Group S8A serine proteases, including a novel enzyme cadeprin, induce long-lasting, metabotropic glutamate receptor-dependent synaptic depression in rat hippocampal slices
Present address: Department of Pathology and Laboratory Medicine, Brown University, Providence, RI 02912, USA.
Abstract
Long-term potentiation and long-term depression (LTD) are forms of synaptic plasticity in the central nervous system. We now report that a group of chymotrypsin-like serine proteases, especially members of the S8A subfamily, induce LTD of evoked potentials in rat hippocampal slices. The proteolytic activity of these enzymes is required for the induction of LTD, as serine protease inhibitors prevent the effect. The depression is partly mediated by the suppression of transmitter release from glutamatergic terminals but also involves an elevation of action potential threshold with no change of post-synaptic membrane potential or input resistance. We have also isolated a novel and more potent related enzyme, cadeprin, from Aspergillus. The LTD produced by all of these proteases is not dependent on receptors for several transmitter systems, including N-methyl-d-aspartate or adenosine receptors, but is prevented by blocking group I metabotropic glutamate receptors. The activity of cadeprin, subtilisin and other S8A serine proteases may shed light on the mechanisms of LTD and a related endogenous molecule could have a physiological or pathological role as a modulator of synaptic plasticity in the mammalian hippocampus.
Introduction
Two forms of synaptic plasticity in the hippocampus have received much attention, i.e. long-term potentiation (LTP) and long-term depression (LTD) (Bramham & Srebro, 1987; Ito, 1989; Stanton & Sejnowski, 1989; Artola & Singer, 1993). LTD is a long-lasting, synapse-specific decrease in the strength of synaptic transmission and has been observed in many areas of the brain (Bliss & Lomo, 1973; Bear & Abraham, 1996). Coexisting in the CA1 region of the hippocampus are two mechanistically distinct forms of LTD, which depend upon the activation of N-methyl-d-aspartate (NMDA) (Dudek & Bear, 1992; Mulkey & Malenka, 1992) or metabotropic glutamate receptors (mGluRs) (Bolshakov & Siegelbaum, 1994; Manahan Vaughan, 1997; Oliet et al., 1997; Anwyl, 1999; Bortolotto et al., 1999), respectively. These changes of synaptic function can last for many months after the delivery of an appropriate pattern of stimuli and are thought to underlie some features of learning and memory. There is therefore great interest in these phenomena both as key processes in cognitive function and as targets for drug development in the treatment of memory dysfunction in ageing and cognitive disorders.
The serine proteases comprise a large and extensive family of enzymes with a complex system of classification based on molecular structure, proteolytic activity and selective inhibition (Barrett & Rawlings, 1995; Clements et al., 2004; Rawlings et al., 2006). In a study of enzymatic effects on transmission in the CA1 region of the hippocampus, we now report that subtilisin, a member of the small S8A subfamily of serine proteases, is able to induce LTD. We have also isolated a novel member of the same S8A subfamily from Aspergillus, characterized on the basis of sequence similarities and susceptibility to protease inhibitors. We show that this molecule, like subtilisin, also induces a profound, long-lasting depression of neuronal function and, in view of this activity, we have called the Aspergillus enzyme ‘cadeprin’ (CA1 depressing protein). The activity is independent of NMDA receptors but is mediated by mGluRs.
Materials and methods
Assay of proteolytic activity
The proteolytic activity of enzymes was tested using a modification of the method described by Larcher et al. (1992) for the detection of p-nitroaniline cleavage from Suc-Ala-Ala-Pro-Phe-p-nitroaniline. In brief, 180 µL of enzyme solution was added to 20 µL 5 mm Suc-Ala-Ala-Pro-Phe-p-nitroaniline in a 96-well plate and incubated for 30 min at 37 °C, before the reaction was terminated with 100 µL 2% acetic acid. The substrate concentration was therefore 500 µm in all assays. Absorbances in the 96-well plate were read on an Opsys MR® microplate reader at 405 nm using the Revelation Quicklink Version v4.03 programme (Dynex Technologies Inc., USA). Several serine proteases were tested using this assay over a concentration range of 30 pm−3 mm. The enzymes were prepared in HEPES buffer (containing 25 mm HEPES, 10 µm EDTA and 100 µm dithiothreitol, adjusted to pH 7.0 with 1 m NaOH), except for furin, thrombin, calpain II and thermolysin, which were prepared in 20 mm HEPES buffer containing 100 mm CaCl2.
For the determination of enzyme inhibitory profiles, enzyme solutions were incubated with 20 µL of the inhibitor solution for 30 min at room temperature (20–22 °C) before the addition of 5 mm Suc-Ala-Ala-Pro-Phe-p-nitroaniline and a further 30 min incubation at 37 °C. The effect of the inhibitor was expressed as a percentage of the relevant internal control (enzyme plus inhibitor vehicle prior to substrate incubation).
Electrophysiological examination of long-term depression
Male Wistar rats (100–150 g) were anaesthetized with urethane (1.5 g/kg) and immediately killed by cervical dislocation. The brain was removed into ice-cold artificial cerebrospinal fluid that contained (in mm): 115 NaCl, 2.2 KH2PO4, 2 KCl, 1.2 MgSO4, 25 NaHCO3, 2.5 CaCl2, 10 glucose, gassed with 5% CO2 in O2. The hippocampi were rapidly removed and chopped into 450 µm transverse slices using a McIlwain tissue chopper. The slices were pre-incubated at room temperature for at least 1 h in a water-saturated atmosphere of 5% CO2 in O2 before individual slices were transferred to a 1 mL capacity superfusion chamber for recording. Slices were superfused at 33 °C using artificial cerebrospinal fluid at a flow rate of 3–4 mL/min. A concentric bipolar electrode was used for stimulation of the Schaffer collateral and commissural fibres in stratum radiatum, using stimuli delivered at 0.1 or 0.05 Hz with a pulse width of 50–300 µs, adjusted to evoke a response amplitude approximately 70% of maximum so as to allow increases or decreases in size to be detected. Extracellular recordings were made via glass microelectrodes containing 150 mm NaCl (tip diameter approximately 2 µm, 2–5 MΩ) positioned in the stratum pyramidale or stratum radiatum of the CA1 region. Potentials were digitized and stored on computer via a micro1401 interface (Cambridge Electronic Design). The population spike was also digitized independently and plotted on-line onto a high-frequency chart recorder (DASH IV, Astromed) for the continuous monitoring of changes in spike amplitude and, where necessary, manual measurement of spike amplitude. The population spike amplitude was measured as the voltage difference between the peak negativity and the corresponding time point on a line drawn between the preceding and succeeding positive peaks of the recording. The population excitatory post-synaptic potential (EPSP) recorded in stratum radiatum was measured as the maximum slope of the negative-going arm of the potential. The axonal volley was monitored to ensure that no change of synaptic input occurred during experiments and the perfusion of compounds. Intracellular recordings were made using sharp electrodes pulled from borosilicate fibre-containing glass blanks (Clark Electromedical, Reading, UK) using a vertical Narashige puller. The electrodes were filled with 1 m potassium chloride or acetate (90–120 MΩ). Potentials were amplified via a Neurolog DC amplifier or Axoclamp-2 system in bridge-balance mode. Neuronal excitability was tested using current pulses between 0.02 and 1.0 nA amplitude applied for 300 ms. Responses were digitized at 20 kHz and stored on disc via a micro1401 interface for later analysis. Input resistance was monitored using 300 ms hyperpolarizing pulses between 0.1 and 1 nA amplitude. All aspects of animal care and use were according to the Animals (Scientific Procedures) Act 1986 in the UK, and were approved by the University of Glasgow Research Ethics Committee.
Isolation of a group S8A serine protease from Aspergillus
At each stage of the development of the purification protocol, fractions were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis, tested for their ability to produce LTD and finally tested for proteolytic activity. Fractions that contained LTD activity were further purified until they contained only a single major band by sodium dodecyl sulphate–polyacrylamide gel electrophoresis.
Sample preparation
From a partially purified extract of Aspergillus melleus, 600 mg was suspended in 5 mL HEPES buffer (25 mm HEPES, 10 µm EDTA and 100 µm dithiothreitol, adjusted to pH 7.0 with 1 m NaOH). A cocktail of four protease inhibitors (inhibitor cocktail IV, Calbiochem) was used during the extraction, with broad specificity for the inhibition of aspartic, cysteine, metalloproteases and serine proteases as recommended for fungal and yeast cell extracts. Each vial of stock solution was diluted 1 : 1000 to yield final effective concentrations as follows: 4-(2-aminoethyl)-benzenesulphonyl fluoride, 100 µm; 1,10-phenanthroline, 500 µm; (2S,3S)-3-(N-{(S)-1-[N-(4-guanidinobutyl)carbamoyl]3-methylbutyl}-carbamoyl)-oxirane-2-carboxylic acid, 1.5 µm; and pepstatin A, 2 µm. Non-soluble contaminants in the crude extract were separated from the solubilized preparation by centrifugation at 15 000 g for 10 min at 4 °C. The pellet was discarded and the supernatant was fractionated by ammonium sulphate precipitation. Tests on hippocampal slices indicated that the 45–55% ammonium sulphate precipitate contained a component able to induce LTD, so the active component was provisionally given the name ‘cadeprin’ (CA1 depression-inducing protein).
The resulting pellet was resuspended in HEPES buffer and then desalted using an HR16/10 column (Amersham Pharmacia) packed with Sephadex G25 (column volume ∼25 mL) (volume 3 mL/run at a flow rate of 2 mL/min). Fractions were pooled on the basis of absorption at 280 nm, although absorption at 254 and 322 nm was also monitored and pooled fractions were injected onto two 5 mL HiTrap® Q HP columns (Amersham Biosciences) connected in series. The columns were run at 4 mL/min with HEPES buffer as the start buffer and eluted off with a gradient of NaCl in HEPES buffer. The gradient conditions were: 0–150 mm NaCl over 12 min, 150 mm−1 m over 25 min and then 1 m for a further 4 min. Fractions (1 mL) were collected and the absorbance measured at 280 nm. Individual fractions were tested for their ability to induce LTD on hippocampal slices and active fractions were pooled and concentrated by centrifugal filtration using 15 mL Amicon Ultra 10 000 MW cut-off filters [Millipore (UK) Ltd] at 4000 g. The retentate (0.25 mL) was brought to 10 mL with HEPES buffer, injected onto a Mono Q® HR10/10 column (Amersham Biosciences) and eluted with an NaCl gradient in HEPES buffer. The gradient was 0–150 mm NaCl in 64 min, 150 mm−1 m in 24 min and then 1 m NaCl for a further 12 min. Fractions (1 mL) were collected and absorbance measured at 280 nm. Fractions producing LTD were again pooled and concentrated by centrifugal filtration using Amicon Ultra filters (15 min, 4000 g, 4 °C) and the retentate (0.25 mL) was diluted with triethylamine (25 mm, pH 8.3). The concentrated sample was subjected to chromatofocusing using a Mono P® column (HR5/5, Amersham Biosciences) with starting conditions of 0.5 mL/min start buffer including 25 mm triethylamine, pH 8.3, and 0.3 m iminodiacetic acid. The eluting mixture (Amersham Biotech) contained 3.0 mL polybuffer 96 and 7 mL polybuffer 74, brought to 100 mL with distilled water and adjusted to pH 5.0 with 0.3 m iminodiacetic acid. Fractions eluted off the column at pH < 6 were neutralized with 10% 0.1 m NaOH. The polybuffer was replaced with HEPES buffer by centrifugal filtration through an AmiconUltra filter.
Biochemical and proteomic analysis of cadeprin
Chromatofocusing of the cadeprin samples resulted in two distinct peaks, the main peak at ∼pH 5.5 and a minor peak at ∼pH 6.0. LTD activity was present in the main but not the minor peak. The major and minor peaks were then subjected to both non-denaturing and denaturing (sodium dodecyl sulphate) polyacrylamide gel electrophoresis. The non-denaturing (native) gel was a 5.3% polyacrylamide gel and the denaturing gel was a sodium dodecyl sulphate + 15% polyacrylamide gel. The Coomassie-stained gel was washed for 15 min with Milli-Q water and then destained by repeated washing in a 50 : 50 mixture of ammonium bicarbonate and methyl cyanide. Each gel band was then treated with dithiothreitol (10 µm) in 100 mm NH4HCO3 and left to incubate for 1 h (56 °C), after which the supernatant was removed and treated with 50 µL of fresh 50 mm iodoacetamide in 100 mm NH4HCO3. Bands were digested by adding to each tube 20 µL of 12.5 µg/mL modified trypsin (bovine; Roche sequencing grade) in 20 mm NH4HCO3/0.1% N-octylglucoside. Excess 20 mm NH4HCO3 was added to cover the band pieces before incubation overnight at 30 °C (> 16 h). An equal volume of CH3CN was added to a digest of each gel band and the mixture left to incubate at 30 °C for 30 min on a shaking platform. The digest supernatant was dried in a Speedvac, resuspended in 10 µL of 50% CH3CN in 0.1% trifluoroacetic acid and incubated at 30 °C for 10 min on a shaking platform. The digest was then analysed by a Q-star I-mass spectrometer using AnalystQS software (Applied Biosytems) or, in some runs, by a SELDI-TOF PSII mass spectrometer using MP20 chips and controlled by Proteinchip Software 3.1 (Ciphergen Biosciences Inc.).
Results
Structural identification of cadeprin
The mass spectrometric analysis generated multiple distinct masses. When the best fit sequence for each mass was calculated using the Mascot Matrix System programme and sequences were compared, six continuous sequences were determined to be present, containing 17, 29, 36, 14, 22 and 14 amino acids (total 132 residues), respectively (Table 1). Basic Local Alignment Search Tool (BLAST) analysis of these sequences indicated that cadeprin is closely related to the precursor of alkaline serine protease of A. fumigatus (strain FRR 1266), also known as oryzin, a member of the S8A subfamily of serine proteases. Comparison of the purified sequences and that of oryzin revealed that the cadeprin sequences included the second (H64) and third (S221) catalytic residues. There was 44% coverage of the peptidase unit (299 residues), of which 126/132 (95%) were conserved.
| Sequence no. | Structure | No. of residues | Residues in oryzin |
|---|---|---|---|
| 1 | LTTQKGAPWGLGSISHK | 17 | 123–139 |
| 2 | ASLAYNAVGGQHVDSVG HGTHVAGTIGGE | 29 | 176–204 |
| 3 | AKKANLLSVKVFQGESS STSIILDGFNWAANDIVSK | 36 | 209–244 |
| 4 | SAPNALTVAASTKS | 14 | 296–308 |
| 5 | SAWIGSTTATNTISGTSMATPH | 22 | 333–354 |
| 6 | IKQLATSGVVTDAQ | 14 | 378–391 |
- The residues are identical to the equivalent residues in oryzin of Aspergillus fumigatus except for those indicated in bold font. The residues shown as italic in sequences 2 and 5 indicate the catalytic site.
Investigation into the nature of this protein using a peptidase data base (MEROPS, Rawlings et al., 2006) revealed that it has several names, including allergen Asp fl 1 (A. flavus) and allergen Asp fl 13 (A. flavus and A. fumigatus), in addition to its more common name of oryzin. Oryzin is a serine endopeptidase, in the small S8A subfamily of the Clan SB, with a MEROPS identifier (S08.053) and the designation EC 3.4.21.63 of the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). It is not reported to be present in the human, mouse or rat genomes (MEROPS). Other members of the S8A subfamily include subtilisin A, proteinase K, proprotein convertase 9 (also referred to as neuronal apoptosis-regulated candidate gene) and subtilisin/kexin isoform 1. Members of the S8A subfamily differ from members of the S8B subfamily in both isoelectric properties and substrate specificity (Siezen & Leunissen, 1997; Hedstrom, 2002; Rawlings et al., 2006).
Protease activity
The synthetic compound sucAPPFpNA is a substrate for chymotrypsin-like enzymes (members of either the S1A or S8A subfamilies). Cadeprin showed clear activity against this substrate, with an EC50 of 33 nm (Fig. 1A). Subtilisin A was the most effective of several proteases tested on this substrate, with an EC50 of 3.6 nm (Fig. 1A). These EC50 values were independent of substrate concentration over the range tested of 3–500 µm. Proteinase K (S8A) and α-chymotrypsin (α-CT) (S1A) had approximately equal activity (EC50s of 180 and 130 nm, respectively) (Fig. 1A). In contrast, the non-serine alkaline proteases calpain-2 (a calcium-dependent cysteine protease of the C2 family) and thermolysin (a metalloprotease of the M04 family) (Fig. 1B), and several non-chymotrypsin-like serine proteases including trypsin (S1A), thrombin and furin (S8B), had little effect (Fig. 1B and C). Most of these enzymes had no detectable activity even up to a concentration of 1 µm. Trypsin (S1A) showed some activity at very high concentrations (over 10 µm, EC50 of 35 µm, Fig. 1C). Thermolysin exhibited only partial activity, with a maximum effect of around 30% activity even at 300 µm.

Proteolytic cleavage of substrate. Graphs showing the proteolytic activity of various subgroups of proteases against the substrate Suc-Ala-Ala-Pro-Phe-p-nitroaniline (sucAAPFpNa). The panels show representative enzymes of (A) chymotrypsin-like serine proteases, (B) non-serine proteases and (C) non-chymotrypsin-like serine proteases. The chymotrypsin-like serine proteases are several orders of magnitude more active than other enzymes tested, with subtilisin A and the novel enzyme cadeprin being the most potent.
Enzyme inhibitor effects on proteolytic activity
Subtilisin and α-CT belong to the small S8A subfamily and very extensive S1A subfamilies of serine proteases, respectively. These families are closely related biochemically, making it difficult to achieve a precise classification of enzyme activity using inhibitors. Although there are some chymotrypsin-specific inhibitors, there are none selective for the subtilisin S8A subfamily, and the approach normally used is to test S8 family enzymes with S1-selective and S1-non-selective inhibitors and determine whether a novel enzyme is or is not a member of the SA family based on the inhibitory profile obtained.
Taking this approach, four serine protease inhibitors were used: phenylmethylsulphonyl fluoride (a non-specific protease inhibitor blocking several protease families in clans SA and SB), chymostatin (an inhibitor with selectivity for the SA clan and thus inhibiting subtilisin A and α-CT), N-tosyl-l-phenylalanyl-chloromethyl-ketone (TPCK) (a selective inhibitor of α-CT in S1A) and N-tosyl-l-lysyl-chloromethyl-ketone, a selective inhibitor for trypsin and related enzymes in S1).
Phenylmethylsulphonyl fluoride produced a concentration-dependent inhibition of the proteolytic effects of the four main proteases compared here. The IC50 values against subtilisin A, proteinase K and α-CT were 3, 4.7 and 21 µm, respectively but it showed greatest activity as an inhibitor of cadeprin (IC50 of 2.2 µm) (Fig. 2A).
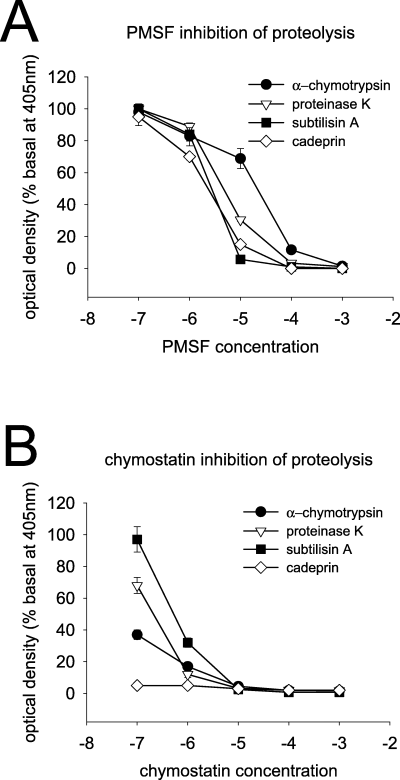
Inhibition of proteolysis. Graphs illustrating proteolysis of the substrate Suc-Ala-Ala-Pro-Phe-p-nitroaniline by four chymotrypsin-like serine proteases and the inhibition of proteolytic activity by phenylmethylsulphonyl fluoride (PMSF) (A) or chymostatin (B). Cadeprin is the most sensitive of these enzymes to inhibition, especially by chymostatin.
Cadeprin was almost fully (94%) inhibited by 100 nm chymostatin (n = 5), the lowest concentration tested, with α-CT being inhibited by 63% by 100 nm (n = 4) (Fig. 2B). Proteinase K and subtilisin A were also inhibited by chymostatin, though less effectively, with EC50 values of 210 and 570 nm, respectively. Both N-tosyl-l-lysyl-chloromethyl-ketone and TPCK failed to inhibit the proteolytic activity of cadeprin, subtilisin A and proteinase K under the experimental conditions used and in the concentration range tested (1 µm−1 mm).
Electrophysiology
As LTD develops slowly, the effects of the enzymes were quantified by measuring the extent of the evoked potential depression either when a maximum response had been induced or at the 60 min time point following the end of superfusion, whichever was the earlier. The time course and major characteristics of the depression induced by a 10 min superfusion of cadeprin (20 nm) are illustrated in Fig. 3. The depression became apparent gradually after beginning enzyme superfusion, increasing over a period of approximately 10–60 min and reaching a plateau at which the original potentials were reduced by 78 ± 4.4% of the initial control size (n = 10 slices). The inhibition persisted for as long as recordings were made. In most cases, the slices were observed for at least 60 min after the application of cadeprin but, in six slices, recordings were followed for up to 4 h with no sign of recovery from the inhibited level. The extent of the depression was concentration-dependent, with the EC50 concentration for cadeprin being calculated as 17 nm (data not shown).
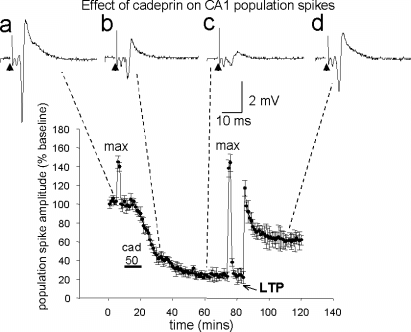
The effect of cadeprin on the amplitude of evoked population field potentials in the CA1 region of hippocampal slices. During the bar below the record, cadeprin was superfused at a concentration of approximately 50 nm (cad). This induced a slow decline in spike amplitude until a new steady state was achieved after about 30 min. The spike amplitudes marked as ‘max’ indicate the maximum size of the evoked population spike recorded before and during cadeprin-induced long-term depression, induced by increasing stimulus strength. The graph also illustrates the result of inducing long-term potentiation (LTP) by a tetanus delivered at 100 Hz for 1 s (LTP). The unchanged maximum spike amplitude and the ability to sustain LTP indicate that cadeprin is not having a toxic action on the slice. The traces (a−d) illustrate representative potentials taken at the times indicated by the dashed lines in an individual experiment, showing absence of any change in the spike volley. The arrowhead indicates the stimulus artefact. Points show the mean ± SEM of 10 slices.
The long-lasting effect of cadeprin was not the result of a toxic effect on the slices. Firstly, the neurones remained capable of a full maximal response. At the start of each experiment, a maximum population potential was obtained and the stimulus strength was then reduced so as to evoke a submaximal response around 70% of the maximum, so that increases or decreases of response size could be detected. Once a stable submaximal evoked potential had been obtained at this reduced stimulus strength, a single test maximal stimulus was delivered before application of cadeprin (‘max’ in Fig. 3). When the same stimulus was presented during the maximal depression of evoked potentials, the maximal response was found to be unchanged from the control period (Fig. 3).
The second evidence for a non-toxic action of cadeprin was that, even when depression of the evoked potentials was fully developed, LTP could still be induced by a standard tetanus of 100 Hz for 1 s (Fig. 3). This LTP was blocked by superfusion of the slices with 2-amino-5-phosphono-pentanoic acid, indicating that it was NMDA receptor dependent (data not shown). Importantly, this also shows that the increased evoked potential amplitude was not merely a reversal of the mGluR-dependent LTD (see below).
α-Chymotrypsin (S1A) and the S8A serine proteases tested electrophysiologically (cadeprin and subtilisin A) were able to induce concentration-dependent LTD. Subtilisin and α-CT were less potent than cadeprin by one and two orders of magnitude, respectively (Fig. 4A and B). Subtilisin induced a concentration-dependent depression of evoked potentials with a plateau inhibition of only 31% at 209 nm (n = 4) and 88% at the maximum concentration tested (349 nm, n = 3) (Fig. 4A). α-CT was much less effective; even at 5 µmα-CT only induced a plateau LTD of 29% at 60 min and concentrations of 16.8 and 168 µm were required to inhibit evoked potentials by >50% and >80%, respectively (Fig. 4B).
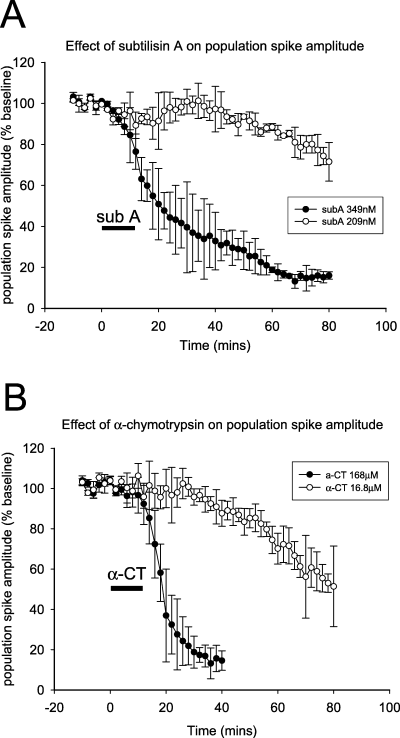
Effect of serine proteases on population spike amplitude. This graph illustrates the long-lasting depression of spike size induced by (A) subtilisin A (subA) and (B) α-chymotrypsin (α-CT) at two of the concentrations tested (for clarity). The time course of depression by both enzymes is similar to that produced by cadeprin (Fig. 3). The graphs illustrate the concentration dependence of the long-term depression (LTD) with a concentration of 349 nm of subtilisin A or 168 µm of α-CT inducing an LTD of at least 80% of the baseline potential size. Symbols show the mean ± SEM.
In contrast, trypsin had little effect on synaptic transmission at concentrations that were not toxic and produced a depression of all electrophysiological parameters, including maximum potential amplitude and the induction of LTP, indicating that it was having a toxic effect on the slice (data not shown).
Excitatory post-synaptic potential/spike coupling
When simultaneous measurements were made of the CA1 population EPSP in stratum radiatum and the population spike amplitude in stratum pyramidale, it was found that the population spike was depressed to a larger extent by cadeprin than the EPSP. The relationship between these two (the EPSP/spike coupling), represented by the ratio of population spike amplitude to EPSP slope, showed a significant decrease from a control ratio of 0.86 ± 0.04 to a value of 0.55 ± 0.12 (P < 0.05, n = 5) measured 30 min after ending the application of cadpeprin (50 nm). This result indicates that cadeprin was reducing the ability of EPSPs to evoke a post-synaptic spike and suggests a post-synaptic site of action This would be consistent with other descriptions of mGluR-dependent LTD (Watabe et al., 2002).
Intracellular recordings
Intracellular recordings from pyramidal neurones in the CA1 stratum pyramidale revealed that cadeprin did not produce any hyperpolarization of the membrane potential that might account for the LTD (Fig. 5A). Little consistent change was seen in the input resistance of the neurones during this time, with three of eight cells showing only a small, progressive increase in resistance in response to the protein. The pooled data from eight cells showed no significant change in membrane input resistance with control values at rest of 41 ± 6.1 and 36 ± 2.1 MΩ (n = 8) at 30 min after protein perfusion (6, 5).
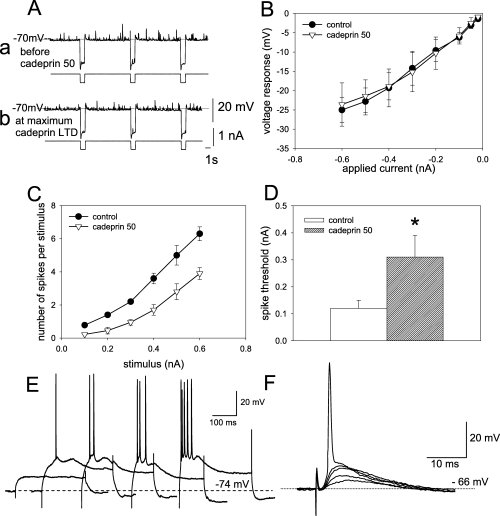
The effects of cadeprin on neuronal properties recorded intracellularly. (A) The absence of effect of cadeprin (50 nm) on resting membrane potential recorded before the application of the enzyme (a) and 60 min after its removal (b) at a time when long-term depression (LTD) was fully established. The records also illustrate the absence of any effect on spontaneous excitatory post-synaptic potentials (the rapid upward deflections on the voltage records) or on membrane input resistance (indicated by the downward deflections resulting from the application of 1 s pulses of hyperpolarizing current delivered as shown in the lower of each pair of traces). Calibration: 20 mV and 1 nA for membrane potential and stimulus amplitude, respectively; time, 1 s. (B) Confirmation of the absence of any change of membrane resistance measured from records such as A, when the current/voltage relationship was examined over a range of current intensities. (C) Evidence for a post-synaptic action of cadeprin was obtained from the change in the number of action potentials evoked by intracellularly applied stimuli of different amplitudes. Symbols indicate mean ± SEM for five slices. All points on the control and cadeprin curves are significantly different at P < 0.05. (D) A post-synaptic action was suggested by evidence that the threshold of cells for spike generation in response to intracellular depolarizing current injection was increased by cadeprin (n = 5, *P < 0.05). (E) Intracellular recordings of a cell responding to stimuli of increasing strength by generating an increased number of spikes. Data from records such as these were used to generate C. Calibrations: 20 mV and 100 ms. (F) Intracellular recordings of a cell responding to stimuli of increasing strength by exhibiting larger amplitude depolarizations, one of which triggers an action potential. Data of spike thresholds from records such as these were used to generate D. Calibration: 20 mV and 10 ms.
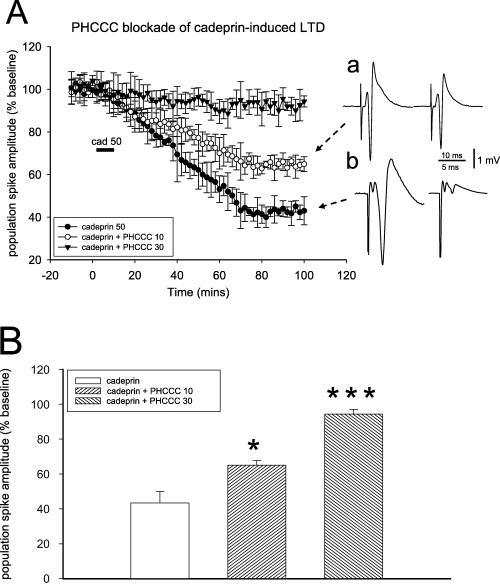
Blockade of cadeprin-induced long-term depression (LTD) by N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide (PHCCC). (A) Graph of the amplitude of evoked population spike potentials in the hippocampal slice, illustrating the long-lasting depression of spike size induced by cadeprin (50 nm) and its antagonism by PHCCC at 10 and 30 µm (n = 4). Points show the mean ± SEM. (a and b) Representative records taken at time points 0 and 100 min after the start of cadeprin perfusion in the presence of PHCCC at 10 µm (a) and 30 µm (b). (B) Quantitative summary of the results obtained with PHCCC by measuring the degree of spike depression 60 min after ending the superfusion of cadeprin. *P < 0.05, ***P < 0.001 (n = 4 slices for each concentration).
The application of intracellular depolarizing current pulses revealed an increase in the threshold for spike initiation, tested at the time of established LTD, 30 min after ending the superfusion of cadeprin. Two protocols were adopted. In the first, the number of spikes generated in response to current pulses of varying intensity was counted during the control period and again 30 min into the establishment of LTD. The resulting plot revealed a significant reduction in the number of spikes per pulse at each stimulus level (Fig. 5C and E). This effect occurred despite no significant change in the membrane potential (controls, 65.4 ± 3.7 mV; cadeprin, 70.1 ± 4.8 mV, n = 10, n.s.) or input resistance (controls, 26.4 ± 6.8 MΩ; cadeprin, 33.2 ± 5.5 MΩ, n = 10, n.s.) of the cells tested. In the second protocol, ramp pulses were applied and the threshold for spike generation interpolated directly from the action potential take-off point on the depolarizing potential record. Superfusion with cadeprin significantly increased the threshold for spike generation (Fig. 5D and F), again despite no change in membrane potential (controls, 63.0 ± 2.8 mV; cadeprin, 66.3 ± 5.8 mV, n = 5, n.s.) or resistance (controls, 22.9 ± 6.5 MΩ; cadeprin, 28.8 ± 4.5 MΩ, n = 5, n.s.).
Receptor mechanisms
The LTD produced by cadeprin was unchanged when experiments were performed in the presence of 2-amino-5-phosphonopentanoic acid (50 µm, a competitive antagonist at NMDA receptors, n = 4), 8-phenyltheophylline (1 µm, an antagonist at A1 and A2 adenosine receptors, n = 4), 1,3-dipropyl-8-cyclopentylxanthine (50 nm, a selective antagonist at adenosine A1 receptors, n = 4), bicuculline methiodide (10 µm, a GABAA receptor antagonist, n = 3), l-nitroarginine-methyl ester (30 µm, an inhibitor of nitric oxide synthase, n = 4), naloxone (10 µm, opiate receptor blocker, n = 3), acetylsalicylic acid (500 µm, n = 5) or indomethacin (50 µm, n = 3) (inhibitors of cyclo-oxygenase), or the protein kinase inhibitor N-(2-aminoethyl)-5-chloro-1-naphthalenesulphonamide (100 nm, n = 4). These experiments rule out any involvement of NMDA, adenosine or GABAA receptors, nitric oxide, cyclo-oxygenase or protein kinases in the LTD.
In contrast, the activity of cadeprin was significantly attenuated by superfusing slices with 10 µmN-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide, an antagonist at group I mGluRs. N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide was able to reduce the magnitude of LTD induced by cadeprin (Fig. 6). In addition, cadeprin-induced LTD could be reversed by superfusion with the selective mGluR5 antagonist 6-methyl-2-(phenylazo)-3-pyridinol (SIB1757) (30 µm for 20 min) (Fig. 7), although the antagonist had no effect itself on baseline spike amplitude. If either N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide or SIB1757 were applied after the ending of protease superfusion, the antagonists induced a transient and partial reversal of the LTD. This is illustrated in Fig. 8 for SIB1757, which reversed LTD to a spike amplitude of 74% of the initial baseline potential (P < 0.05). When either antagonist was removed from the perfusate the LTD was re-established and, in the case of SIB1757, eventually stabilized at an average of 25% of the baseline (n = 4).
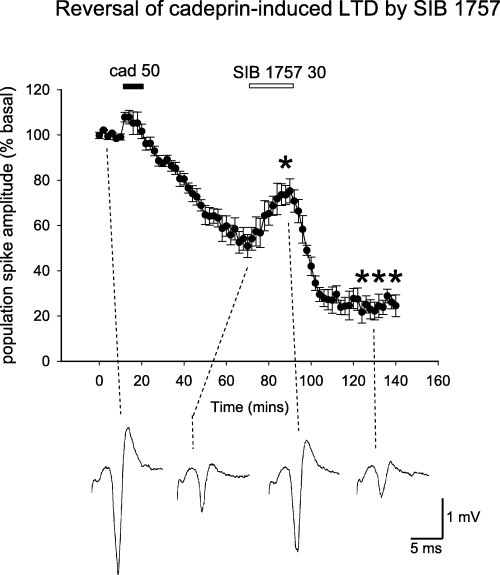
Reversal of cadeprin-induced long-term depression (LTD) by 6-methyl-2-(phenylazo)-3-pyridinol (SIB1757). Graph of the amplitude of evoked population spike potentials in the hippocampal slice, illustrating the long-lasting depression of spike size induced by cadeprin (50 nm) and its reversal by SIB1757 (30 µm). Points show the mean ± SEM. *P < 0.05 between the population spike size immediately before the application of SIB1757 and at the end of its application. ***P < 0.001 between population spike size at the end of the application of SIB1757 and the attainment of maximum LTD. The traces below the graph illustrate representative recordings taken at the time points indicated by the dashed line. Calibration: 1 mV and 5 ms.
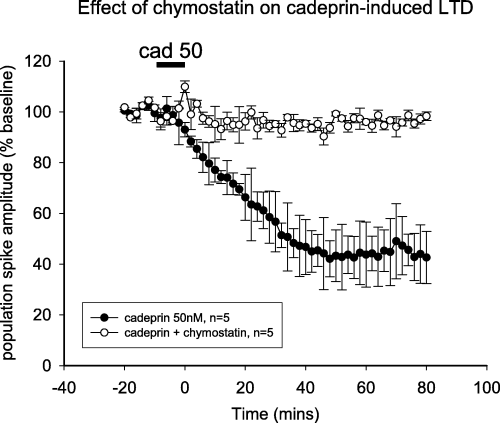
Effect of chymostatin on cadeprin-induced long-term depression (LTD). Graph of the amplitude of evoked population spike potentials in the hippocampal slice and the long-lasting depression induced by cadeprin (50 nm). The depression is prevented by superfusion of the slices with chymostatin (94 nm). Bars show the mean ± SEM for five slices.
Other proteases and inhibitors
The enzyme inhibitors tested against the proteolytic activity of subtilisin A or cadeprin (PMSF, TLCK and TPCK) were also tested against their electrophysiological actions. Subtilisin A or cadeprin were incubated with each of the inhibitors for 30 min before perfusion over the brain slices, in case enzyme inhibition developed too slowly to allow the simultaneous addition of enzyme and inhibitor to the slices. A similar pattern was obtained for the blockade of LTD and of proteolysis; chymostatin (94 nm) (Fig. 8) and phenylmethylsulphonyl fluoride (10 µm) completely inhibited the induction of LTD by cadeprin at concentrations that had abolished it's proteolytic activity. The inhibitors alone had no effect on the population spike amplitude and the inhibitor vehicles failed to attenuate the ability of subtilisin A or cadeprin to induce LTD. This strongly suggests that the same protease activity was responsible for both the proteolytic activity and the electrophysiological induction of LTD in brain slices. Neither TPCK nor TLCK inhibited the induction of LTD, consistent with their failure to prevent the proteolytic activity (see above).
Discussion
Long-term depression is a well-documented phenomenon in the hippocampus and, together with LTP, may reflect long-term aspects of synaptic plasticity relevant to learning and memory (Bramham & Srebro, 1987; Ito, 1989; Stanton & Sejnowski, 1989; Artola & Singer, 1993). Recent reports suggest that LTD may be associated specifically with certain aspects of cognitive function, such as the exploration and familiarization of novel environments and stimuli (Manahan-Vaughan & Braunewell, 1999; Kemp & Manahan-Vaughan, 2004, 2007).
Proteases and long-term depression
There are two major groups of findings in this report. Firstly, LTD can be induced by serine proteases in the small S8A subfamily. Both the proteolytic activity of these enzymes and their induction of LTD are prevented by chymostatin. This compound is recognized as an inhibitor of the actions of α-CT, which was itself able to induce LTD. However, α-CT was far weaker than S8A enzymes and the latter proteases were not inhibited by TPCK, a selective inhibitor of chymotrypsin-like serine proteases in subfamily S1A. Thus, the ability to induce LTD seems to be effectively restricted to chymotrypsin-like serine proteases of the small S8A subfamily, such as subtilisin A, proteinase K and cadeprin.
The net result of applying cadeprin is a substantial and prolonged depression of synaptic transmission at the glutamate-releasing synapses formed by the Schaffer collateral and commissural axons onto CA1 pyramidal neurones. The fact that control responses could be restored by increasing the stimulation current and that NMDA-dependent LTP could be induced after the establishment of LTD indicates that the cells were not damaged in any way by the protease activity. Trypsin, however, induced a long-lasting suppression of hippocampal activity that was accompanied by a loss of responsiveness to increased stimuli and an inability to sustain LTP, suggesting a generalized depression of cellular function possibly of a toxic nature.
The mechanism of the LTD produced by S8 proteases remains uncertain. Changes in paired-pulse inhibition and facilitation are believed to reflect changes of transmitter release from pre-synaptic terminals (Mennerick & Zorumski, 1995; Debanne et al., 1996). There is, however, also a clearly demonstrable post-synaptic component involved in the effect of cadeprin, as the protein induced a significant change of EPSP/spike coupling measured extracellularly and an increase in action potential threshold measured intracellularly. This effect was again not associated with any change in membrane potential or conductance.
This dichotomy of pre- and post-synaptic actions reflects many previous studies of LTD, which can have both pre-synaptic (Bolshakov & Siegelbaum, 1994; Oliet et al., 1997; Fitzjohn et al., 2001; Watabe et al., 2002; Nosyreva & Huber, 2005) and post-synaptic (Otani & Connor, 1998; Nosyreva & Huber, 2005) components. The locus of initial events, such as calcium influx, leading to LTD induction is generally considered to be post-synaptic, whereas expression is both pre- and post-synaptic (Mulkey & Malenka, 1992). The release of calcium from both pre- and post-synaptic stores is required (Mulkey & Malenka, 1992; Reyes Harde & Stanton, 1996), with a rise in post-synaptic calcium being considered necessary for the induction of LTD (Mulkey & Malenka, 1992; Bolshakov & Siegelbaum, 1994; Neveu & Zucker, 1996; Oliet et al., 1997; Kemp & Bashir, 1999; Huber et al., 2000; Fitzjohn et al., 2001). Interestingly, cadeprin-induced LTD was dependent on electrical stimulation of the afferent Schaffer collateral and commissural pathway (our unpublished observations). This could reflect the need for a particular stimulus-dependent process, such as activation of T-type calcium channels as suggested by Oliet et al. (1997).
Several reports of LTD have indicated the possible involvement of a retrograde messenger, such as nitric oxide (Reyes Harde et al., 1999; Izumi & Zorumski, 1993; Gage et al., 1997). In the present study, the selective nitric oxide synthase inhibitor Nω-nitro-l-arginine did not affect cadeprin-induced LTD, eliminating this as a possible mechanism.
Our pharmacological analysis excludes the involvement of several major neurotransmitter and transduction systems, including NMDA receptors, which are known to mediate some forms of LTD. The blockade or temporary reversal of cadeprin-induced LTD by the mGluR1 receptor antagonist N-phenyl-7-(hydroxyimino)cyclopropa[b]chromen-1a-carboxamide suggests a role for group I mGluRs but these include both mGlu1 and mGlu5 subtypes. In the present work, LTD was also reversed by SIB1757 (30 µm), a selective mGluR5 antagonist (Varney et al., 1999; Bonsi et al., 2007), strongly suggesting a dominant role for this subtype.
Group I mGluRs are mostly found post-synaptically (Lujan et al., 1997), where they are known to initiate the release of calcium from post-synaptic endosomal stores and activate protein kinase C in the post-synaptic expression of LTD (Schnabel et al., 1999) but they have also been observed pre-synaptically (Romano et al., 1995). Of the two subtypes of group I receptors, mGluR5 is especially enriched in the post-synaptic CA1 pyramidal neurones, whereas mGluR1 expression is low and diffuse (Lujan et al., 1997; Shigemoto et al., 1997). The application of group I mGluR agonists has previously been shown to induce LTD in the CA1 region of the adult and juvenile rat hippocampus by activation of mGluR1 and mGluR5 (Ito et al., 1992; Palmer et al., 1997; Huber et al., 2000, 2001). The expression of agonist-induced mGluR LTD is mediated by the endocytosis and persistent decrease in the surface expression of post-synaptic 3-hydroxy-5-methylisoxazole-4-propionic acid receptors (Snyder et al., 2001; Xiao et al., 2001).
Metabotropic glutamate receptors are known to be involved in the induction of LTD that can be induced by electrical stimulation under specific conditions (Bolshakov & Siegelbaum, 1994; Manahan Vaughan, 1997; Oliet et al., 1997; Anwyl, 1999; Bortolotto et al., 1999). Indeed, mGluR LTD has been reported to be absent in mGluR5 knockout mice, suggesting a major requirement for this subtype for either the induction or expression of LTD (Huber et al., 2000, 2001; Faas et al., 2002).
The involvement of mGluRs in serine protease-induced LTD may indicate one of two main possibilities. S8A protease activity may generate a secondary molecule that then activates the metabotropic receptors. Alternatively, the proteases may themselves activate the metabotropic receptors directly. It is very unlikely that the proteases act by releasing glutamate itself onto receptors as this would be expected to yield a mixture of effects including both LTP and LTD, whereas we have only ever observed LTD with chymotrypsin-like proteases.
Cadeprin
The second major aspect of this report is that we have added a novel enzyme to the small S8A subfamily of serine proteases, with the isolation of cadeprin from A. melleus. Sequence analysis shows the very close similarity between cadeprin and oryzin isolated from A. fumigatus, raising the possibility that cadeprin may be the functional equivalent of oryzin in A. melleus. Comparison of the six peptide sequences generated in this study with the reported sequences of neuropsin and serpin B6 indicates no sequence homology, indicating that cadeprin is not related to these two proteins that are involved in the generation of LTP. The presence in Aspergillus of a cerebrally active protease may explain the very high (> 90%) mortality rate associated with central nervous system aspergillosis in humans or animals (Guppy et al., 1998; Schwartz & Thiel, 2003; Tattevin et al., 2004) and an animal model (Chiller et al., 2002).
In addition to the novelty of cadeprin, however, it is interesting to note that it is far more potent than subtilisin in producing LTD, with an EC50 of around 17 nm compared with 300 nm for subtilisin A. Molecules of this potency frequently prove to have a similar endogenous molecule and a search for related compounds in the mammalian brain might be rewarding.
The S8 family is subdivided into two subfamilies based on sequence homology. The S8A subfamily, of which subtilisin A is the most prominent member, are alkaline proteases. The S8B subfamily, of which kexin is representative, are acidic proteases, cleaving at carboxyterminal residues. The S8B subfamily contains the majority of the known proprotein convertases, many of which are secreted proteases. The biological role of many of these is largely unknown, although some members can, for example, metabolize opioid peptides (Breslin et al., 1993; Peinado et al., 2003). Again based on sequence homology, the S8A subfamily is itself considered to include five subgroups, the subtilisin, thermitase, proteinase K, lantibiotic and pyrolysin groups. Oryzin and cadeprin belong to the proteinase K subgroup.
Tissue plasminogen activator, thrombin and neuropsin are all serine proteases that have been reported to have a role in synaptic plasticity and especially LTP. Although they are all members of the S1A subfamily, they are not chymotrypsin-like and have different substrate and inhibitor specificity; chymostatin is only a weak inhibitor of tissue plasminogen activator. Tissue plasminogen activator is able to influence LTP by a variety of means, including acting to increase levels of cAMP, proteolysis of the NMDA receptor and proteolysis of plasminogen that results in plasmin-induced degradation of laminin. Thrombin acts via protease-activated receptor-1, which leads to an increased protein kinase C activation and a reduction of the Mg2+ blockade of the NMDA receptor, leading to LTP (Turgeon & Houenou, 1997). Neuropsin (also known as brain serine protease 1 or human kallikrein 8) does not appear to act directly on any receptor to induce LTP, although it does act on the extracellular matrix and fibronectin is reported to be one of its targets (Tani et al., 2001). The absence of this particular protease seems to increase susceptibility to seizure activity (Davies et al., 2001), further emphasizing the possible importance of serine proteases in the regulation of neuronal excitability.
In summary, the present results report the discovery of cadeprin, a serine protease related to known members of the S8A subtilase subfamily and with a high probability that it is closely related to oryzin. Cadeprin and other members of the S8A subfamily can induce LTD in the hippocampal CA1 region, probably by the proteolysis of a substrate that is sensitive to this family of enzymes. As abnormalities of synaptic plasticity may underlie the cognitive decline of ageing and neurodegenerative disease, it might be valuable to develop inhibitors to probe the biological and possible pathological roles of these enzymes or endogenous counterparts or as potential therapeutic agents.
Acknowledgements
We are grateful to Dr Fiona Ross and Dr E. Martin O'Kane for some of the preliminary experiments on this project. We are also grateful to Scottish Enterprise (Proof of Concept) and The Cunningham Trust for financial support.
Abbreviations
-
- α-CT
-
- α-chymotrypsin
-
- EPSP
-
- excitatory post-synaptic potential
-
- LTD
-
- long-term depression
-
- LTP
-
- long-term potentiation
-
- mGluR
-
- metabotropic glutamate receptor
-
- NMDA
-
- N-methyl-d-aspartate
-
- SIB1757
-
- 6-methyl-2-(phenylazo)-3-pyridinol
-
- TPCK
-
- N-tosyl-l-phenylalanyl-chloromethyl-ketone.