GABAergic neurosteroids mediate the effects of ethanol on long-term potentiation in rat hippocampal slices
Abstract
We previously found that ethanol has complex effects on hippocampal synaptic plasticity, inhibiting long-term potentiation (LTP) and long-term depression by different mechanisms. The block of long-term depression appears to be mediated by effects on N-methyl-d-aspartate receptors, whereas the block of LTP involves augmented inhibition via γ-aminobutyric acid-A receptors (GABAARs). To pursue factors contributing to effects on LTP, we examined the ability of various concentrations of ethanol to block LTP in the CA1 region of rat hippocampal slices. Complete LTP block required 60 mm ethanol. LTP block was enhanced at lower ethanol concentrations in the presence of (3α5α)-3-hydroxypregnan-20-one, a GABAAR-potentiating neurosteroid, suggesting that neurosteroids may be important contributors to the effects of ethanol on LTP. Consistent with this, we found that block of LTP by 60 mm ethanol was overcome by coadministration of a cyclodextrin that binds and removes lipophilic neurosteroids. More specifically, treatment of slices with finasteride, an agent that inhibits the synthesis of 5α-reduced neurosteroids, or with an agent that inhibits the effects of 5α-reduced neurosteroids on GABAARs overcame the effects of 60 mm ethanol on LTP. Taken together, these results indicate that acute production of GABAAR-enhancing neurosteroids plays a key role in mediating the effects of ethanol on LTP.
Introduction
Acute ethanol intoxication is associated with memory impairment. When severe, this includes an inability to register new information, a state known as a ‘blackout’ (White, 2003). Ethanol has multiple effects that could contribute to these cognitive changes (Chandler, 2003), including inhibition of N-methyl-d-aspartate receptors (NMDARs) and potentiation of γ-aminobutyric acid-A receptors (GABAARs). NMDARs play critical roles in the induction of hippocampal long-term potentiation (LTP), a form of synaptic plasticity thought to be important for memory processing (Martin et al., 2000). Thus, direct effects on NMDARs could play a major role in alcohol-associated memory impairment. Similarly, GABAAR-mediated inhibition is important in regulating regional activity and in modulating the induction of synaptic plasticity (Steele & Mauk, 1999).
Previous studies have found that ethanol inhibits LTP in the CA1 region of the hippocampus. The concentrations required have varied, with most studies reporting that 50 mm ethanol or greater is needed (Morrisett & Swartzwelder, 1993; Sugiura et al., 1995; Schummers et al., 1997), although another study found that 5 mm is sufficient to diminish LTP (Blitzer et al., 1990). The reasons for these discrepancies and the mechanisms responsible for the block of LTP are uncertain. We previously found that 60 mm ethanol acutely inhibits CA1 LTP and that the effects are overcome by picrotoxin, a GABAAR inhibitor (Izumi et al., 2005b). The involvement of GABAARs in the effects of ethanol on LTP is consistent with previous studies (Morrisett & Swartzwelder, 1993; Schummers et al., 1997; Schummers & Browning, 2001) and suggests a prominent role for enhanced GABAergic inhibition in mediating memory impairment.
The actions of ethanol on GABAARs are complex and depend on receptor subtype (Criswell & Breese, 2005; Breese et al., 2006). Ethanol directly augments extrasynaptic GABAARs expressing δ-subunits (Wallner et al., 2003; Wei et al., 2004; but see Borghese et al., 2006) that mediate tonic inhibition in some brain regions (Hanchar et al., 2004). Effects on other GABAARs are less direct and, in some cases, involve GABAAR-potentiating neurosteroids (Morrow et al., 1999, 2001; VanDoren et al., 2000; Sanna et al., 2004). Acute ethanol exposure increases levels of (3α5α)-3-hydroxypregnan-20-one (3α5αP or allopregnanolone), a neurosteroid that enhances γ-aminobutyric acid actions (Zorumski et al., 2000; Belelli & Lambert, 2005). In some cases, including effects on memory (Matthews et al., 2002), neurosteroids mediate the ability of ethanol to alter central nervous system function (VanDoren et al., 2000).
We recently found that ethanol enhances hippocampal feedforward inhibition following orthodromic stimulation of stratum radiatum when administered in the presence of nanomolar concentrations of 3α5αP (Murayama et al., 2006). This suggests that GABAergic actions resulting from the combined effects of ethanol and neurosteroids may be important for effects on synaptic plasticity. In this study, we examined the effects of ethanol on LTP in greater detail and probed the potential involvement of neurosteroids.
Materials and methods
Hippocampal slice physiology
Hippocampal slices were prepared from post-natal day 30–32 Sprague-Dawley rats using standard methods (Zorumski et al., 1996). Protocols for animal use were approved by the Washington University Animal Studies Committee in accordance with the NIH guidelines for care and use of laboratory animals. Rats were anesthetized with isoflurane and decapitated. Hippocampi were rapidly dissected and placed in artificial cerebrospinal fluid containing (in mm): 124 NaCl, 5 KCl, 2 MgSO4, 2 CaCl2, 1.25 NaH2PO4, 22 NaHCO3, 10 glucose, bubbled with 95% O2/5% CO2 at 4–6 °C and cut transversely into 450 µm slices using a vibrotome. Acutely prepared slices were placed in an incubation chamber containing gassed artificial cerebrospinal fluid and maintained for at least 1 h at 30 °C before synaptic studies.
At the time of study, slices were transferred individually to a submersion-recording chamber. Experiments were performed at 30 °C with continuous bath perfusion of artificial cerebrospinal fluid at 2 mL/min. Extracellular recordings were obtained from the apical dendritic layer of the CA1 region for analysis of population excitatory post-synaptic potentials (EPSPs) and from the cell body layer for analysis of population spikes. EPSPs were measured by their maximal slopes, whereas population spikes were measured as a maximal height from the apex of the first positive peak to the most negative point of the spike. Isolated NMDAR synaptic responses were recorded in artificial cerebrospinal fluid containing 0.1 mm Mg2+, 2.5 mm Ca2+ and 30 µm 6-cyano-7-nitroquinoxaline-2,3-dione.
For most experiments, evoked synaptic responses were elicited with 0.1 ms constant current pulses through a bipolar stimulating electrode (Rhodes Medical Instruments) placed in the Schaffer collateral/commissural (stratum radiatum) pathway. Synaptic responses in CA1 were monitored by applying single stimuli to the Schaffer collaterals every 60 s at an intensity sufficient to elicit half maximal responses. After establishing a stable baseline for at least 10 min and a control input/output curve, LTP was induced by applying a single 100 Hz × 1 s tetanus using the same intensity stimulus. Input/output curves were repeated 20 and 60 min following tetanic stimulation. In studies of synaptic plasticity, values reported in the text represent percent changes in EPSP slopes measured 60 min following tetanic stimulation relative to baseline responses in the same slice.
To study recurrent inhibition in the CA1 region, we used a paired-pulse stimulation paradigm in which antidromic stimulation in the alveus was paired 15 ms later with orthodromic stimulation in stratum radiatum (Petrides et al., 2007). For these studies, orthodromic stimuli were applied at an intensity that activated 90% maximal population spikes and the antidromic stimuli were administered at an intensity that diminished baseline orthodromic population spikes by 30–50%. The effects of ethanol and 3α5αP, alone and in combination, were determined by changes in the degree of antidromically induced paired-pulse spike depression. We also examined the effects of ethanol on proximal inhibitory inputs to CA1 by stimulating adjacent to the pyramidal cell layer and determining effects on population spikes using a paired-pulse paradigm similar to the one described above with a 15 ms interpulse interval. Previous studies indicated that these proximal inhibitory inputs differ in their time course and pharmacology, including ethanol sensitivity, compared with distal dendritic or recurrent inhibitory inputs (Pearce, 1993; Weiner et al., 1997).
Statistical analysis
Data in the text and figures are expressed as mean ± SEM. Student's t-test was used for comparisons between groups where appropriate. Otherwise, the non-parametric Mann–Whitney U-test was used. Statistical comparisons in studies of synaptic plasticity and the values reported in the text are based on analysis of input/output curves at baseline and 60 min following tetanic stimulation, representing the degree of change at the half-maximal point on the input/output curves compared with baseline responses. Data shown in the figures represent averaged values of responses from continuous monitoring during the experiments. P-values of less than 0.05 were considered statistically significant.
Materials
β-Cyclodextrin sulfobutyl ether (ADVASEP7) was obtained from CyDex (Lenexa, KS, USA). (3α5α)-17-phenylandrost-16-en-3-ol was synthesized as reported previously (Mennerick et al., 2004). All other chemicals were obtained from Sigma Chemical Company (St Louis, MO, USA).
Results
Effects of ethanol on CA1 long-term potentiation
To determine whether neurosteroids modulate the ability of ethanol to inhibit LTP, we initially examined the effects of ethanol in the absence and presence of exogenous 3α5αP in the CA1 region of hippocampal slices from 30-day-old rats. In this preparation, a single 100 Hz × 1 s tetanus reliably induced NMDAR-dependent LTP. We found that ethanol alone inhibited this LTP in a concentration-dependent fashion (Fig. 1), with little effect at 20 mm (+32 ± 8% change in EPSP slopes measured 1 h after the tetanus, n = 5 vs. control; +45 ± 5% change in EPSP slopes, n = 10, P > 0.05) and partial, but significant, block at 40 mm (+19 ± 4% change, n = 8, P < 0.01 by t-test) (Fig. 1). Consistent with our previous studies (Izumi et al., 2005b), complete inhibition of LTP was observed with 60 mm ethanol (−4 ± 2% change in EPSPs, n = 6, P < 0.01).
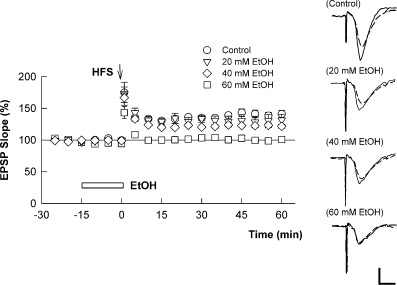
High concentrations of ethanol (EtOH) are required to block long-term potentiation (LTP) acutely under basal conditions in hippocampal slices. The graph shows the time course of change in field excitatory post-synaptic potentials (EPSPs) in control slices (○) and in the presence of 20 mm (), 40 mm (◊) and 60 mm (□) EtOH. A 100 Hz × 1 s high-frequency stimulus (HFS) was administered at time 0 (arrow) and EtOH was administered for the time denoted by the white bar. As reported in the text, significant reductions in LTP were observed with 40 mm EtOH but complete block required 60 mm. Traces to the right show representative EPSPs prior to (dashed lines) and 60 min following (black lines) HFS in the various conditions. Calibration: 1 mV, 5 ms.
A neurosteroid enhances ethanol-mediated long-term potentiation inhibition
To determine whether neurosteroids modulate the effects of ethanol on LTP, we pre-incubated hippocampal slices with 100 nm 3α5αP. For these studies, 3α5αP was administered for 2 h prior to study and continued throughout the recording period. A period of pre-incubation was used to mimic the fact that steroids are normally present in the central nervous system at nanomolar levels (Purdy et al., 1991). At 100 nm, 3α5αP caused no change in basal transmission and did not alter orthodromically induced paired-pulse depression of CA1 population spikes evoked by stimulation of stratum radiatum (Murayama et al., 2006). Furthermore, 3α5αP had no effect on the ability to generate LTP when administered alone at either 100 nm (+39 ± 6% change in EPSPs, n = 5, P > 0.05, Fig. 2A) or 1 µm (+37 ± 4% change, n = 4). In the presence of 100 nm 3α5αP, however, 20 mm ethanol, which had no effect on LTP under control conditions, decreased the magnitude of LTP by more than 50% (+15 ± 4% change in EPSPs, n = 6, P < 0.05 by t-test, Fig. 2B). Similarly, 40 mm ethanol depressed LTP by about 50% in control slices but inhibited LTP completely in the presence of the steroid (−1 ± 4% change, n = 6, P < 0.01 by t-test, Fig. 2C).
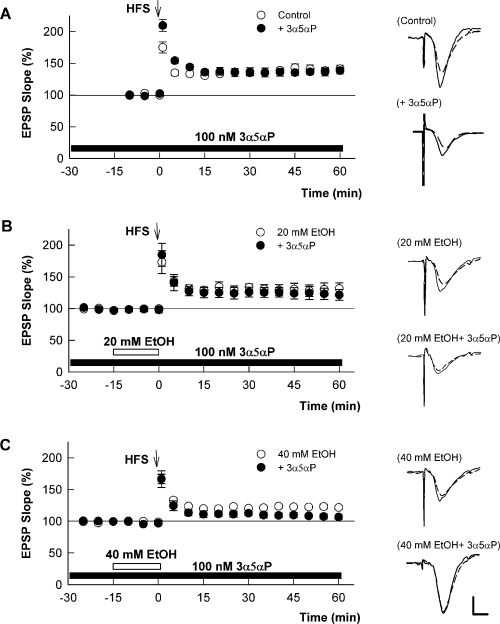
(3α5α)-3-hydroxypregnan-20-one (3α5αP) augments the ability of ethanol (EtOH) to block long-term potentiation (LTP). (A) The graph shows the change in excitatory post-synaptic potentials (EPSPs) in the absence (○) and presence (•) of 100 nm 3α5αP. 3α5αP was administered for 2 h prior to and during the experiments as described in the text (black bar). High-frequency stimulus (HFS) was administered at time 0 (arrow). In the presence of 3α5αP, 20 mm (B) and 40 mm (C) EtOH show enhanced block of LTP (black circles) compared with the effects of EtOH administered in the absence of 3α5αP (white circles). White symbols are the same data as shown in Fig. 1. Traces to the right show representative EPSPs before (dashed lines) and 60 min following (black lines) HFS. Calibration: 1 mV, 5 ms.
The ability of 3α5αP to augment the block of LTP by ethanol cannot be explained by effects on NMDARs because 100 nm 3α5αP alone had no effect on NMDAR-mediated EPSPs (−4 ± 4% change, n = 3) and did not augment the block of NMDAR EPSPs by 60 mm ethanol (−35 ± 13% decrease in NMDAR-mediated EPSPs, n = 4). Based on our previous studies, showing that the effects of ethanol on LTP were overcome by picrotoxin (Izumi et al., 2005b), we also examined interactions between 3α5αP and ethanol on recurrent network inhibition in the CA1 region. Using a paired-pulse stimulation paradigm in which antidromic stimulation of the alveus is paired 15 ms later with orthodromic stimulation of the Schaffer collateral inputs to evoke CA1 population spikes (Petrides et al., 2007), we found that neither 100 nm 3α5αP (−40 ± 10% paired-pulse depression in controls vs. −33 ± 10% depression in 3α5αP, n = 3, P > 0.05) nor 60 mm ethanol (−35 ± 9% depression in controls vs. −41 ± 7% depression in ethanol, n = 4, P > 0.05) altered paired-pulse depression when administered alone. In contrast, ethanol augmented antidromic paired-pulse depression when administered in the presence of 3α5αP (−32 ± 5% depression in 3α5αP vs. −65 ± 8% depression in 3α5αP plus ethanol, n = 5, P < 0.01, Fig. 3A). This antidromically evoked depression was blocked by 1 µm picrotoxin, reducing the effect of ethanol plus 3α5αP to −7 ± 6% depression and indicating the involvement of GABAARs.
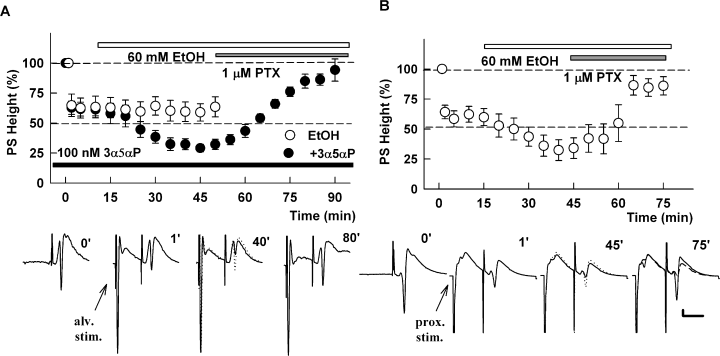
(A) (3α5α)-3-hydroxypregnan-20-one (3α5αP) augments the effects of ethanol (EtOH) on antidromically evoked inhibition of CA1 population spikes (PSs). The graph shows paired-pulse depression of CA1 PSs evoked by stimulation of Schaffer collateral inputs in the presence of 100 nm 3α5αP (black bar) when preceded 15 ms earlier by antidromic stimulation of the alveus (alv. stim.) to evoke recurrent inhibition. The initial point at 100% represents the amplitude of control PSs evoked by a single Schaffer collateral pathway stimulation (trace at time 0 shown below the graph). In the presence of 100 nm 3α5αP (black bar, black circle), 60 mm EtOH (white bar) enhanced this paired-pulse depression and the paired-pulse depression was reversed by 1 µm picrotoxin (PTX) (gray bar). In contrast, EtOH alone had no effect on this form of inhibition (○). Traces below the graph show representative waves at the times indicated. In the third panel the 1′ trace is shown as a dotted trace to highlight the effects of EtOH plus 3α5αP. (B) EtOH enhances paired-pulse inhibition resulting from stimulation of proximal inputs to CA1 (prox. stim.). The graph shows the depression of CA1 PSs following stimulation of proximal inhibitory inputs using a stimulation paradigm in which activation of the proximal inputs was evoked 15 ms prior to stimulation of Schaffer collateral inputs to evoke PS firing. This paired-pulse depression is augmented by 60 mm EtOH (open bar) and reversed to near baseline by 1 µm PTX (gray bar). Traces show representative waveforms obtained at the times indicated. In the 45′ and 75′ traces, the prior trace is shown as a dotted or dashed line to highlight the change. Calibration: 1 mV, 15 ms.
We also examined the effects of ethanol on proximal inhibitory inputs to CA1 pyramidal neurons by stimulating adjacent to the pyramidal cell layer and examining effects on population spikes evoked 15 ms later by Schaffer collateral stimulation (Pearce, 1993). Consistent with a previous report (Weiner et al., 1997), we found that 60 mm ethanol alone enhanced paired-pulse spike depression following activation of proximal inputs (−36 ± 6% paired-pulse depression in control conditions vs. −64 ± 9% depression in the presence of ethanol, n = 6, P < 0.01; Fig. 3B). This form of paired-pulse inhibition and the effects of ethanol were largely reversed by 1 µm picrotoxin, indicating the involvement of GABAARs (−14 ± 8% depression).
Inhibitors of neurosteroids overcome the effects of ethanol on long-term potentiation
As ethanol is known to increase neurosteroid levels acutely in brain slices (Belelli & Herd, 2003; Sanna et al., 2004), we examined the effects of ADVASEP7 (Kay et al., 1999), a sulfobutylated β-cyclodextrin that can act as a molecular sponge to remove steroids from their sites of action (Shu et al., 2004, 2007). At 300 µm, ADVASEP7 alone had no effect on basal CA1 transmission or on LTP induction (+35 ± 3% change in EPSPs, n = 5, Fig. 4A) but significantly diminished the block of LTP by 60 mm ethanol (+29 ± 6% change, n = 5, P < 0.01 compared with ethanol alone, Fig. 4B). The effects of ADVASEP7 on LTP cannot be explained by the cyclodextrin binding and removing ethanol because we found that ADVASEP7 had no effect on the block of synaptic NMDARs by 60 mm ethanol (−36.2 ± 2.1% inhibition of NMDAR EPSPs by 60 mm ethanol in the presence of cyclodextrin, n = 3; no change from the degree of block in the absence of cyclodextrin). We also found that 300 µm ADVASEP7 overcame the effects of 40 mm ethanol plus 100 nm 3α5αP on LTP induction (+40 ± 11% change in EPSPs, n = 6, P < 0.01 vs. 40 mm ethanol plus 3α5αP, Fig. 4C). The combination of ethanol, 3α5αP and ADVASEP7 did result in a depression of basal CA1 transmission that was not observed with any of the agents alone. Despite this depression, a 100 Hz × 1 s high-frequency stimulus resulted in clear LTP over original baseline levels of transmission.
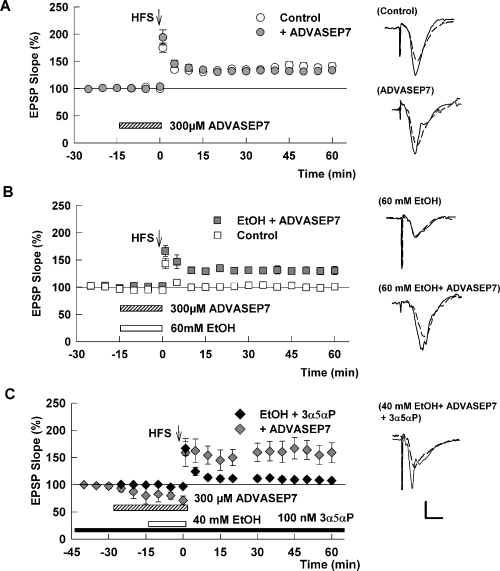
A β-cyclodextrin overcomes the block of long-term potentiation (LTP) by ethanol (EtOH) in the presence of a neurosteroid. (A) The graph shows the change in excitatory post-synaptic potentials (EPSPs) in control slices (○) and slices treated with 300 µmβ-cyclodextrin sulfobutyl ether (ADVASEP7) 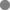 . ADVASEP7 was administered for the duration noted by the bar. White circles showing control LTP are the same data as shown in Fig. 1.
(B) ADVASEP7 significantly reduces the effects of 60 mm EtOH on LTP. In the presence of cyclodextrin (hatched bar), 60 mm EtOH (white bar) fails to inhibit LTP (
. ADVASEP7 was administered for the duration noted by the bar. White circles showing control LTP are the same data as shown in Fig. 1.
(B) ADVASEP7 significantly reduces the effects of 60 mm EtOH on LTP. In the presence of cyclodextrin (hatched bar), 60 mm EtOH (white bar) fails to inhibit LTP ( ). White squares show the effects of 60 mm EtOH alone for comparison and are the same data as shown in Fig. 1.
(C) The graph shows the effects of high-frequency stimulation (HFS) (arrow) administered in slices treated with 100 nm (3α5α)-3-hydroxypregnan-20-one (3α5αP) starting 2 h prior to recording and maintained throughout the experiment (black bar) and subsequently treated with 300 µm ADVASEP7 (hatched bar) and 40 mm EtOH (white bar). In the presence of the cyclodextrin, the combination of EtOH and 3α5αP failed to inhibit LTP, despite a depression of baseline transmission (
). White squares show the effects of 60 mm EtOH alone for comparison and are the same data as shown in Fig. 1.
(C) The graph shows the effects of high-frequency stimulation (HFS) (arrow) administered in slices treated with 100 nm (3α5α)-3-hydroxypregnan-20-one (3α5αP) starting 2 h prior to recording and maintained throughout the experiment (black bar) and subsequently treated with 300 µm ADVASEP7 (hatched bar) and 40 mm EtOH (white bar). In the presence of the cyclodextrin, the combination of EtOH and 3α5αP failed to inhibit LTP, despite a depression of baseline transmission ( ). Black diamonds show the effects of 40 mm EtOH with 3α5αP alone for comparison and are the same data as shown in Fig. 2C. Traces to the right of the graph show representative EPSPs obtained before (dashed line) and 60 min following (black line) HFS. Calibration: 1 mV, 5 ms.
). Black diamonds show the effects of 40 mm EtOH with 3α5αP alone for comparison and are the same data as shown in Fig. 2C. Traces to the right of the graph show representative EPSPs obtained before (dashed line) and 60 min following (black line) HFS. Calibration: 1 mV, 5 ms.
These results indicate that a neurosteroid enhances the effects of ethanol on LTP and that higher concentrations of ethanol probably result in the production of a lipophilic agent that is removed by cyclodextrin treatment. To determine whether this agent is a GABAergic neurosteroid, we examined finasteride, an inhibitor of 5α-reductase, a key enzyme involved in the synthesis of endogenous 3α5αP and other 5α-reduced neurosteroids. When administered at 1 µm, finasteride alone had no significant effect on baseline transmission. However, finasteride overcame the ability of 60 mm ethanol to inhibit LTP (+30 ± 4% change in EPSPs, n = 6, P < 0.01 compared with ethanol alone, Fig. 5A).
(A) A 5α-reductase inhibitor overcomes the effects of 60 mm ethanol (EtOH) on long-term potentiation (LTP). The experimental protocol is similar to other figures. In this case, 1 µm finasteride (hatched bar), an inhibitor of 5α-reduced steroid synthesis, blocked the ability of 60 mm EtOH (white bar) to inhibit LTP (□). High-frequency stimulation (HFS) was delivered at the time denoted by the arrow.
(B) An antagonist of 5α-reduced steroids overcomes the effects of 60 mm EtOH on LTP. In this set of studies, slices were treated with 10 µm (3α5α)-17-phenylandrost-16-en-3-ol (17-PA), a synthetic steroid that has been shown to inhibit the ability of 5α-reduced neurosteroids to potentiate γ-aminobutyric acid-A receptors (Mennerick et al., 2004) (hatched bar) during administration of 60 mm EtOH (white bar). The steroid site antagonist overcame the effects of EtOH on LTP ( ). White squares show the effects of 60 mm EtOH alone for comparison and are the same data as shown in Fig. 1. Traces to the right of the graph show representative excitatory post-synaptic potentials (EPSPs) before (dashed line) and 60 min following (black line) the HFS. Calibration: 1 mV, 5 ms.
). White squares show the effects of 60 mm EtOH alone for comparison and are the same data as shown in Fig. 1. Traces to the right of the graph show representative excitatory post-synaptic potentials (EPSPs) before (dashed line) and 60 min following (black line) the HFS. Calibration: 1 mV, 5 ms.
We also examined the effects of (3α5α)-17-phenylandrost-16-en-3-ol, a synthetic steroid that we have previously shown to act as an antagonist of the potentiation of GABAARs by 5α-reduced neuroactive steroids (Mennerick et al., 2004). At 10 µm, (3α5α)-17-phenylandrost-16-en-3-ol completely overcame the block of LTP produced by 60 mm ethanol (+39 ± 9% change in EPSP slope 60 min after the tetanus, n = 5, P < 0.01 compared with ethanol alone; Fig. 5B).
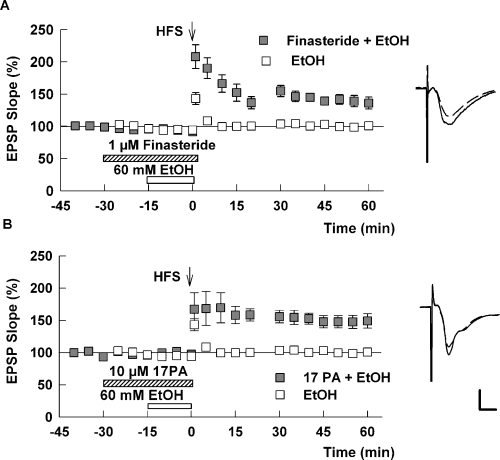
Taken together, these results indicate that ethanol blocks CA1 LTP in a neurosteroid-dependent fashion and that a 5α-reduced neurosteroid augments the effects of ethanol on both CA1 LTP and CA1 network inhibition. As ethanol alone enhances inhibition following stimulation of proximal inputs (Fig. 3), we also examined whether these inhibitory effects are mediated by a 5α-reduced neurosteroid. We found that treatment of slices with 1 µm finasteride both diminished baseline paired-pulse inhibition of CA1 population spikes following proximal stimulation (49 ± 7% inhibition at baseline vs. 7 ± 6% inhibition in the presence of finasteride, P < 0.01, n = 5) and eliminated the enhancement of this inhibition by 60 mm ethanol (8 ± 5% inhibition in the presence of ethanol plus finasteride, P < 0.01 compared with ethanol alone; no difference from finasteride alone; n = 5; Fig. 6A). In contrast to these effects on proximal inhibition, we found that finasteride alone had no effect on recurrent paired-pulse spike inhibition mediated by stimulation of the alveus (Fig. 6B).
A 5α-reduced steroid contributes to the effects of ethanol (EtOH) on proximal inhibition in the CA1 region. (A) Finasteride overcomes the depression produced by activation of proximal CA1 inhibitory inputs and overcomes the enhancement by EtOH. For these studies, stimulation of proximal inhibitory inputs was administered 15 ms prior to stimulation of the Schaffer collaterals. Traces below the graphs show representative waveforms at the times indicated. The initial control responses are shown as dotted traces for comparison. (B) The graph shows the effect of 1 µm finasteride (white bar) on paired-pulse depression of population spike (PS) firing induced by stimulation of alveus 15 ms prior to stimulation of the Schaffer collaterals to evoke spike firing. Although finasteride has no effect on this form of inhibition, 1 µm picrotoxin (PTX) completely reverses the inhibition. Calibration: 1 mV, 15 ms.fa
Discussion
The present studies indicate that, under baseline conditions, block of LTP in hippocampal slices from 30-day-old rats by acute ethanol requires high concentrations of ethanol. Partial LTP inhibition was observed at 40 mm but complete block required 60 mm ethanol. Although these are high concentrations, they are within the range of blood alcohol levels observed during intoxication. Intoxication is typically defined as a blood alcohol level of 0.08–0.1 mg/dL (about 20–25 mm), whereas levels of 60 mm or greater (> 200 mg/dL) represent severe intoxication that is usually associated with marked behavioral and cognitive impairment (White, 2003). When ethanol is administered in the presence of a nanomolar concentration of 3α5αP, however, block of LTP occurs at lower ethanol concentrations, with significant inhibition observed at 20 mm and complete block at 40 mm. Given that neurosteroids are present ambiently at nanomolar levels in the brain (Purdy et al., 1991), this suggests that, under normal conditions, effects on synaptic plasticity and presumably on memory (Martin et al., 2000) are likely to occur at ethanol levels observed in social and clinical settings.
Our results further support the idea that block of LTP by ethanol involves effects on GABAAR-mediated inhibition (Schummers & Browning, 2001; Izumi et al., 2005b). However, because LTP usually requires activation of NMDARs, it is also important to consider the effects of ethanol on these receptors. In our slice preparation, we previously found that inhibition of synaptic NMDARs by 60 mm ethanol overlaps strongly, if not completely, with the block produced by 10 µm ifenprodil (Izumi et al., 2005a,b), a relatively selective inhibitor of NR1/NR2B NMDARs at low micromolar concentrations (Williams, 1993; Priestley et al., 1995). The role of ifenprodil-sensitive NMDAR subtypes in synaptic plasticity is controversial. Some, but not all, studies indicate that selective NR1/NR2B antagonists preferentially block long-term depression but not LTP (Liu et al., 2004; Massey et al., 2004; Izumi et al., 2006; but see Bartlett et al., 2007 and Morishita et al., 2007). LTP, in contrast, probably involves multiple NMDAR subtypes, based on studies using reasonably selective subtype antagonists (Berberich et al., 2005; Weitlauf et al., 2005). In our preparation, we have found that ifenprodil, at low micromolar concentrations, blocks long-term depression but not LTP (Izumi et al., 2006). Thus, it seems likely that the ability of ethanol to alter GABAergic function is primarily responsible for effects on LTP and that effects on NMDARs play a less prominent role.
The effects of ethanol on GABAARs are complex. Some GABAARs, particularly those containing δ-subunits that are expressed at extrasynaptic sites, are enhanced by low millimolar concentrations of ethanol (Wallner et al., 2003; Wei et al., 2004; but see Borghese et al., 2006). δ-Subunit-containing receptors are also highly sensitive to the potentiating actions of 5α-reduced neurosteroids (Wohlfarth et al., 2002; Stell et al., 2003). Other GABAARs, including those expressing γ2 subunits at synapses, are less sensitive to ethanol or not affected at all. Previous studies have demonstrated that ethanol promotes the accumulation of GABAAR-enhancing neurosteroids (Barbaccia, 2004; Sanna et al., 2004) and that these endogenous steroids mediate at least some behavioral and physiological effects of ethanol, including effects on hippocampal neuronal firing (Tokunaga et al., 2003) and spatial memory (Matthews et al., 2002). Sanna et al. (2004) reported that ethanol acutely increases 3α5αP levels in rat hippocampal minces in a concentration- and time-dependent fashion with significant effects at 50 mm ethanol following a 20 min incubation and even greater effects during 30 min incubations. There is also evidence that the presence of relatively low concentrations of 5α-reduced neuroactive steroids facilitates the potentiation of GABAARs by ethanol (Akk & Steinbach, 2003), suggesting that interactions between the two classes of agents may be important. These latter interactions could be important for effects on hippocampal GABAARs that contain α5 subunits and that are important for modulating hippocampal-dependent memory (Collinson et al., 2002) and the amnestic effects of anesthetic drugs (Cheng et al., 2006).
Based on the effects of a cyclodextrin and agents that inhibit either the synthesis of 5α-reduced neurosteroids or the actions of these neurosteroids on GABAARs (Mennerick et al., 2004; Shu et al., 2004), our studies are consistent with the idea that a 15 min incubation with 60 mm ethanol promotes the generation of a 5α-reduced GABAergic neurosteroid and that modulation of GABAARs by a steroid participates in the block of LTP. Furthermore, in the presence of a nanomolar concentration of exogenous 3α5αP, ethanol is more potent in its ability to block LTP, exerting effects at ethanol concentrations that are readily achieved during intoxication. Our studies focused on the role of γ-aminobutyric acid-potentiating neurosteriods in mediating effects of ethanol on LTP. It is also possible that ethanol acutely promotes the generation of other agents, including other neurosteroids that modulate CA1 transmission. Although none of the agents that we used to inhibit neurosteroid effects altered baseline transmission when administered alone, we did observe that a combination of ethanol, exogenous 3α5αP and the cyclodextrin depressed basal transmission in addition to overcoming the inhibition of LTP (Fig. 4C), suggesting that other lipophilic molecules may be generated under certain circumstances.
The ability of ethanol to enhance network inhibition in the CA1 region, like the ability of ethanol to block LTP, is augmented in the presence of 3α5αP (Murayama et al., 2006; present study), although neither ethanol nor 3α5αP alone altered orthodromic paired-pulse depression of population spikes following stimulation of stratum radiatum (Murayama et al., 2006) or antidromic spike depression following stimulation of the alveus (present study). Consistent with previous work (Weiner et al., 1997), however, we found that ethanol alone does enhance γ-aminobutyric acid-mediated inhibition evoked by stimulation of proximal inputs to CA1 pyramidal neurons. Furthermore, we found that this proximal inhibition and its enhancement by ethanol are overcome by finasteride, providing a link between enhanced CA1 network inhibition and LTP block by ethanol. This also suggests that the GABAergic effects of ethanol that contribute to LTP inhibition probably result from actions on a restricted set of inhibitory inputs in CA1. We cannot exclude other inhibitory effects, including changes in tonic conductances, and detailed studies examining tonic and phasic inhibitory conductances will be needed to address these issues.
Our group has previously shown that certain cyclodextrins, including β-cyclodextrins like ADVASEP7, are effective membrane-impermeant molecular sponges that act extracellularly to remove neurosteroids rapidly and greatly accelerate the termination of steroid effects on GABAARs (Shu et al., 2004, 2007; Akk et al., 2005; Murayama et al., 2006). The block of ethanol's inhibition of LTP by the 5α-reductase inhibitor, finasteride, suggests that the acute local production of neurosteroids is facilitated by exposure to high concentrations of ethanol. Recent studies suggest that the source of these neurosteroids may be the principal (pyramidal) neurons in the local CA1 circuit (Agis-Balboa et al., 2006), although contributions from glial cells are also possibly based on the expression of important steroidogenic enzymes (Pelletier et al., 1994; King et al., 2002). Local production of 3α5αP is also consistent with the ability of ethanol to facilitate miniature GABAergic inhibitory post-synaptic currents in CA1 pyramidal neurons (Sanna et al., 2004).
Taken together, our results indicate that 5α-reduced neurosteroids are critical participants in the ability of ethanol to inhibit LTP but not in the ability of ethanol to inhibit NMDARs. To the extent that LTP is a mechanism underlying certain forms of learning, this suggests that treatments that alter neurosteroid actions during acute ethanol intoxication may help to preserve some aspects of cognitive function and enhance memory processing.
Acknowledgements
This work was supported by grants AA12951, GM47969, MH077791 and the Bantly Foundation.
Abbreviations
-
- 3α5αP
-
- (3α5α)-3-hydroxypregnan-20-one (allopregnanolone)
-
- ADVASEP7
-
- β-cyclodextrin sulfobutyl ether
-
- EPSP
-
- excitatory post-synaptic potential
-
- GABAAR
-
- γ-aminobutyric acid-A receptor
-
- LTP
-
- long-term potentiation
-
- NMDAR
-
- N-methyl-d-aspartate receptor.