Disruption of maternal behaviour by acute conspecific interaction induces selective activation of the lateral periaqueductal grey
Abstract
Maternal behaviour is sensitive to stress and opioidergic activation. The periaqueductal grey (PAG) is involved in coping strategies to stress, whereas morphine inhibition of maternal behaviour depends on the activation of the PAG. The aim of this study was to investigate whether the PAG is activated by disrupting maternal behaviour. Lactating Wistar rats were assigned to four groups: C (control); E1 (acute exposure to a male rat); E2 (daily 2-h exposure to another lactating female and a male rat from Day 3 to 6 of lactation); and E1 + 2 (treated first as E2 and, on Day 9, as E1). Maternal behaviour was recorded on Day 9 of lactation and analysed for 1 h. The E1 group spent more time retrieving their pups, took longer to initiate nursing, had shorter nursing bouts and spent more time in non-maternal activities compared with control. Rats submitted to E2 or E1 + 2 did not differ from the control. In another experiment, lactating rats were treated as above, except that 90 min after the end of the observation period the rats were killed and their brains were processed for immunohistochemical detection of Fos protein in the PAG. Fos increased in the lateral PAG only in the E1 group. We also observed that neurons activated by acute conspecific interaction in the PAG could be responsible for an opioid-dependent decrease in maternal behaviour as this effect was reversed by a microinjection of naltrexone, nor-binaltorphimine or naloxonazine into the lateral PAG. Chronic conspecific interaction alters the way this circuitry responds to acute conspecific interaction.
Introduction
Maternal behaviour encompasses a series of stereotypical behaviours that range from direct interaction of the mother with the litter (retrieval, crouching, grooming of the pups, nursing) to behaviours aimed at protecting the litter (aggressive behaviour towards intruders, nest building; Numan, 1994). Maternal behaviour can, however, be disrupted if the dam is exposed to stressful events during lactation (Leonhardt et al., 2007). Also, chronic stress during pregnancy decreases maternal aggressiveness in pregnant mice (Pardon et al., 2000) and reduces maternal care in rats (Champagne & Meaney, 2006). Lactating rats briefly confronted with an unfamiliar male rat show delayed retrieval of the pups and take longer to initiate nursing (Medeiros, Pavesi & Terenzi, unpublished observations). Several brain regions have been shown to be involved in regulating maternal behaviour, for example, the medial preoptic area (Stack et al., 2002), the bed nuclei of the stria terminalis (Numan & Numan, 1996; Housham et al., 1997), the amygdala (Wartella et al., 2003) and the periaqueductal grey (PAG). Lactating rats show increased expression of Fos protein in the PAG when allowed to interact with their pups (Lonstein et al., 1998b). Suckling is also a potent stimulus for Fos expression in the lateral and ventrolateral PAG, areas that are important for the correct posture during nursing (crouching; Lonstein et al., 1998a) and pup retrieval to the nest (Lonstein & Stern, 1998).
Neurons in the medial preoptic area and the bed nuclei of the stria terminalis that express Fos in female rats when they are actively caring for their pups have been shown to project to the PAG (Numan & Numan, 1997). This reinforces the involvement of the PAG in pup maternal care (Lonstein & Stern, 1998). Miranda-Paiva et al. (2003) have shown that pregnant rats injected with morphine intraperitoneal (i.p.) present activation of the rostral-lateral PAG correlated with inhibition of maternal behaviour later in lactation. The decrease in maternal behaviour is blocked in these animals by unilateral microinjection of naloxone into the lateral PAG. Therefore, considering that the PAG is involved in stress-coping strategies (Keay & Bandler, 2001) and normal maternal behaviour, the studies here reported aimed to determine whether the inhibition of maternal behaviour caused by a form of stress (conspecific interaction) would also involve the activation of the PAG. For this purpose, two models of conspecific interaction were used (acute and chronic) in lactating rats. Immunohistochemical detection of Fos protein was performed in order to determine which areas within the PAG are likely to be involved in these responses. Finally, another aim was to attempt to block or reduce any conspecific interaction-induced disruption of maternal behaviour by microinjecting opioid antagonists into those areas of the PAG activated by conspecific interaction.
Materials and methods
Behavioural protocols
Twenty female (180–220 g) and 10 male Wistar rats (280–320 g) were used. All animals were acquired from the University (UFSC) Animal House and kept in the Department of Physiological Sciences during the experiments. All animals were maintained under a 12 h light : dark cycle (lights on at 7:00 h), at 22–24 °C, and had free access to food and water. All experiments were performed from 10:00 to 16:00 h. Animals were treated in accordance with the guidelines of the Committee on Animals of the Colégio Brasileiro de Experimentação Animal (COBEA, Brazil). The experimental protocols had been previously approved by the Federal University of Santa Catarina (UFSC) Ethics Committee for the Use of Animals (CEUA). The female rats were mated with experienced male rats and placed in an individual cage when sperm was noticed in their vaginal smears. The day of parturition was considered to be Day 1 of lactation. On the second day of lactation, the litters were adjusted to eight pups each. On the day prior to the experiment, the pups were separated from the dams for a period of 24 h. They were placed in a clean Tupperware box, containing woodshavings and paper towel as bedding. In order to prevent the mothers from smelling or hearing their litters, the pups were removed to a room adjacent to the one where the dams were kept. Four experimental groups were used (n = 5 female rats on each): C = control group (maternal behaviour was monitored in a clean cage for 1 h on Day 9 of lactation); E1 = acute conspecific interaction (maternal behaviour was monitored in a clean cage, for 1 h on Day 9, in the presence of an unfamiliar male rat that was separated from the dam and litter by a wire mesh), this model was used previously in our lab (Medeiros, Pavesi & Terenzi, unpublished observations) and shown to disrupt maternal behaviour; E2 = chronic conspecific interaction (the rats were transferred to a clean cage for a 2-h period from Day 3 to 8 in the presence of other female and male rats; the male rat was separated from the females by a wire mesh and its space was gradually increased, whereas the space for the females was proportionally diminished), this model was based on the social instability model of stress, which has been shown to cause higher corticosterone release in females than in males (Haller et al., 1998). After this period of social instability, the animals were returned to their pups. On Day 9, maternal behaviour was monitored in a clean cage as in C; E1 + 2 = acute conspecific interaction in rats previously submitted to chronic conspecific interaction (these animals were treated as the E2 group, on Day 9 lactation they were exposed to an unfamiliar male as in E1). This group was planned to determine whether a period of social instability would affect the response to an acute stressor.
On the day of the experiment (Day 9 of lactation), the pups were returned to the mother's cage, spread as widely as possible, and the dams reunited to the litter to begin the 1-h monitoring of maternal behaviour that would typically start with pup retrieval and nest building. The following parameters of maternal behaviour were measured: time course of pup retrieval (eight pups); latency to onset of the first nursing bout; number of pups at start of nursing; total time of nursing; time spent in other maternal behaviours (pup grooming/licking, nest building); time spent in non-maternal behaviours (cage exploration, self-grooming). The experiments were filmed onto VHS tapes, and the behaviour analysed and quantified using the Etholog 2.25 freeware.
Immunohistochemistry
Ninety minutes after the end of the 1-h observation of maternal behaviour, all dams were overdosed with sodium thiopental (40 mg/kg, i.p., Abbott). Their chests were opened for transcardial perfusion with phosphate-buffered saline (PBS; pH 7.4, 0.1 m) followed by 4% paraformaldehyde in PBS (pH 7.4, 0.1 m). The brains were removed and kept overnight in the same fixative, and transferred to a 30% sucrose solution for cryoprotection for a minimum of 24 h. The brains were sectioned on a cryostat (Leica CM 3050). Two series of six mesencephalic 40-µm sections through the PAG were collected from each animal, cut caudal to rostral. The sections were collected from the level of −8.0 mm caudal to bregma (recognized by the caudal end of the decussation of the superior cerebellar peduncle) up to the level of −5.6 mm caudal to bregma (between the level of the superior colliculus/posterior commissure and the caudal end of the medial geniculate nucleus). The first section that contained the decussation of the superior cerebellar peduncle, and every 10th section after the first, were immunostained for Fos protein. Adjacent sections to those above were stained with thionine (Nissl). The Nissl-stained sections were delineated by comparison to the atlas of Paxinos & Watson (1998). Fos counts were performed on the immunostained sections. Unused sections were discarded. The sections in the immunostained set were incubated for 12 h with 1 : 1000 dilution of anti-Fos primary antibody (rabbit IgG, Santa Cruz Biotechnology) in 0.1 m PBS with 0.3% Triton X-100. After three PBS washes (10 min each), the sections were incubated with a biotinylated secondary antibody (anti-rabbit IgG, H+L, Jackson ImmunoResearch) for 60 min. After three more 10-min washes in PBS, another incubation with the avidin-biotin complex (ABC Elite, Vector Laboratories) was done. The reagents were washed with PBS and the reaction product visualized with 0.05% diaminobenzidine (DAB) + 0.01% hydrogen peroxide in PBS. Five minutes later, the sections were once again washed to stop the reaction and mounted onto gelatinized slides and left to dry at room temperature for 48 h. After this period, the sections were further stained with osmium tetroxide for enhanced contrast as follows: the slides with the sections were washed in distilled water for 1 min, and briefly immersed in a OsO4 solution (0.05% for 20–60 s as required), washed again in distilled water (1 min) and dehydrated (standard alcohol and xylol washes). Finally, the slides were left to dry at room temperature and coverslipped with DPX. A set of adjacent sections, not immunoreacted, was mounted and stained with thionine for comparison and identification of the Fos labelling within the subdivisions of the PAG.
The PAG region was subdivided as described by Bandler et al. (1991) and Comoli et al. (2003): dorsal, lateral and ventrolateral. The divisions were identified as described in the atlas of Paxinos & Watson (1998). All Fos-stained neurons were counted in each subdivision (bilaterally) of the six immunostained sections. A cell was considered positive for Fos-immunoreactivity when presenting a well-defined oval or round nucleus 8–15 µm in diameter at × 10 magnification, and showing the characteristic dark brown stain of DAB. Nuclei counts were done using a Nikon Eclipse E600 microscope with a camera lucida attachment.
Microinjection of opioid antagonists into the PAG
A group of 61 female rats was anaesthetized on Day 14–15 of gestation with sodium thiopental, 35 mg/kg i.p. (Thionembutal, Abbott Laboratories) and xylazine, 10 mg/kg intramuscular (i.m.; Virbaxil, Virbac Laboratories.). They were then placed in a stereotaxic frame (Insight Equip., Brazil) to implant a steel cannula (G25, 12 mm length) 3 mm above the lateral PAG, using the coordinates described by Paxinos & Watson (1998) at an angle: anterior/posterior plane, −6.0 mm from bregma, lateral plane, 1.5 mm and ventral plane, 2.7 mm. The cannula was introduced at an angle of 10° to the right of the midline to avoid perforating the sagittal sinus. The cannula was secured with polyacrylic cement, and this cement was anchored to the skull with stainless steel screws. After parturition, 30 of the dams were submitted to the acute conspecific interaction protocol (E1s, n = 8 for saline control microinjection; E1n, n = 7 for naltrexone; E1b, n = 7 for nor-binaltorphimine; E1z, n = 8 for naloxonazine), as reported in the section ‘Conspecific interaction disruption of maternal behaviour’, and 31 were not stressed (Cs, n = 8 for saline control microinjection; Cn, n = 7 for naltrexone; Cb, n = 8 for nor-binaltorphimine; Cz, n = 8 for naloxonazine).
Both groups of rats (stressed and not stressed) were randomly assigned to be microinjected with one of three opioid antagonists: non-selective naltrexone (Sigma), 2.5 µg/0.5 µL (12 mmol/L); κ-selective nor-binaltorphimine (Tocris), 4 µg/0.5 µL (10 mmol/L); µ-selective naloxonazine (Tocris), 5 µg/0.5 µL (13 mmol/L) or saline (0.5 µL NaCl 0.9%) as a control. The animals were gently held during the microinjection, which was performed introducing a 15-mm injector needle (G30) into the guide cannula. This was connected to a 5-µL Hamilton syringe by a polythene tube (PE10). The microinjection lasted 30 s, and the needle was held in place for another 30 s before withdrawal. Behavioural observations were begun 10 min after the microinjection. The behavioural tests are described at the start of the Materials and methods. At the end of the tests, all dams were killed with an overdose of thiopental and perfused transcardially with saline followed by formaline 10%. The brains were removed and the position of the injection needle checked in 100-µm fixed tissue sections.
Statistical analysis
Two-way anova, followed by Duncan's post hoc test (Statistica 5.0) was used to detect differences between treatments. The progress of pup retrieval was analysed with the repeated measures two-way anova, followed by Duncan's. When P ≤ 0.05 was obtained, the difference between the compared values was taken as significant.
Results
Conspecific interaction disruption of maternal behaviour
After a 24-h separation period, reuniting the dam to her litter induced very clear expression of maternal behaviour. The female rats started by retrieving their pups to a nest area, concomitantly, the nest area was improved with the addition of woodshavings and when most of the pups were in the nest, the female crouched over the litter to start nursing. Control (kept away from other adult rats) animals spent 4.0 ± 2.6 min (mean ± SEM) to retrieve the eight pups. Rats that were exposed to an unfamiliar male during the observation period (acute conspecific interaction, E1) took much longer to complete retrieval (F3,14 = 7.30, P = 0.003, Duncan's post hoc: P = 0.002; Fig. 1). This group took 14.5 ± 6.9 min to gather all pups to the nest. Animals submitted to chronic conspecific interaction (E2) or a double procedure (acute + chronic, E1 + 2) did not show any alteration in pup retrieval compared with control animals. As this was true for all the other parameters observed, the following description will only deal with the control and acute conspecific interaction (E1) animals. However, the figures show all the data from the four groups.
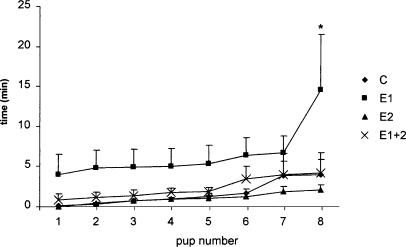
Progress of pup retrieval under different conspecific interaction protocols. Dams were submitted to acute (E1), chronic (E2) or both (E1 + 2) conspecific interaction. Group C are control not exposed rats. Each point shows the mean of five animals. *Indicates a difference (P = 0.003, repeated measures anova) between E1 and C.
At the start of the first nursing bout, control rats had 6.0 ± 0.3 pups in the nest, from a maximum of eight. There was no significant difference in the number of pups retrieved by the time of onset of nursing between the control and any of the experimental groups (F3,14 = 0.66, P = 0.58). The lactators initiated nursing behaviour by crouching over the pups and becoming almost immobile for short periods of time in which the pups were seen to be suckling. Control females started nursing 3.2 ± 0.5 min after the beginning of the experiment. Female rats of the E1 group were slower (7.8 ± 2.4 min, Duncan's post hoc: P = 0.02) than controls (F3,14 = 3.08, P = 0.05; Fig. 2A).
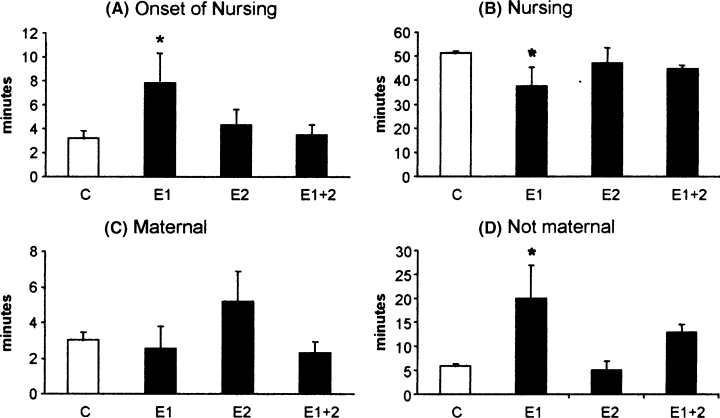
Effect of conspecific interaction on the onset of nursing (A), time spent in nursing position (B), time spent in maternal behaviours other than nursing (C) and time spent in non-maternal behaviours (D). Not exposed controls (C), acute conspecific interaction (E1), chronic conspecific interaction (E2), and acute and chronic conspecific interaction (E1 + 2). Each bar represents the mean ± SEM. *Indicates a difference (P < 0.05) between E1 and C.
Control rats spent a total of 51.1 ± 0.6 min crouching over the litter (nursing), whereas the E1 group spent less time nursing (37.4 ± 7.5 min, F3,14 = 2.24, Duncan's post hoc: P = 0.03; Fig. 2B). Maternal behaviour other than nursing (grooming, nest building, pup gathering after the onset of nursing) was 2.9 ± 0.4 min. No conspecific interaction protocol caused changes in this parameter (F3,14 = 1.03, P = 0.40; Fig. 2C). Non-maternal behaviour (cage exploration, self-grooming) in control rats was 5.8 ± 0.2 min. E1 rats spent longer than controls (F3,14 = 6.05, P = 0.007, Duncan's post hoc: P = 0.003). These rats spent 19.9 ± 6.8 min in non-maternal behaviours (Fig. 2D).
Fos expression induced by conspecific interaction in maternal rats
Immunohistochemical detection of Fos protein showed a homogeneous pattern of distribution of positive perikarya within the PAG subdivisions in control (C group) rats 3-5). However, lactators submitted to exposure to a strange male (E1 group) showed increased density of Fos-positive nuclei in the lateral PAG, at all levels of its rostral-caudal extent (F3,10 = 10.33, P < 0.002). The number of Fos-positive neurons is shown in Fig. 3 (bregma −5.6 = C: 97.7 ± 16.3 vs E1: 165.0 ± 19.1, P = 0.02; bregma −6.0 = C: 66.2 ± 6.5 vs E1: 210.5 ± 32.4, P = 0.0003; bregma −6.4 = C: 65.2 ± 5.8 vs E1: 178.5 ± 14.5, P = 0.0001; bregma −6.8 = C: 69.0 ± 3.9 vs E1: 171.0 ± 25.7, P = 0.0004; bregma −7.2 = C: 70.7 ± 7.7 vs E1: 176.2 ± 20.0, P = 0.0002; bregma −7.6 = C: 70.7 ± 5.6 vs E1: 167.2 ± 10.2, P = 0.0001).
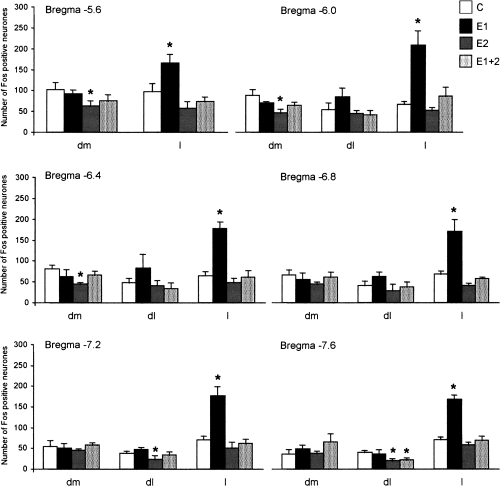
Effect of acute (E1), chronic (E2) or both (E1 + 2) conspecific interaction protocols on Fos expression in the PAG (dl, dorsolateral; dm, dorsomedial; l, lateral subdivision). Not exposed controls (C, n = 5), acute conspecific interaction (E1, n = 5), chronic conspecific interaction (E2, n = 5), and acute and chronic conspecific interaction (E1 + 2, n = 5). Fos expression was compared separately within each subdivision at each bregma level. Each bar represents the mean ± SEM. *Indicates a difference (P < 0.01) compared with C.
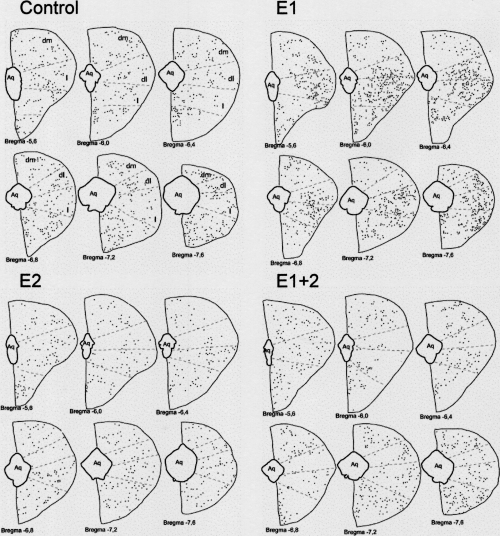
Camera lucida diagrams showing the localization of Fos-stained nuclei in not exposed control, acute (E1), chronic (E2) or both (E1 + 2) conspecific interaction protocols. Each set of six rostro-caudal bregma levels shows the result of one animal. Bregma levels and PAG subdivisions are based on the atlas of Paxinos & Watson (1998). Aq, aqueduct; dl, dorsolateral; dm, dorsomedial; l, lateral.
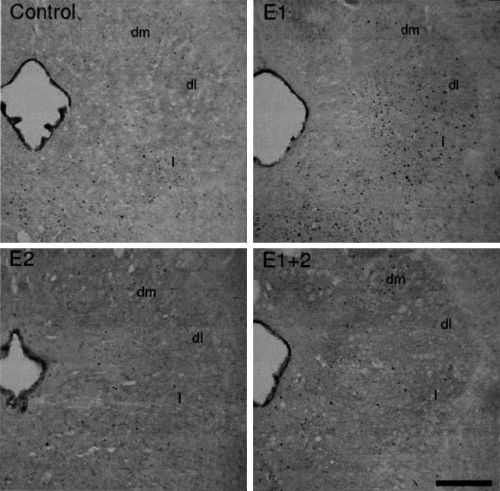
Bright-field photomicrographs from the PAG (at level −6.4 mm caudal to bregma) of four rats submitted to different conspecific interaction protocols [not exposed control, acute (E1), chronic (E2) or both (E1 + 2)]. The sections show the Fos protein-immunostained nuclei as black round-shaped dots, and were counterstained with osmium tetroxide. Scale bar: 300 µm. dl, dorsolateral; dm, dorsomedial; l, lateral.
Rats exposed to the chronic conspecific interaction protocol (E2) did not show changes in Fos expression in the lateral PAG, but a significant decrease was observed in the rostral dorsomedial PAG compared with control (F3,10 = 9.02, P < 0.003; Fig. 3; bregma −5.6 = C: 102.5 ± 14.1 vs E2: 62.6 ± 11.2, P = 0.02; bregma −6.0 = C: 87.7 ± 12.8 vs E2: 46.6 ± 6.3, P = 0.004; bregma −6.4 = C: 81.0 ± 6.9 vs E2: 44.3 ± 3.1, P = 0.01). This group also showed a decrease in Fos expression in the caudal dorsolateral subdivision (F3,10 = 34.92, P < 0.03; bregma −7.2 = C: 38.7 ± 3.4 vs E2: 24.0 ± 7.4, P = 0.03; bregma −7.6 = C: 39.5 ± 4.2 vs E2: 19.0 ± 3.9, P = 0.01).
The animals submitted to the double protocol (E1 + 2) were the least affected in the PAG Fos expression, only the very caudal end of the dorsolateral PAG showed a decrease in Fos-positive perikarya (bregma −7.6 = C: 39.5 ± 4.2 vs E1 + 2: 21.3 ± 4.0, P = 0.03; Fig. 3). Figure 4 shows diagrams of the pattern of Fos expression in one animal for each group. Figure 5 illustrates the pattern of Fos expression at level −6.4 bregma.
Microinjection of opioid antagonists into the PAG
All 61 microinjections were placed in the lateral subdivision of the PAG, as this was the area activated by acute conspecific interaction (E1), which was effective in disrupting maternal behaviour. Figure 6A illustrates a representative injection site. The following description refers to the results obtained in these animals. Microinjections of opioid antagonists 1 mm dorsal to the PAG did not reverse the disruption of maternal behaviour caused by acute conspecific interaction observed in saline-injected animals (data not shown). The responses of the eight saline animals (Cs) were not different to those five of the control for Fos (C). The same is true for the behaviour of the eight E1s animals compared with the five E1.
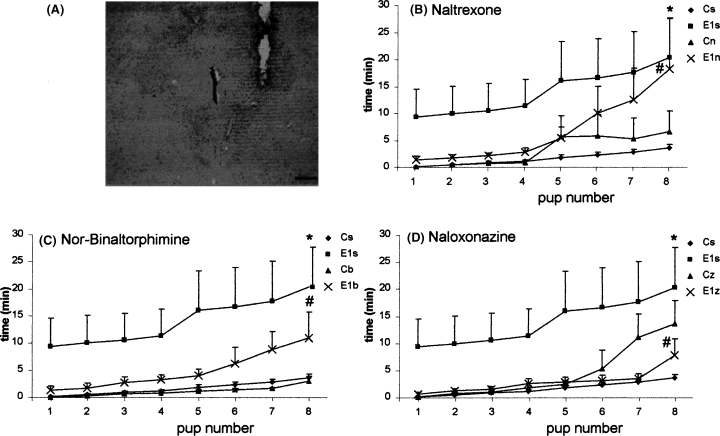
(A) Photomicrograph of a Nissl-stained section showing the position of the injection cannula into the lateral PAG (scale bar: 300 µm). (B–D) Progress of pup retrieval under different conspecific interaction protocols. Dams were submitted to acute conspecific interaction (E1). Group C is not exposed control rats. Cs (n = 8) and E1s (n = 8) are the same for all groups. Each point shows the mean ± SEM of seven–eight animals. (B) Effect of naltrexone (Cn n = 7 and E1n, n = 7) or saline microinjected into the PAG. (C) Effect of nor-binaltorphimine (Cb n = 8 and E1b n = 7) or saline. (D) Effect of naloxonazine (Cz n = 8 and E1z n = 8). *Shows the difference between E1s and Cs (repeated measures anova, P = 0.02). #Shows the difference between the acute conspecific interaction groups injected with antagonist (E1n, E1b and E1z) and the saline control (E1s; repeated measures anova, P = 0.002).
In this experiment, rats acutely exposed (E1s) microinjected with saline into the lateral PAG were slower than saline-injected not exposed (Cs) rats to complete pup retrieval (F3,28 = 3.48, P = 0.02, Duncan's post hoc: P = 0.01; Fig. 6B–D). These animals took 20.3 ± 7.4 min to gather the pups to the nest. On the other hand, acutely exposed rats microinjected with naltrexone (E1n), nor-binaltorphimine (E1b) or naloxonazine (E1z) into the lateral PAG spent the same amount of time retrieving pups as the control rats (Cn, Cb and Cz; F15,108 = 2.59, P = 0.002).
Acute conspecific interaction also interfered with the onset of nursing (Fig. 7A). Saline-microinjected (C, not exposed) rats began nursing at 5.3 ± 0.9 min from the start of the test. Exposed rats on the other hand (E1) started nursing at 15.2 ± 4.5 min. This was a significant difference (F3,28 = 4.58, P = 0.009, Duncan's post hoc: P = 0.007). Opioid antagonists injected into the lateral PAG reversed the delay to onset of nursing caused by conspecific interaction (F15,108 = 3.64, P = 0.00004). The time spent in crouching posture (nursing) was also affected by conspecific interaction (Fig. 7B). Saline-injected, not exposed rats spent a total of 42.0 ± 2.7 min crouching over the litter. E1 dams spent less time (34.4 ± 3.4 min, Duncan's post hoc: P = 0.05) nursing the pups compared with C. Microinjection of opioid antagonists into the lateral PAG reverted the decrease in nursing time caused by acute conspecific interaction (F15,108 = 2.82, P = 0.0009).
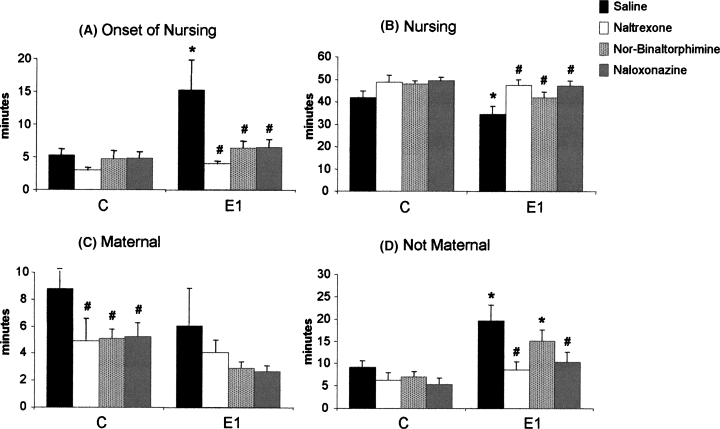
Effect of conspecific interaction (n numbers are the same as those in Fig. 6) on the onset of nursing (A), time spent in nursing position (B), time spent in maternal behaviours other than nursing (C) and time spent in non-maternal behaviours (D). Not exposed controls (C) and acute conspecific interaction (E1). Each bar represents the mean ± SEM. *Indicates a difference (P < 0.05) between saline-injected E1 and C (black bars). #Shows the difference between animals injected with antagonist (lighter bars) and their respective saline controls (black bar; P < 0.01).
Time spent in maternal behaviour, other than nursing, was not affected by conspecific interaction (Fig. 7C). However, not exposed rats microinjected with any of the opioid antagonists showed a decrease in this parameter compared with saline controls (F15,108 = 2.21, P = 0.009, Duncan's post hoc: P < 0.05). Acute conspecific interaction caused an increase in the time spent in non-maternal behaviour in saline-injected rats (Duncan's post hoc: P = 0.007; Fig. 7D). Naltrexone and naloxonazine, but not nor-binaltorphimine, reversed this effect (F15,108 = 2.54, P = 0.002, Duncan's post hoc: P < 0.01). Exposed rats injected with nor-binaltorphimine spent longer than saline-injected rats in non-maternal behaviours (Duncan's post hoc: P = 0.04).
A further eight animals were tested on the E2s protocol and eight animals on the E1 + 2s protocol. The response profile of the latter groups was similar (no statistical difference) to the behaviour of the five E2 and five E1 + 2. Also, another 47 rats (7 to 9 per group) were submitted to the E2 and E1 + 2 protocols and microinjected with the three opioid antagonists (not illustrated), the drugs did not affect the behavioural profile of these groups.
Discussion
Disruption of maternal behaviour by acute conspecific interaction
The data reported in this study show that acute conspecific interaction disrupts maternal behaviour in early lactating rats. Dams that were briefly exposed to an unfamiliar male rat on Day 9 of lactation took longer to retrieve all their pups to a nest and to assume a crouching position to start nursing compared with unstressed controls. They also spent less time crouching over the litter, engaging instead in non-maternal activities (cage exploration, self-grooming).
The decreased maternal behaviour as a result of stress could occur as a result of the inhibition of a brain circuit that normally permits maternal behaviour to be expressed. This circuit is activated during pregnancy and lactation, and is inhibited by certain types of stress. Hasen & Gammie (2005) showed that lactators are more aggressive towards intruders than virgin mice, especially in the presence of the litter. Also, female rats release ACTH and corticosterone when confronted with an intruder in the presence of their pups (Deschamps et al., 2003). The aggressive behaviour is stronger with unfamiliar males (used in our acute conspecific interaction experiment), decreasing with repeated exposure to the same animal. In our experiments, the female rat was not allowed to attack the male because a wire mesh was placed between them, therefore no aggression was observed, but pup retrieval and nursing were clearly delayed. There is a possibility that this alteration in behaviour was caused by a brief attentional shift, because once the pups are gathered and suckling is established, maternal behaviour proceeds as normal. However, the following discussion will describe the immediate effect of the ‘distractor’ as ‘maternal care disruption’ as this was the parameter analysed in our studies.
Interestingly, lactators exposed to a chronic conspecific interaction protocol for 6 days before testing on Day 9 did not show any significant disruption of maternal behaviour, although it has been shown that female rats can be very sensitive to social instability (Haller et al., 1998), the model that was the basis of our chronic conspecific interaction paradigm. Further, lactators that suffered both stressors (chronic social instability for 6 days followed by acute presentation of an unfamiliar male on Day 9) were resistant to the effects of acute conspecific interaction. Social instability appears to be stressful only to virgin female rats, as the lactators used in our experiments did not show altered behaviour. Although we did not measure plasma levels of corticosterone in these animals, it is possible that they would not be greatly altered by stress once it has been reported that the hypothalamus/pituitary/adrenal (HPA) axis activity is low in lactators (Wigger et al., 1999; Neumann et al., 2000), which might explain the absence of altered maternal care in the case of chronic conspecific interaction. We postulate that acute conspecific interaction is less dependent upon the activity of the HPA axis, which would be more involved in long-term responses to stress.
Another possibility that might explain our results is the observation that repeated exposure to conspecifics might induce tolerance in parts of the neuronal circuits involved in stress responses. Slamberova et al. (2001) reported that maternal behaviour is inhibited by opioids. Pre-treatment with morphine sensitizes the female rats to the inhibitory effect of opioids on maternal behaviour (Miranda-Paiva et al., 2001). Kinsley (1994) showed that multiparous females are more resistant to the disruptive effects of opioids on their maternal behaviour than primiparous dams. A systemic injection of morphine was effective in 88% of primiparous dams, whereas the same dose only affected 38% of the multiparous dams. Therefore, plastic changes in opioid receptors could reflect differences in sensitivity to the behavioural changes induced by morphine or by the manipulation of a brain circuit that involves opioidergic synapses. Miranda-Paiva et al. (2003) observed that systemic administration of morphine activates the lateral PAG and decreases maternal behaviour. Acute conspecific interaction could activate this circuit, whereas chronic conspecific interaction might depress its function by inducing tolerance in opioid synapses encountered in various areas, like the PAG, making the dams more resistant to stress.
Fos expression in the PAG induced by conspecific interaction in maternal rats
Our results show that acute but not chronic conspecific interaction induces activation of the lateral PAG. This increased expression of Fos in the lateral PAG correlates with the exclusive effect of acute conspecific interaction on maternal behaviour, and is in accordance with Miranda-Paiva et al. (2003) studies. These authors showed that morphine injected systemically in pregnant rats increases Fos expression in the lateral PAG and inhibits maternal behaviour when injected during lactation. Moreover, lesions of the lateral PAG prevent the disruption of maternal behaviour caused by morphine injection (Sukikara et al., 2006). It has been proposed that the PAG plays an important role in the choice of stress-coping behavioural strategies (Keay & Bandler, 2001). In the presence of an unfamiliar male, the dam needs to decide between an active aggressive attack and the care of the litter, this conflicting situation leads to the activation of areas crucial for decision making.
The PAG has a multifactorial role in pregnant rats, and mediates behaviours such as nursing, sexual activity, aggression and fear responses. Lesions of the caudal PAG increase aggression and the frequency of attacks to a male intruder (Lonstein & Stern, 1998). The PAG integrates defence behaviour. Electrical or chemical stimulation of the PAG induces defence reactions, like freezing, whereas lesions of the PAG reduce those responses (Bandler et al., 1985; Vianna et al., 2003). The PAG also integrates fight or flight responses and defensive rage, in which autonomic and somato-motor activation are very strong. Several limbic regions involved in defence/rage responses and maternal behaviour send afferents to the PAG (Greg & Siegel, 2001). In particular, the lateral PAG is activated when a rat is exposed to situations that induce active behaviour, such as flight or fight reactions (Keay & Bandler, 2001). In this case, the animal shows increased locomotor activity, hypervigilance and sympathetic autonomic activity (hypertension, redistribution of blood flood, etc.). In our experiments, only acute conspecific interaction caused some of those reactions (increased exploration, hypervigilance). The animals submitted to chronic conspecific interaction were not affected in the same manner and, even more striking, animals submitted to acute conspecific interaction after chronic conspecific interaction did not show changed activity, or did they show increased Fos expression in the lateral PAG.
In addition to stress activation of the PAG, it has been reported by others (Gregg & Siegel, 2001) that electrical stimulation of the amygdala activates opioid receptors in the PAG. The importance of this connection rests on the fact that stressors can excite parts of the amygdala. For instance, the medial amygdala is activated in lactating rats when exposed to an intruder conspecific, the odour of a predator or even the presence of the pups (Deschamps et al., 2003). The amygdala plays a role in the modulation of maternal behaviour and lactation. Terenzi & Ingram (2005) showed that medial and central amygdala neurons respond to oxytocin, and the proportion of responses varies within the reproductive cycle, with lactators presenting the largest proportion. We suggest that acute conspecific interaction could activate an enkephalinergic projection from the amygdala to the PAG and other areas (for example, the bed nuclei of the stria terminalis, unpublished observations). As a result, increased activity in a subpopulation of neurons in the lateral PAG would lead to a temporary switch of behaviour from maternal to hypervigilance and increased locomotion, perhaps reflecting a switch towards maternal aggression (towards a male intruder). The medial preoptic area (MPOA) of the hypothalamus is a region that is heavily involved in several maternal behaviours (see review by Gammie, 2005). Relevant to our observations, pup retrieval and maternal aggression are both processed by circuits that involve the MPOA and the PAG, and these two areas have reciprocal connections (Numan & Numan, 1997). Activation of the MPOA occurs during pup retrieval and other maternal behaviours (Lonstein et al., 1998b), and inhibits the PAG (Stack et al., 2002), whereas systemic morphine, which disrupts maternal behaviour, inhibits MPOA neurons. Activation of the PAG (as seen in our acute conspecific interaction group) might, on the other hand, inhibit the MPOA and the bed nuclei of the stria terminalis, as there are important numbers of PAG-hypothalamic and bed nuclei of the stria terminalis efferents (Cameron et al., 1995).
Reversal of conspecific interaction disruption of maternal behaviour by microinjection of opioid antagonists into the PAG
Our results showed that the non-selective opioid antagonist naltrexone was able to block the disruption of maternal behaviour caused by acute conspecific interaction when microinjected into the lateral PAG region, but not when injected outside the PAG. The opioid sensitivity of this effect was seen also when more selective antagonists were used: nor-binaltorphimine (κ antagonist) or naloxonazine (µ1 antagonist). The only parameter more sensitive to µ1 antagonism was non-maternal behaviour, which suggests that maternal behaviour including nursing is dependent on at least two opioid receptor types: µ1 and κ. Regarding the influence of the µ receptor on the onset and maintenance of maternal behaviour, it has been shown that systemic morphine interrupts the onset of maternal behaviour during lactation in a naloxone- (Bridges & Grimm, 1982) or naloxonazine- (Mann et al., 1990) sensitive manner.
Kinsley et al. (1995) reported that unstressed dams given systemic naloxone spend more time in the nest but less time nursing their pups, suggesting that opioids might help nursing but not other behaviours like nest building. However, these data were contradicted by Byrnes et al. (2000) who did not show changes in retrieval or crouching, or even pup grooming and nest building, but observed that females injected with i.p. or intracerebroventricular (i.c.v.) naloxone spent longer time nursing the pups. Recently, Dobryakova et al. (2005) showed that naloxone (i.p. or intranasal) accelerates the gathering of the pups to a nest (retrieval) and increases the number of crouching bouts over the litter, which tends to confirm Kinsley et al. (1995) results. The effects reported in those studies are almost certainly mediated by structures outside the PAG, as we did not see effects of the opioid antagonists when given to unstressed females. We suggest that when the PAG is activated by acute conspecific interaction, opioids are released within this region to inhibit maternal behaviour. The source of the opioid input is probably outside the PAG, as both the central amygdala (Rizvi et al., 1991) and the medial hypothalamus (Canteras, 2002) are known to project to the PAG and play a role in the behavioural responses to aversive stimuli. Also, as shown by Chiou & Huang (1999), the overall effects of opioids in the PAG could result in excitation, as many opioid receptors are presynaptic onto tonically active GABAergic neurons, therefore leading to an excitatory effect by disinhibition.
Naloxone microinjected into the lateral PAG also blocks the inhibition of maternal behaviour caused by systemic administration of morphine (Miranda-Paiva et al., 2003). Morphine interacts with the µ subtype receptor, but it can activate other subtypes. The PAG is rich in opioid receptors (Mansour et al., 1995), and Mann et al. (1991), attempted to establish which opioid receptor subtypes would be involved in the disruption of maternal behaviour using a variety of µ, κ, δ and σ (i.c.v.) on Day 6 of lactation. They reported that only the activation of the µ receptor with DAGO was able to block maternal care, and it was more potent than morphine. Our results indicate that acute conspecific interaction, on the other hand, appears to recruit the activation of at least two subtypes: µ and κ in the lateral PAG.
On a final note, a further eight animals were tested on the E2s protocol and eight animals on the E1 + 2s protocol. The response profile of the latter groups was similar (no statistical difference) to the behaviour of the five E2 and five E1 + 2 (reported on the previous section). Also, another 47 rats were submitted to the E2 and E1 + 2 protocols and microinjected with the three opioid antagonists, with the result that the drugs did not affect the behavioural profile of these groups compared with the dams not submitted to stereotaxic surgery in the previous section (data not illustrated). This means that the disturbance of maternal behaviour is robust and withstands stereotaxic surgery and microinjection manipulations.
In conclusion, our results suggest that enkephalinergic inputs to the PAG are activated by acute but not chronic conspecific interaction in maternal rats. The functional relevance of this activation is to induce the female to switch its attention towards the immediate source of disturbance in order to resolve a conflict between two behavioural strategies: the care of the litter or the confrontation with the intruder. Chronic conspecific interaction does not affect the dam in the same way, and even prevents future conflicting behaviours when acute conspecific interaction is presented. When no conflict is evident, the lateral PAG is not activated. It remains to be seen whether other models of stress, where conflicting behavioural strategies are presented, would also activate the lateral PAG.
Acknowledgements
This research was funded by grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) and CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior). We are also indebted to Drs A.P. Carobrez, J. Marino Neto and N. Canteras for sharing their time and laboratories for the completion of this work.
Abbreviations
-
- C
-
- control group
-
- DAB
-
- diaminobenzidine
-
- E1
-
- acute conspecific interaction group
-
- E2
-
- chronic conspecific interaction group
-
- i.p.
-
- intraperitoneal
-
- i.c.v.
-
- intracerebroventricular
-
- MPOA
-
- medial preoptic area
-
- PAG
-
- periaqueductal grey
-
- PBS
-
- phosphate-buffered saline.