadpoles, staged according to Nieuwkoop & Faber (1956), and obtained by induced breeding following injection of human chorionic gonadotrophin (1000 U/mL, Sigma, UK) into pairs of adults from a laboratory colony. Eggs were kept in de-chlorinated tap water at 17–23 °C until they had reached the desired stage of development. This work was performed in accordance with the UK Animals (Scientific Procedures) Act 1986.
Preparation
Animals were immobilized by immersion in 10 µmα-bungarotoxin for approximately 30 min following gashing of the dorsal fin with a tungsten needle to aid toxin penetration. Animals were then removed from the α-bungarotoxin and transferred to an experimental chamber (∼2 mL volume) containing re-circulating frog ringer solution (ionic composition in mm: NaCl, 120; KCl, 2.5; CaCl2, 2; MgCl2, 1; NaHCO3, 2.5; Hepes, 10; pH 7.4). For a more detailed description of the preparation procedure, see Reith & Sillar (1997). A schematic diagram of the experimental preparation is shown in Fig. 1A. Fictive swimming episodes were evoked by a brief (1-ms) current pulse delivered by a Digitimer DS2 isolated stimulator (Digitimer Ltd, Welwyn Garden City, UK) applied to the tail skin via a glass suction stimulating electrode. Ventral root activity was recorded by positioning glass suction electrodes (∼50 µm tip opening) in the intermyotomal clefts. Activity was routinely recorded simultaneously from rostral and caudal ventral roots on the left side (Fig. 1A and B).
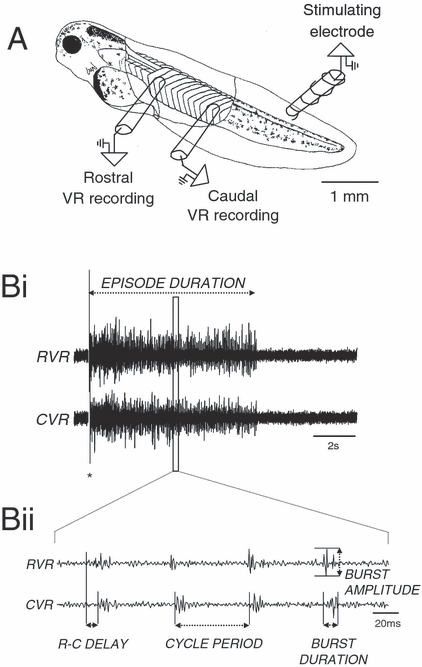
The Xenopus tadpole preparation. (A) The standard Xenopus experimental preparation for recording from ipsilateral ventral roots. Extracellular suction electrodes are placed rostrally and caudally over myotomal clefts following removal of flank skin. Swimming activity is induced by a brief current pulse (1 ms) applied to the tail skin via a stimulating electrode. (Bi) Extracellular recordings showing a whole episode of swimming from a rostral (RVR) and a more caudal ventral root (CVR). The asterisk represents stimulus artefact. (Bii) Trace expanded out from (Bi) demonstrating the parameters of ventral root activity measured.
Pharmacological agents
Stock concentrations of drugs were added to the recirculating saline (100 mL) and agitated to achieve the desired final concentration. With the exception of α-bungarotoxin (Sigma, UK), all drugs were purchased from Tocris Cookson (Bristol, UK). Preparations were only ever exposed to one class of mGluR agonist and antagonist during any one experiment. Fictive swimming activity was monitored throughout drug applications, but effects normally became apparent after approximately 20–40 min.
Parameters of fictive swimming
The following parameters of the fictive swimming rhythm (Fig. 1B) were measured before, during and after applications of metabotropic glutamate receptor agonists and antagonists: burst durations; burst amplitudes; cycle periods; episode durations; and rostro-caudal delays. Delays were measured as the interval between the onsets of ventral root bursts recorded rostrally and at least five or six clefts more caudally.
Data and statistical analysis
All electrophysiological data were recorded via a Digidata 1322 A analog-to-digital converter (Axon Instruments) and analysed using Dataview software (courtesy of W. J. Heitler, University of St. Andrews; version 1.2 h). The first 500 ms of each episode of swimming activity was excluded from analysis to rule out any contribution from sensory stimulus-evoked potentials (∼200 ms in duration; Sillar & Roberts, 1988). Unless stated otherwise, all samples of swimming analysed are taken 500 ms from the onset of swimming and measurements comprised 30 consecutive cycles of motor activity from at least four episodes of swimming. Where stated, data for experiments were pooled and some figures show ‘representative’ individual experiments where ventral root data recordings are shown. For statistical analyses, comparisons with equal variances were carried out using one-way analysis of variance (anova) or Students t-test; and where data were not normally distributed according to Anderson–Darling tests, Kruskal–Wallis tests were used. Normally distributed parametric data are presented as means and standard error of the mean. Non-parametric data are presented as medians and the standard errors bars represent the difference between lower and upper quartile ranges (interquartile range, IQR). Statistical analysis was performed using Minitab (version 13) and differences were considered to be significant at P < 0.05. n equals the number of animals.
Results
The data presented derive from experiments on both embryonic (stage 37/38) and larval (stage 42) Xenopus. Unless stated, results were qualitatively the same for both stages of development studied.
Group I modulation of swimming
Initial experiments involved the application of the general agonist DHPG [(S)-3,5-dihyroxyphenylglycine], to selectively activate group I mGluRs and thereby determine what influence these receptors have on the motor network. Experiments were first carried out to determine at what concentration DHPG produced effects (data not shown). The optimum concentration was established as being between 5 and 10 µm; higher concentrations produced episodes of swimming that were either too short to analyse accurately or that became highly irregular. Therefore, all of the experiments used 10 µm DHPG, giving maximum effect without compromising the ability to analyse the data accurately. This concentration of agonist is similar to that used in other motor systems [e.g. neonatal rat, 5–50 µm (Taccola et al., 2004a); lamprey, 20–100 µm (Krieger et al., 1996, 1998, 2000; Cochilla & Alford, 1998; Kettunen et al., 2002)].
In data pooled from ten stage 37/38 embryos and seven stage 42 larvae, bath application of 10 µm DHPG significantly reduced cycle periods, from 64.42 ± 0.35 ms in control saline to 59.26 ± 0.28 ms (one-way anova: O-A, P < 0.05, n = 17; Fig. 2, Ai and Aii). A significant reversal of this facilitatory effect on swimming frequency was achieved following washout in nine animals (62.53 ± 0.46 ms; O-A, P < 0.05, n = 9; data not shown) and in eight animals following application of the general group I mGluR antagonist AIDA (1-aminoindan-1,5-dicarboxylic acid, 100 µm; 67.32 ± 0.51 ms; Kruskal–Wallis: K-W, P < 0.05, n = 8; Fig. 2, Ai and Aii). The shorter cycle periods following activation of group I mGluRs persisted throughout episodes of swimming (t-test, P < 0.05, n = 17; Fig. 2, Aiii). Ventral root burst durations and amplitudes were not significantly affected following application of DHPG in either stage 37/38 embryos (n = 8) or stage 42 larvae (n = 7; O-A, P > 0.05; Fig. 2, Bi and Bii) and, similarly, neither washout (data not shown) nor application of AIDA affected burst durations or amplitudes (O-A, P > 0.05, n = 8 embryos, 7 larvae). Rostro-caudal (R-C) delays decreased, but not significantly, in the presence of DHPG (3.36 ± 0.21 ms in control saline to 2.9 ± 0.18 ms; O-A, P > 0.05, n = 17; Fig. 2C) and did not change with application of AIDA (3.19 ± 0.16 ms; O-A, P > 0.05, n = 8; Fig. 2C). However, R-C delays significantly increased during washout (3.8 ± 0.18 ms; O-A, P < 0.05, n = 9; data not shown), suggesting that although the decrease in R-C delays under DHPG was insignificant, the trend was in the expected direction, and such a decrease would be hypothesized to accompany the decrease in cycle periods (cf. Tunstall & Sillar, 1993). Lastly, episode durations were not significantly affected with application of DHPG (O-A, P > 0.05, n = 17; Fig. 2D), AIDA (O-A, P > 0.05, n = 8; Fig. 2D), or during washout (O-A, P > 0.05, n = 9; data not shown).
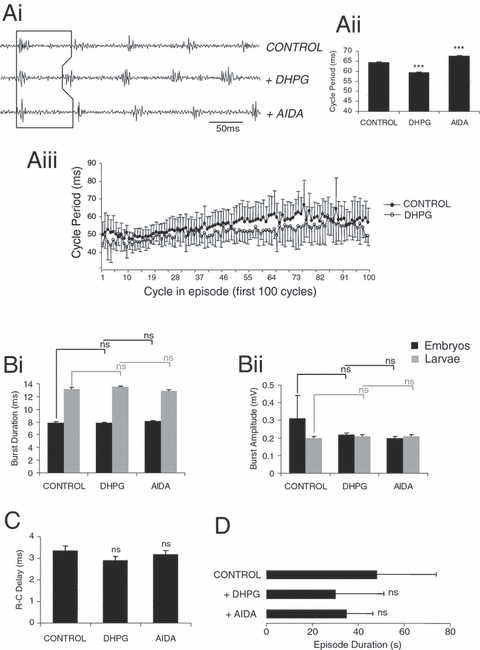
Effects of DHPG on Xenopus fictive swimming. Ventral root recording (Ai; from one representative stage 42 animal) and graphical representation (Aii and iii) showing reduced cycle periods following application of the specific group I receptor agonist DHPG (10 µm; P < 0.05). Subsequent application of the general group I antagonist AIDA (100 µm) reversed this effect on swimming frequency (P < 0.05). (Aiii) Graph illustrating the decrease in cycle periods induced by DHPG over the first 100 cycles of a swimming episode (average of four episodes per condition). Burst durations (Bi) and amplitudes (Bii) were not significantly altered in either stage 37/38 embryos or stage 42 larvae after activation of group I receptors (P > 0.05) or following application of AIDA (P > 0.05). R–C delays (C) and episode durations (D) are unaffected by DHPG (P > 0.05) and following AIDA application (P > 0.05). *** = significant, ns = not significant. Values are means ± SEM, except for Aiii which for clarity shows + SEM for control and –SEM for DHPG.
These initial experiments suggest that the selective activation of group I receptors leads to an increase in swimming frequency, similar to the effects seen in other vertebrate locomotor systems (El Manira et al., 2002; Marchetti et al., 2003; O'Neill et al., 2003; Taccola et al., 2003). In addition, another repeatable effect observed with bath application of 10 µm DHPG is an increase in the number of spontaneously occurring swimming episodes, consistent with an increase in network excitability. This effect was also reversed by subsequent application of AIDA (100 µm; Fig. 3, Ai–Aiii). In eight animals, during the periods between experimentally evoked swimming, the number of spontaneous swim episodes significantly increased from an average of 0.9 ± 0.29 episodes/min in control saline to 2.6 ± 0.49 episodes/min following DHPG application (O-A, P < 0.05, n = 8; Fig. 3, Ai and Aii). This effect was significantly reversed following application of AIDA (to a mean of 0.87 ± 0.39 episodes/min; O-A, P < 0.05, n = 8; Fig. 3, Aiii).
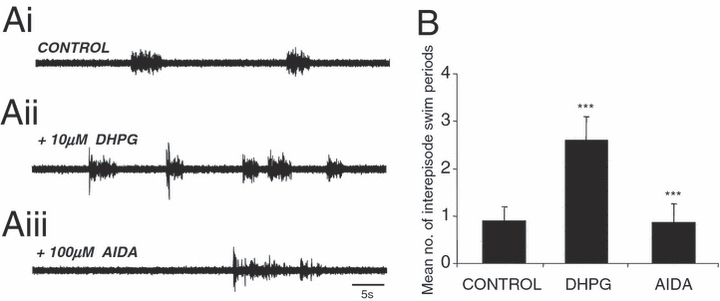
DHPG increases the rate of spontaneous swimming episodes. (Ai and ii) Ventral root recording from a representative animal showing an increase in the number of spontaneous swimming episodes between periods of evoked swimming following application of 10 µm DHPG compared with control. (Aiii, B) The mean number of spontaneously occurring episodes (per minute) of swimming increased significantly under DHPG (P < 0.05) and was reversed following application of AIDA (100 µm; P < 0.05). *** = significant. Values are means ± SEM.
Group II modulation of swimming
To assess the influence of group II mGluR activation on the locomotor network, the general agonist APDC [(2R,4R)-aminopyrrolidine-2,4-dicarboxylic acid] was bath applied. APDC (100 µm; n = 11) produced a profound inhibitory effect on many of the measured parameters of swimming. Pooled data demonstrate that cycle periods were significantly increased, from 56.56 ± 0.4 ms in control saline to 67.54 ± 0.4 ms with APDC (O-A, P < 0.05, n = 11; Fig. 4, Ai and Aii). This effect was significantly reversed in four animals following washout (to 60.8 ± 0.5 ms; O-A, P < 0.05, n = 4; data not shown) and in seven animals following application of the group II specific antagonist EGLU (1-ethyl-2-benzimidazolinone, 100 µm; 60.8 ± 0.5 ms with EGLU; O-A, P < 0.05, n = 7; Fig. 4, Ai and Aii). The increase in cycle periods persisted throughout episodes of swimming following APDC application compared with control (first 100 cycles: t-test, P < 0.05, n = 7; Fig. 4, Aiii). However, the range of cycle periods did not significantly differ from the beginning to the end of an episode of swimming; cycle periods ranged on average from 44 to 94 ms in control conditions, and between 47 and 98 ms under APDC, suggesting that the cycle periods may have increased, in part, due to a shortening of swimming episode durations (see Discussion).
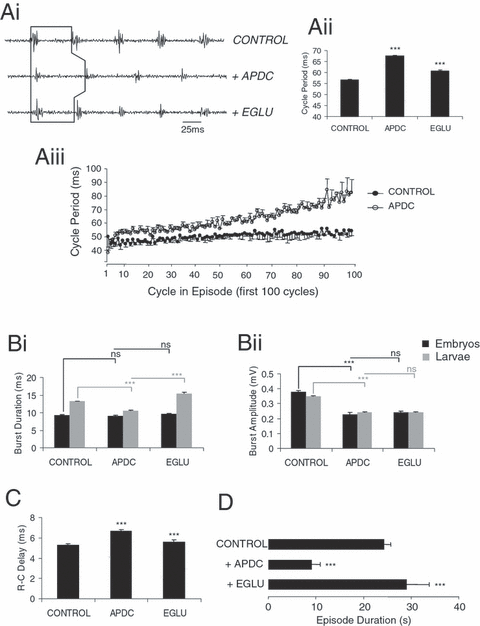
Effects of group II mGluR activation with APDC are partially reversed with the antagonist EGLU. Effects on swimming frequency as shown by representative ventral root recording (Ai; stage 42 larvae) and graphical representation (Aii and iii). Cycle periods increased following application of APDC (100 µm; P < 0.05) and reversed with application of the antagonist EGLU (100 µm; P < 0.05). (Aiii) Graph illustrating on a cycle-by-cycle basis the increase in cycle periods induced by APDC (open circles) over the first 100 cycles, compared with control (closed circles). (Bi) APDC reduced ventral root burst durations in stage 42 larvae (P < 0.05) but not in stage 37/38 embryos (P > 0.05). This effect on burst durations in larvae could be reversed by EGLU (P < 0.05). (Bii) Burst amplitudes were also reduced following APDC application in both larvae (P < 0.05) and embryos (P < 0.05). EGLU did not antagonize the APDC-induced reduction in burst amplitudes in either embryos or larvae (P > 0.05). (C) In eight larvae and three embryos, R–C delays increased following APDC application (P < 0.05) and were significantly reversed by EGLU (P < 0.05). (D) Episode durations were significantly decreased by APDC (P < 0.05) and were reversed by EGLU (P < 0.05). *** = significant, ns = not significant. Values are means ± SEM, except in Aiii which for clarity shows –SEM for control and +SEM for APDC.
APDC also produced a developmental stage-specific decrease in burst durations. Thus, in three stage 37/38 embryos, ventral root burst durations were not affected by applications of APDC, EGLU or following washout (O-A, P > 0.05; Fig. 4, Bi). Burst amplitudes, however, decreased in embryos with application of APDC (from 0.38 ± 0.01 mV in control saline to 0.23 ± 0.01 mV; O-A, P < 0.05, n = 3; Fig. 4, Bii), and could be reversed during washout (0.37 ± 0.01 mV; O-A, P < 0.05, n = 1; data not shown), but not following application of the antagonist EGLU (0.24 ± 0.01 mV; O-A, P > 0.05, n = 2; Fig. 4, Bii). In contrast, by larval stage 42, APDC application caused a significant decrease in burst durations (from 13.23 ± 0.11 ms in control saline to 10.59 ± 0.14 ms; O-A, P < 0.05, n = 8; Fig. 4, Bi) that could be reversed following EGLU application (to 15.52 ± 0.24 ms; O-A, P < 0.05, n = 5; Fig. 4, Bi). Burst durations following EGLU application were greater than in control prior to APDC, suggesting endogenous activation of group II receptors. However, during washout of APDC an additional decrease in burst duration is observed (to 9.63 ± 0.1 ms; O-A, P < 0.05, n = 3; data not shown), suggesting a continued effect of APDC and/or incomplete washout. Similar to embryos, larval burst amplitudes also decreased after group II receptor activation (from 0.35 ± 0.004 mV in control saline to 0.24 ± 0.004 mV; O-A, P < 0.05, n = 8; Fig. 4, Bii) but this effect was not reversed during washout (O-A, P > 0.05, n = 3; data not shown) or following EGLU application (O-A, P > 0.05, n = 5; Fig. 4, Bii).
R-C delays increased following the activation of group II mGluRs (from 5.27 ± 0.14 ms in control saline to 6.65 ± 0.13 ms; O-A, P < 0.05, n = 11; Fig. 4C) and this effect was reversed during washout in four animals (to 5.27 ± 0.14 ms; O-A, P < 0.05, n = 4; data not shown) and with EGLU application in seven animals (to 5.62 ± 0.18 ms; O-A, P < 0.05, n = 7; Fig. 4C). Lastly, episode durations decreased in the presence of APDC (from 24.37 ± 4.67 s in control saline to 9.13 ± 1.72 s with APDC; O-A, P < 0.05, n = 11; Fig. 4D), but this effect did not reverse during washout (6.28 ± 1.27 s; O-A, P > 0.05, n = 4; data not shown). However, the reduction in episode durations was fully reversed following the application of EGLU (29.15 ± 1.42 s with EGLU; O-A, P < 0.05, n = 7; Fig. 4D) and sometimes increased beyond measurements taken in control, again suggesting endogenous activation of group II receptors.
Group III modulation of swimming
The effects of activating group III mGluRs on the neural network underlying swimming were investigated by bath applying the specific agonist L-AP4 (l-2-amino-4-phosphonobutanoate). During these experiments it became apparent that only a narrow range of agonist concentrations was able to affect the motor network without abolishing motor activity completely. The optimum concentration of L-AP4 found to be effective during these experiments was 50 µm, but even at this concentration swimming activity was completely abolished in four out of 15 animals. By comparison, in the lamprey model, L-AP4 was used at far higher concentrations of between 100 and 500 µm (Krieger et al., 1996, 1998). Thus, the Xenopus tadpole swimming network seems particularly sensitive to the activation of group III receptors.
In data pooled from the remaining 11 (of 15) animals, bath application of 50 µm L-AP4 caused a decrease in swimming frequency, with cycle periods increasing significantly, from 53.75 ± 0.47 ms in control saline to 59.97 ± 0.34 ms under L-AP4 (O-A, P < 0.05, n = 11; Fig. 5, Ai and Aii). This effect persisted through episodes; cycle periods following L-AP4 application were consistently longer when compared with control (first 100 cycles: t-test, P < 0.05, n = 11; Fig. 5, Aiii). The range of cycle periods over the whole episode of swimming was wider following L-AP4 application (ranging on average from 42 to 91 ms in control and from 43 to 119 ms under L-AP4), suggesting that L-AP4 application can affect cycle periods independently from episode duration. In eight animals (two stage 37/38 embryos and six stage 42 larvae), the increased cycle periods under L-AP4 could not be reversed with application of the general group III antagonist MAP4 [(S)-2-amino-2-methyl-4-phosphonobutanoate, 100 µm; to 58.17 ± 0.36 ms; O-A, P > 0.05, n = 8; Fig. 5, Ai and Aii). Nevertheless, in a further three animals (all stage 42 larvae), this effect could be significantly reversed with washout (to 55.8 ± 0.36 ms; O-A, P < 0.05, n = 3; data not shown).
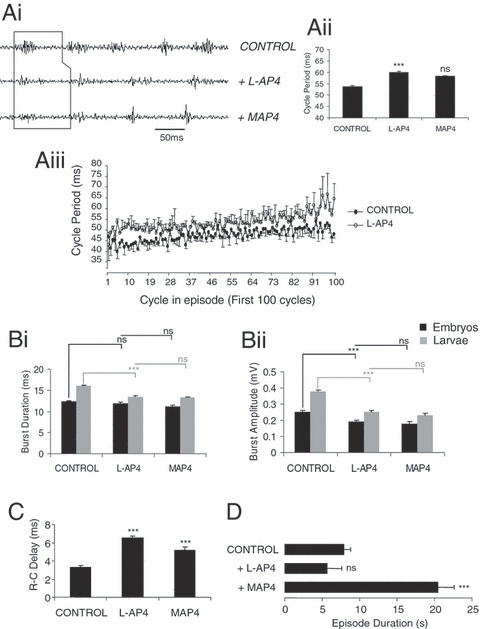
Effects of L-AP4 on the locomotor rhythm are not always reversed with the general group III mGluR antagonist MAP4. Cycle periods increased following L-AP4 (50 µm) application as demonstrated by representative ventral root recording (Ai) and graphs (Aii and iii; P < 0.05). This effect did not reverse in the presence of the general group III receptor antagonist MAP4 (100 µm; P > 0.05). (Aiii) Graph illustrating the L-AP4-induced increase in cycle periods over the first 100 cycles of an episode of swimming. In stage 42 larvae, L-AP4 application significantly decreased burst durations (Bi; P < 0.05) and burst amplitudes (Bii; P < 0.05), which did not reverse in the presence of MAP4 (P > 0.05). In stage 37/38 embryos, L-AP4 application did not significantly affect burst durations (P > 0.05), but reduced burst amplitudes (Bi and ii; P < 0.05). The reduced burst amplitudes in embryos also did not reverse following MAP4 application. (C) In nine larvae and two embryos, R–C delays significantly increased in the presence of L-AP4 and were partially reversed with antagonist application (P < 0.05). (D) L-AP4 (50 µm) does not greatly affect the duration of episodes in the majority of preparations (P > 0.05). *** = significant, ns = not significant. Values are means ± SEM, except Aiii which for clarity shows –SEM for control and + SEM for L-AP4.
There was a significant decrease in burst durations following L-AP4 application, but only in stage 42 larval animals. Thus, ventral root burst durations decreased with 50 µm L-AP4 in nine larval animals from 16.03 ± 0.22 ms in control saline to 13.5 ± 0.2 ms (O-A, P < 0.05, n = 9; Fig. 5, Bi), an effect which did not reverse during washout in three animals (O-A, P > 0.05, n = 3; data not shown) or following application of MAP4 in six animals (O-A, P > 0.05, n = 6; Fig. 5, Bi). In two stage 37/38 embryos, there was no change in burst durations with either L-AP4 or MAP4 application (O-A, P > 0.05, n = 2; Fig. 5, Bi). The amplitude of the ventral root bursts also significantly decreased after L-AP4 application, both in larvae (from 0.38 ± 0.01 mV in control saline to 0.25 ± 0.01 mV with L-AP4; O-A, P < 0.05, n = 9; Fig. 5, Bii) and in two embryonic animals (from 0.25 ± 0.01 mV in control saline to 0.19 ± 0.01 mV with L-AP4; O-A, P < 0.05, n = 2; Fig. 5, Bii). The effect on burst amplitudes could not be reversed by washout (O-A, P > 0.05, n = 3; data not shown) or by MAP4 application (O-A, P > 0.05, n = 6 larvae and two embryos, respectively; Fig. 5, Bii). R-C delays also significantly increased in the presence of L-AP4 from 3.34 ± 0.18 ms in control saline to 6.52 ± 0.19 ms under L-AP4 (O-A, P < 0.05, n = 11; Fig. 5C). Although this effect on R-C delays could not be reversed during washout, they displayed a further increase with the antagonist MAP4 (to 5.23 ± 0.31 ms; O-A, P < 0.05, n = 8; Fig. 5C).
Episode durations appeared to be reduced in an ‘all-or-nothing’ manner following application of L-AP4. At 50 µm L-AP4, episode durations, although shorter, were not significantly affected in 11 animals (from 7.95 ± 2.12 s in control saline to 5.73 ± 1.86 s with L-AP4; O-A, P > 0.05, n = 11; Fig. 5D). Subsequent application of MAP4 in eight (of 11) animals significantly increased episode durations, even beyond the value of control condition (to 20.51 ± 0.87 s; O-A, P < 0.05, n = 8; Fig. 5D), suggesting endogenous activation of these receptors. However, washout was without effect on episode durations in three (of 11) animals (5.57 ± 0.84 s; O-A, P > 0.05, n = 3; data not shown). In a further four animals swimming activity was completely abolished by application of 50 µm L-AP4, yet in these cases application of MAP4 was always able to restore activity.
Endogenous and subtype-specific activation of mGluRs
In order to assess the contribution, if any, of endogenous activation of mGluRs during swimming activity, group- and subtype-specific mGluR antagonists were bath applied. The general group I receptor antagonist AIDA (100 µm), when bath applied alone, caused no significant changes in cycle periods in any of the seven animals tested (three embryos and four larvae; from 55.96 ± 0.35 ms in control to 55.4 ± 0.32 ms with AIDA; O-A, P > 0.05, n = 7; Fig. 6, Ai and Aii) and all other measured parameters of swimming were similarly unaffected (data not shown). Attempts to enhance endogenous activation of group I mGluRs by increasing the extracellular levels of glutamate using the glutamate uptake inhibitor L-AHM (l-β-aspartic acid hydroxymate, 100 µm), produced the expected increase in swim frequency with cycle periods decreasing from 71 ± 11 ms in control saline to 61.5 ± 6.125 ms (K-W, P < 0.05, n = 7; Fig. 6, Ai and ii) in the presence of the uptake inhibitor. However, the application of AIDA in the presence of L-AHM still caused no change in cycle periods (K-W, P > 0.05, n = 7; Fig. 6, Ai and ii). The resultant rise in extracellular glutamate due to L-AHM will not only cause an activation of all mGluRs subtypes, but also ionotropic glutamate receptors and other glutamate transporters not blocked with L-AHM. Therefore, in this instance, the effects of AIDA on group I receptors may be masked by the additive effects of glutamate acting simultaneously on other glutamate receptors and transporters. The lack of effect of AIDA suggests that either group I receptors are not normally activated during swimming or that AIDA is not potent enough to counteract the effects of group I receptors activated by endogenously released glutamate (however, see below). Alternatively, AIDA may not be so general or specific an antagonist at the group I mGluRs as previously assumed; for example, there is evidence that AIDA may be more selective for the group I subtype, mGluR1, over mGluR5 (Moroni et al., 1997). If this is the case in Xenopus then AIDA could have equal and opposing effects on different receptors, which cancel out the expected increase in cycle periods. Alternatively, group I mGluRs may have no endogenous contribution motor activity under normal experimental conditions.
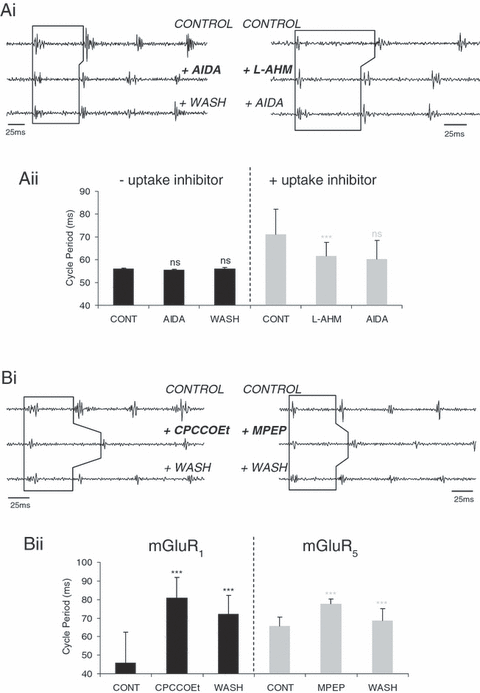
Evidence for endogenous activation of group I mGluRs. Ventral root recording and graphical representation (Ai and ii) showing no significant change in cycle periods following application of the general group I mGluR antagonist AIDA alone (100 µm; O–A, P > 0.05, n = 7) or with prior application of the glutamate uptake inhibitor L-AHM (100 µm; K–W, P > 0.05, n = 7). All other parameters of swimming activity were unaffected in the presence of AIDA (data not shown). (Bi) Cycle periods increased following application of the mGluR1-specific antagonist CPCCOEt (50 µm: K–W, P < 0.05, n = 7). (Bii) Similarly, application of the mGluR5 antagonist MPEP also increased cycle periods (100 µm; K–W, P < 0.05, n = 6). The effects induced by these two antagonists were reversed during washout (K–W, P < 0.05, n = 7 and n = 6, respectively). *** = significant, ns = not significant. Values are means ± SEM or medians ± IQR.
To explore these possibilities, the subtype-specific antagonists CPCCOEt (mGluR1; 50 µm) and MPEP (mGluR5; 100 µm) were bath applied individually. CPCCOEt application (50 µm) profoundly affected swimming frequency, increasing cycle periods by ∼46% from 45.75 ± 16.62 ms in control saline to 81 ± 11 ms (in three embryos and four larvae; K-W, P < 0.05, n = 7; Fig. 6, Bi and Bii), an effect which was partially but significantly reversed after washout (to 72 ± 10.25 ms; K-W, P < 0.05, n = 7). The mGluR5 subtype-specific antagonist MPEP (100 µm) also increased cycle periods, but only by ∼14% from 65.5 ± 5 ms in control saline to 77.5 ± 2.75 ms (in three embryos and three larvae; K-W, P < 0.05, n = 6; Fig. 6, Bi and Bii), which was also reversed successfully after washout (to 68 ± 6.5 ms; K-W, P < 0.05, n = 6). Thus, it appears from these data that both group I subtypes are indeed present and can be activated endogenously, although there do not appear to be differential roles for the two subtypes on motor activity as both increase cycle periods (cf. lamprey: Kettunen et al., 2002, 2003; see Discussion).
The general group II and III antagonists, EGLU (100 µm) and MAP4 (100 µm), were similarly bath applied to explore whether these receptors are normally activated during swimming. In five animals EGLU application caused a decrease in cycle periods (from 48.24 ± 0.49 ms in control saline to 41.1 ± 0.5 ms under EGLU; O-A, P < 0.05, n = 5; Fig. 7A and B), although the other measured parameters of swimming remained unaffected. Similarly, in eight animals MAP4 caused a significant reduction in cycle periods (from 54.84 ± 1.28 ms in control saline to 51.55 ± 0.55 ms under MAP4; O-A, P < 0.05, n = 8; Fig. 7A and B), and thus increased swimming frequency. These results suggest that group II and group III receptors are both activated by endogenously released glutamate during swimming and that the major effect is to cause a reduction in swimming frequency.
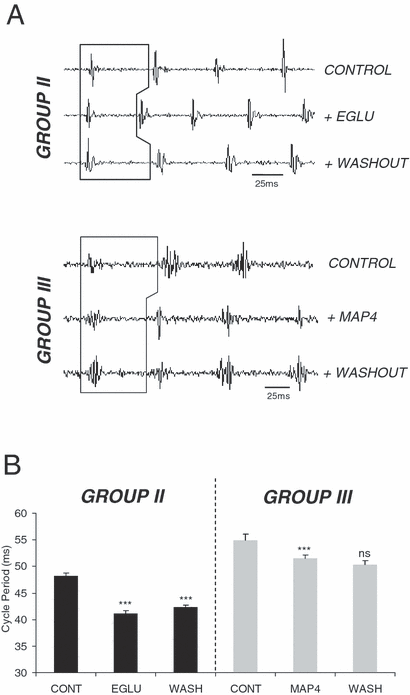
Effects of group II/III receptor antagonists on network activity. Ventral root recording (A) and graphical representation (B) showing reduced cycle periods with individual applications of the group II antagonist EGLU (100 µm; O–A, P < 0.05, n = 5) and the general group III antagonist MAP4 (100 µm; O–A, P < 0.05, n = 8). This effect on swimming frequency only partially reversed during washout from EGLU, but the effects of MAP4 could not be counteracted on washout (O–A, P > 0.05, n = 5 and 8, respectively). *** = significant, ns = not significant. Values are means ± SEM.
Discussion
The notion that glutamate acts exclusively as a neurotransmitter through ionotropic GluRs to generate the excitatory drive to initiate and maintain locomotion in vertebrates has radically changed in recent years due to the identification and characterization of mGluRs. The physiological roles of mGluR activation within the complex neuronal systems underlying motor behaviour have only recently begun to be investigated (El Manira et al., 2002; Marchetti et al., 2003; O'Neill et al., 2003; Taccola et al., 2003, 2004a, b). The present series of experiments provides the first evidence for the presence and activation of all three mGluR groups in the locomotor network generating swimming in Xenopus laevis tadpoles.
As glutamate is such a ubiquitous excitatory neurotransmitter within the central nervous system it was necessary to use specific pharmacological agonists to activate the different mGluR groups. Group I receptor activation with the general agonist DHPG significantly increased both swimming frequency and the number of spontaneously occurring swimming episodes. These excitatory effects of group I mGluRs could potentially be accounted for by a shift in the balance between synaptic excitation and inhibition in the spinal network. DHPG application does not affect motor burst durations or amplitudes, so it is unlikely that group I receptors have a direct effect on motorneurons during the modulation of swimming. Although there are several possible explanations for the changes in the strengths of the synaptic drive during swimming there is precedence in the tadpole for suggesting that DHPG shifts the balance by reducing inhibition rather that increasing excitation. Thus, fast inhibitory pathways have been shown to be common targets for various neuromodulators regulating motor pattern generation in Xenopus (for a review see Sillar et al., 2002). For example, the biogenic amines noradrenaline and 5-HT are known to facilitate and depress glycinergic transmission, respectively, effects which have been shown to occur presynaptically via amine receptors located on the terminals of the inhibitory interneurons (McDearmid et al., 1997; Merrywest et al., 2002; Sillar, et al., 2002). Glycinergic transmission mediates the reciprocal, mid-cycle inhibition that is important for the alternating activity between the two locomotor half-centres during both rhythmic swimming (Dale et al., 1986; Soffe, 1987) and struggling (Soffe, 1993) in Xenopus. There is also evidence that nitric oxide, a free radical, can potentiate both GABAergic and glycinergic synaptic inhibition causing swimming to slow down and stop prematurely (McLean & Sillar, 2000; McLean et al., 2001). The effects of DHPG in Xenopus are largely negated in the presence of strychnine (our unpublished observations), so it will be of considerable interest in the future to determine whether, and by what mechanism, group I mGluRs affect inhibitory synaptic transmission in the Xenopus tadpole.
These findings broadly parallel what has been found in the lamprey, where group I receptor activation both increases the frequency of the locomotor rhythm and promotes motorneuron excitability (Krieger et al., 1998, 2000). In the lamprey locomotor network, group I receptors mediate their effect through multiple mechanisms which converge to increase excitability. For example, activation of mGluR1 causes a potentiation of NMDA receptors (Krieger et al., 1998, 2000), and inhibits a leak current, which boosts depolarization and facilitates ventral root bursting (Kettunen et al., 2003), both leading to a profound increase in locomotor frequency. Furthermore, mGluR1 activation induces the release of endocannabinoids that act in a retrograde fashion to cause a depression of glycinergic inhibitory transmission onto motorneurons and crossed-caudal interneurons, increasing network excitability (Kettunen et al., 2005). Whether similar mechanisms underlie the observed group I mGluR-mediated increase in network excitability in Xenopus must now be explored.
In contrast to the effects induced by group I receptor activation in Xenopus, applications of the group II and III agonists, APDC and L-AP4, respectively, caused a net inhibitory effect on swimming frequency and also reduced episode durations. These two effects may be linked because swimming frequencies within an episode change dynamically, starting rapidly, quickly reaching a plateau then gradually slowing before swimming terminates. Consequently, during the shorter episodes following activation of group II or III mGluRs, the proximity of the cycles measured to the end of the episode increases. Nevertheless, it is clear from the plots of cycle periods over the first 100 cycles that the effect on frequency is present from the start and persists throughout each episode. Interestingly, group III mGluRs severely affected swimming episode durations, often in an ‘all-or-nothing’ manner, to the extent that L-AP4 often completely abolished activity. Additionally, unlike group I mGluRs, activation of both groups II and III significantly decreased ventral root burst durations and amplitudes in larval animals, whilst in stage 37/38 embryos only burst amplitudes decreased (2, 3). It should be noted that burst durations in larvae are much longer than in embryos (Sillar et al., 1991). In larvae, motorneurons can fire multiply in each cycle of swimming and consequently any inhibitory effect of group II or III agonists on this firing might be expected to reduce the overall ventral root burst duration. In embryos, ventral root bursts reflect the synchronous firing of single spikes in each motorneuron in each cycle so that there is little or no scope for any reduction under APDC or L-AP4.
The excitatory drive to the locomotor half-centres is mediated partly by the actions of glutamate, released from premotor interneurons, at the NMDA and non-NMDA ionotropic glutamate receptors (Dale & Roberts, 1984, 1985; Zhao & Roberts, 1998; Li et al., 2001), although there is also evidence for both cholinergic and electrical components to the excitation of motorneurons in each cycle of swimming (Perrins & Roberts, 1995a, b; Li et al., 2004). A reduction in glutamatergic transmission would compromise the ability of neurons to reach the threshold for firing. Both motorneurons and premotor interneurons generally fire only once per cycle in the Xenopus embryo and the number of interneurons active per episode of swimming influences the frequency of swimming (Sillar & Roberts, 1993). Thus, if glutamatergic transmission was reduced following the activation of group II and/or III mGluRs, then the number of excitatory interneurons firing could decrease, such that the number of neurons contributing to the synaptic drive underlying swimming falls below the level required to sustain rhythm generation. A reduction in excitatory transmission could explain why the Xenopus swimming network was unable to sustain rhythmic activity for long periods of time in the presence of APDC and L-AP4. Interneuronal drop-out has been proposed as a mechanism for the gradual reduction in frequency and eventual termination of swimming, implying that a critical number of active neurons are required for the maintenance of the rhythm (Sillar & Roberts, 1993).
The effects of group II and III receptor activation at the network level are directly comparable with the group II and III mGluR-mediated depression of motor activity in the lamprey (Krieger et al., 1996), neonatal rat (Taccola et al., 2004b) and, for group II only, mouse (O'Neill et al., 2003). Studies on the lamprey locomotor network have revealed that activation of presynaptic group II and III mGluRs causes a reduction in excitatory transmitter release from descending reticulospinal neurons onto the motor network (Krieger et al., 1996), yet the underlying mechanisms behind this effect remain to be elucidated. This depression of excitation by group II receptors also holds true in the spinal networks of the mouse and rat where group II antagonist applications consistently enhance locomotor activity (O'Neill et al., 2003; Taccola et al., 2004b). Labelling studies indicate that group II mGluRs are not always closely associated with glutamatergic synapses and often have a perisynaptic localization (Lujan et al., 1997; Shigemoto et al., 1997), suggesting that these receptors act as negative feedback autoreceptors to reduce glutamate release. Similarly, in the larval lamprey spinal cord, there is electrophysiological evidence that presynaptic group III mGluRs modulate glutamate transmitter release in an autoreceptor-mediated fashion from reticulospinal neurons via the L-AP4-induced activation of a presynaptic K+ current that depresses the release of transmitter (Cochilla & Alford, 1998).
In addition to the effects on burst durations and amplitudes, R-C delays in the presence of both APDC and L-AP4 increased in tandem with the increased cycle periods, an effect not observed with applications of the group I agonist DHPG. In larval (stage 42) Xenopus tadpoles, cycle periods and R-C delays are known to be positively correlated, creating a phase-lag along the body, presumably to maintain undulatory body movements during fictive swimming (Tunstall & Sillar, 1993). Thus, it appears from this present study that the different groups of mGluRs have different effects on the various parameters of the swimming rhythm, with groups II and III having a direct effect on both swimming frequency, intersegmental delay and episode duration, whilst group I mGluRs affect swimming frequency independent of intersegmental co-ordination and episode duration.
So far, this study has provided evidence for the functional presence of all three groups of mGluRs in the Xenopus swimming system via the pharmacological activation of each mGluR group. However, applications of group-specific and subtype-specific antagonists have also provided evidence that these receptors can be activated endogenously during normal network activity. Group I mGluRs consist of two subtypes, mGluR1 and mGluR5, and applications of subtype-specific antagonists revealed that a blockade of either of these receptor subtypes slowed swimming frequency, suggesting a level of endogenous activation of the two receptor subtypes. Interestingly, blocking each group I subtype individually produced a similar decrease in swim frequency, in contrast to the effects observed in lamprey, where mGluR1 activation increases burst frequency, whilst mGluR5 activation causes a reduction (Krieger et al., 2000; Kettunen et al., 2002). The reason why both group I receptor subtypes produced the same effect in the Xenopus spinal network cannot be determined at this stage, but it is possible that their expression may be developmentally regulated. By comparison, blockade of group II and III receptors with their respective group antagonists, EGLU and MAP4, increased swimming frequency in Xenopus, again implying that these two groups are also activated endogenously, presumably as a result of glutamate released when the network is cycling. However, it should be noted that previous studies have proposed that this endogenous activation by glutamate may only occur during periods of high activity or pathological conditions when this transmitter is in excess. For example, there are many pharmacological studies reporting that group II compounds are only active in animal models under situations of anxiety, ischaemia and psychosis (for a review see Schoepp et al., 1999). This seems consistent with studies showing group II and III receptors are located perisynaptically (Petralia et al., 1996; Shigemoto et al., 1997; Cartmell & Schoepp, 2000), and are activated when glutamate ‘spills over’ from the synaptic cleft.
It is clear from these data that activation of each group of mGluRs exerts a significant modulatory influence on Xenopus swimming activity and therefore glutamate acts not only as a neurotransmitter, but as a potent neuromodulator within the Xenopus spinal cord. Precisely how each receptor group functions remains unclear and further experiments will be necessary to determine the precise mechanisms of action of each mGluR group, including in particular the possibilities that group I receptors reduce glycinergic transmission while groups II and III act as negative-feedback autoreceptors to reduce excitatory transmission.
Acknowledgements
This work was supported by the BBSRC and the Wellcome Trust. R.J.C. held a BBSRC studentship. We thank Abdel El Manira and Jon Issberner for comments on the manuscript.
Abbreviations
-
- 5-HT
-
- 5-hydroxytryptamine (serotonin)
-
- AIDA
-
- 1-aminoindan-1,5-dicarboxylic acid
-
- APDC
-
- (2R,4R)-aminopyrrolidine-2,4-dicarboxylic acid
-
- DHPG
-
- (S)-3,5-dihyroxyphenylglycine
-
- EGLU
-
- 1-ethyl-2-benzimidazolinone
-
- K-W
-
- Kruskal–Wallis
-
- L-AHM
-
- l-β-aspartic acid hydroxymate
-
- L-AP4
-
- l-2-amino-4-phosphonobutanoate
-
- MAP4
-
- (S)-2-amino-2-methyl-4-phosphonobutanoate
-
- mGluR
-
- metabotropic glutamate receptor
-
- O-A
-
- one-way anova
-
- R-C delay
-
- rostro-caudal delay.