Substance P excites globus pallidus neurons in vivo
Abstract
Substance P is a member of the neurokinin family. Previous studies have reported the existence of substance P and its high-affinity receptor, neurokinin-1 receptor, in globus pallidus. Employing in vivo extracellular recording combined with behavioural tests, the effects of substance P in globus pallidus of rats were studied. Micropressure ejection of the selective neurokinin-1 receptor agonist [Sar9,Met(O2)11] substance P increased the spontaneous firing rate of pallidal neurons in a concentration-dependent manner, with increases of 27.3% at 0.01, 33.4% at 0.03, 45.5% at 0.1, 38.4% at 0.3 and 36.4% at 1.0 mm. The selective neurokinin-1 receptor antagonist SR140333B prevented the excitatory effects induced by [Sar9,Met(O2)11] substance P. In behaving rats, we observed the postural effects of neurokinin-1 receptor activation in the globus pallidus. Consistent with electrophysiological results, unilateral microinjection of [Sar9,Met(O2)11] substance P (0.1 mm) led to a SR140333B-sensitive contralateral deflection in the presence of systemic haloperidol administration. Combining electrophysiological and behavioural findings, we concluded that substance P produces excitatory effects on globus pallidus neurons via neurokinin-1 receptors.
Introduction
The globus pallidus occupies a critical position in the indirect pathway of the basal ganglia. By innervating the striatum, subthalamic nucleus and substantia nigra pars reticulata, the globus pallidus plays an important role in normal movement regulation and in the pathophysiology of basal ganglia disorders (DeLong, 1990; Bergman et al., 1998; Hebb & Robertson, 1999). In Parkinson's disease and its animal model, the depletion of dopamine in the substantia nigra pars compacta results in abnormal hypoactivity of the globus pallidus, which leads to the akinesia and hypokinetic symptoms of Parkinson's disease. Moreover, synchronized oscillating bursts produced by the globus pallidus and subthalamic nucleus are considered to be responsible for tremor presented in parkinsonian patients (Plenz & Kital, 1999; Raz et al., 2000). Furthermore, it has been reported that there is a significant relationship between the firing variability of globus pallidus neurons and the severity of Parkinson's disease (El-Deredy et al., 2000). Therefore, modification of the firing patterns of globus pallidus neurons is an origin of parkinsonian symptoms.
Substance P is a member of the neurokinin family. Since it was identified (Chang & Leeman, 1971), substance P has been reported to be involved in a variety of physiological and pathological processes including pain, inflammation, antianxiety, antidepression, learning, neuroprotection and neurodegeneration (Yankner et al., 1990; Kowall et al., 1991; Gerhardt et al., 1992; Nikolaus et al., 1997; Kramer et al., 1998; Hill & Oliver, 2007). It is well known that substance P is widely distributed in both the central nervous system and peripheral tissues (Arai & Emson, 1986; Cinzia et al., 2002). Pharmacological studies have confirmed the neurokinin-1 receptor as the high-affinity receptor of substance P, which belongs to G protein coupled receptor (Glowinski & Beaujouan, 1993). By using immunohistochemistry, autoradiography and in situ hybridization, abundant substance P and neurokinin-1 receptors have been observed in basal ganglia (Elde et al., 1990; Gallagher et al., 1992; Sutoo et al., 2000; Saffroy et al., 2003). The altered expression of substance P in Parkinson's disease implies its possible involvement in the basal ganglia disorders (Mauborgne et al., 1983; de Ceballos et al., 1993). It has been shown that substance P is involved in the control of spontaneous firing in the basal ganglia. For example, substance P could depolarize neostriatal cholinergic cells and somatostatin- and nitric oxide synthase-positive cells in a dose-dependent manner (Aosaki & Kawaguchi, 1996). In the guinea-pig substantia nigra, substance P evoked excitatory responses in both dopaminergic and nondopaminergic neurons (Nalivaiko et al., 1997). The tonic activation of neurokinin-1 receptors may be necessary to maintain the spontaneous activity of a proportion of midbrain dopaminergic neurons (Minabe et al., 1996). In addition, substance P has also been involved in the release of neurotransmitters. It has been reported that neurokinin-1 receptor activation induced the release of both dopamine and acetylcholine in striatum, as well as the release of both γ-aminobutyric acid (GABA) and glutamate in nucleus tractus solitarius (Arenas et al., 1991; Humpel et al., 1991; Glowinski et al., 1993; Aosaki & Kawaguchi, 1996; Bailey et al., 2004). Intracerebroventricular injection of substance P could increase dopamine levels in the brain of 6-hydroxydopamine (6-OHDA)-lesioned rats (Krasnova et al., 2000).
Much evidence indicated the expression of neurokinin-1 receptor in globus pallidus (Mantyh et al., 1984; Mounir & Parent, 2002; Levesque et al., 2006). It has been reported that substance P-positive fibers from the striatum are closely apposed to pallidal neurons that express neurokinin-1 receptors (Mounir & Parent, 2002; Levesque et al., 2003). Calcium-imaging studies in rats have revealed that nigral and pallidal neurons are responsive to substance P (Levesque et al., 2003). In Parkinson's disease, the levels of substance P and neurokinin-1 receptors display different changes in globus pallidus (Mauborgne et al., 1983;Rioux & Joyce, 1993; Fernandez et al., 1994; Martorana et al., 2003). However, up to now, the function of substance P in globus pallidus is little known. In the present study, we employed both extracellular recordings and postural tests to study the effects of substance P in globus pallidus. Our results indicated that activation of neurokinin-1 receptors excites pallidal neurons.
Materials and methods
Animals
Adult male Wistar rats (250–300 g) were used. The animals were housed in a temperature controlled (22 ± 1 °C) room and maintained on a 12-h light–dark cycle. Food and water were available throughout the experiment. Tests were performed in strict accordance with the University guidelines on animal ethics.
Electrophysiological recordings
Rats were anaesthetized with 20% urethane (1 g/kg, i.p.; supplemented as needed) and placed gently in a stereotaxic apparatus (Narishige SN-3, Tokyo, Japan). A total of 65 adult rats (250–300 g) were used in the electrophysiological recordings. A heating pad was used to maintain rectal temperature at 36–38 °C. A craniotomy was performed at coordinates of 0.8–1.2 mm posterior, 2.5–3.5 mm lateral from the bregma according to a standard stereotaxic atlas (Paxinos & Watson, 1986).
Three-barrel microelectrodes were fastened at each end with metal tubing and prepared using a Stoelting pipette puller (Illinois, USA). The electrodes were stereotaxically positioned into the globus pallidus for single-unit recordings and micropressure ejection, and the tips were broken back under a microscope to 3–10 µm and had a resistance of 10–20 MΩ. The recording barrel of the electrode was filled with 0.5 m sodium acetate containing 2% pontamine sky blue dye. The other two micropressure ejection barrels connected to a four-channel pressure injector (PM2000B; Micro Data Instrument, Inc., USA) contained, respectively: (i)[Sar9,Met(O2)11]substance P (SMSP) and vehicle; (ii) (S)1-(2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidin-3-yl]ethyl)-4-phenyl-1-azoniabicyclo[2.2.2]octane chloride (SR140333B) and SR140333B with SMSP. Neurons were identified as pallidal neurons on the basis of their location and electrophysiological features. Drugs were ejected onto the surface of firing cells with a short gas pressure pulse (1500 ms, 5.0–15.0 psi).
The recorded electrical signals were amplified by a microelectrode amplifier (MEZ-8201; Nihon Kohden, Tokyo, Japan) and displayed on a memory oscilloscope (VC-11; Nihon Kohden) while being fed to an audiomonitor. The amplified electrical signals were passed through low- and high-pass filters into a signal analyser and computer. Cumulative rate histograms were compiled in real time from discriminated action potentials and accumulated in 10-s intervals. Spike times were preprocessed online and further analysed offline using the program of Histogram ver. 1.00 (Shanghai Medical University, Shanghai, China) for spike data analysis. A change of at least 20% from basal firing rate during drug application was considered significant for an individual neuron (Querejeta et al., 2005). Drug infusion was performed only once for each recording. A period of 30 min after the first drug application was allowed to move the electrode to search for another neuron in the same track.
At least 5 min of steady basal firing were collected from each cell before drug ejection into the globus pallidus. The frequency of control was determined by the average frequency of a 120-s period before drug application. The maximal change of frequency within 50 s following drug administration was considered as the drug effect.
Lesions of pallidal cholinergic neurons
The rats were anaesthetized with chloral hydrate (400 mg/kg, i.p.) and positioned in a stereotaxic apparatus. Immunotoxin 192 IgG-saporin solution (0.1 µg in 0.5 µL of 0.1 m PBS) was unilaterally or bilaterally microinjected into the globus pallidus, according to the following coordinates based on the atlas of Paxinos & Watson (1986): 1.0 mm posterior, 3.0 mm lateral to the bregma and 6.8 mm ventral to the skull surface. The rats were then allowed a recovery of 10–14 days from the surgery before electrophysiological recording. After recording, choline acetyltransferase (ChAT) immunohistochemistry was performed to verify the lesion. The lesions produced > 80% loss of ChAT-positive neurons in the globus pallidus. Only the recordings obtained from rats with successful cholinergic neuron lesion were included for statistical analysis.
Postural test
The rats were anaesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic apparatus. A guide cannula constructed from stainless steel (o.d., 0.4 mm; i.d., 0.3 mm) was implanted into a region above the globus pallidus on either side. The cannulae were fixed to the skull with stainless steel screws and dental acrylic. Stainless steel stylets were used to keep the cannulae sealed.
Following at least 3 days of recovery, the rats were tested for postural behaviour. They were placed in an observation cage to which they had already become habituated. Postural behaviour was recorded with a digital camera. Through the injection cannula connected to a 1.0-µL microsyringe, a total volume of 0.5 µL of the drug or vehicle was injected into the globus pallidus of awake animals over 2 min. After completion of infusion, the cannula was left in place for an additional 1 min. Ten minutes after the intrapallidal injection, haloperidol (5 mg/kg), a potent D2 receptor antagonist, was administrated intraperitoneally. Based on the angle between the nose–back line and the back–tail line, the postural asymmetry was quantitatively scored as follows: 0, no fixed postural alteration; 1, < 30°; 2, 30–59°; 3, 60–89°; 4, ≥ 90° (Miwa et al., 2000).
Histological controls
To verify the position of a single-unit recording, pontamine sky blue was ejected from the recording electrode tip by iontophoresis (10 µA, 20 min). To assess the extent of drug spread, 0.5 µL pontamine sky blue was microinjected into the unilateral globus pallidus. All the rats used in electrophysiological and behavioural experiments were killed with chloral hydrate (600 mg/kg, i.p.) and perfused with 4% paraformaldehyde solution transcardially. Then, the brains were dissected out and incubated in paraformaldehyde overnight. After that, the brains were frozen and sectioned at 50 µm, and all the recording and microinjection sites were identified under a light microscope.
Drugs and statistics
SMSP was obtained from Tocris (Avonmouth, UK). SR140333B was kindly provided by Sanofi–Aventis–Chilly Mazarin. 192 IgG-saporin was purchased from Chemicon (Califonia, USA). Haloperidol was obtained from Sigma (St Louis, MO, USA).
The data are expressed as mean ± SEM. Paired t-tests were used to compare the difference in firing rate before and after treatment. Statistical comparisons between or among groups were determined with Student's t-test and one-way anova. The scores of deviant posture were analysed by the nonparametric one-way Kruskal–Wallis test followed by the Mann–Whitney test. The level of significance was preset by using a P-value of 0.05.
Results
SMSP increased the spontaneous firing of pallidal neurons
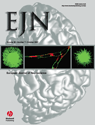
The neurons recorded in the present experiment were type II globus pallidus neurons, which had biphasic positive–negative waveforms (Kelland et al., 1995; Ruskin et al., 1998). The basal spontaneous firing rate of globus pallidus neurons ranged from 6 to 50 Hz. To ascertain the effects of neurokinin-1 receptor activation in globus pallidus, local administration of SMSP was performed in 16 rats. As shown in Fig. 1A, micropressure ejection of 0.1 mm SMSP significantly increased the spontaneous firing rate of pallidal neurons. In 31 out of 53 (58.5%) pallidal neurons, 0.1 mm SMSP increased the firing rate from 14.4 ± 2.1 to 19.2 ± 2.6 Hz (P < 0.001; Fig. 1B). The average increase was 45.5 ± 5.4% with regard to basal values. The mean duration of the excitatory effect was 90.2 ± 13.8 s. In another 21 pallidal neurons (39.6%), micropressure ejection of SMSP did not alter the firing rate significantly. Local application of SMSP decreased the firing rate in only one pallidal neuron. In the vehicle group, normal saline was micropressure-ejected into the globus pallidus. In the 15 pallidal neurons recorded, normal saline did not change the firing rate (basal, 15.0 ± 2.5 Hz; vehicle, 15.7 ± 2.6 Hz; P > 0.05; Fig. 1B). The average increase was 6.0 ± 2.2%.
Effects of micropressure ejection of SMSP on the spontaneous firing rate of globus pallidus neurons. (A) Typical frequency histograms showing that 0.1 mm SMSP increased the firing rate of a globus pallidus neuron by 49.3%. The firings of the neuron, displayed at a faster time base, at different stages of the experiment are shown in the lower trace. (B) Pooled data summarizing the effects of SMSP and vehicle on the firing rate of globus pallidus neurons. ***P < 0.001; ns, not significant.
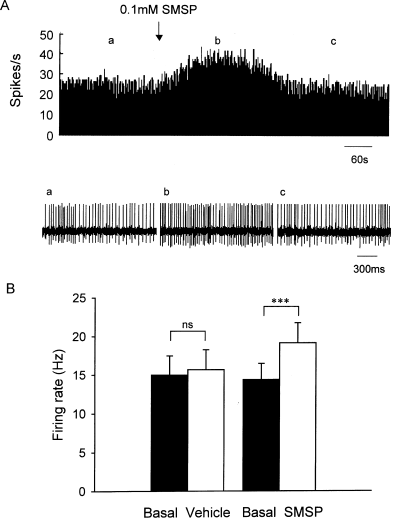
It has been reported that substance P receptors are richly distributed in cholinergic neurons of many brain nuclei (Gerfen, 1991; Pickel et al., 2000; Chen et al., 2001). To observe the effects of substance P on GABAergic neurons, intrapallidal microinjection of 192 IgG-saporin was performed to lesion cholinergic neurons in seven rats. In this case, SMSP still increased the firing rate of pallidal neurons (Fig. 2A). In 12 out of 23 neurons recorded, 0.1 mm SMSP increased the firing rate from 11.1 ± 2.1 to 15.2 ± 2.6 Hz (P < 0.001; Fig. 2B). The average increase was 42.5 ± 6.5%, which was not statistically significantly different from that without 192 IgG-saporin treatment (P > 0.05). In the control group, normal saline was ejected into the globus pallidus with cholinergic neuron lesion. In the 14 neurons recorded, normal saline did not change the firing rate (basal, 11.6 ± 1.9 Hz; vehicle, 11.6 ± 1.8 Hz; P > 0.05; Fig. 2B). The average increase was 2.8 ± 2.1%.
SMSP increased the pallidal firing of rats with cholinergic lesions. (A) Typical frequency histograms showing that 0.1 mm SMSP increased the firing rate of a globus pallidus neuron by 71.5% in the presence of 192 IgG-saporin. Lower trace shows the firing of the neuron displayed at a faster time base. (B) Pooled data summarizing the effects of SMSP and vehicle on the firing rate of globus pallidus neuron. ***P < 0.001; ns, not significant.
Concentration-dependent response induced by SMSP
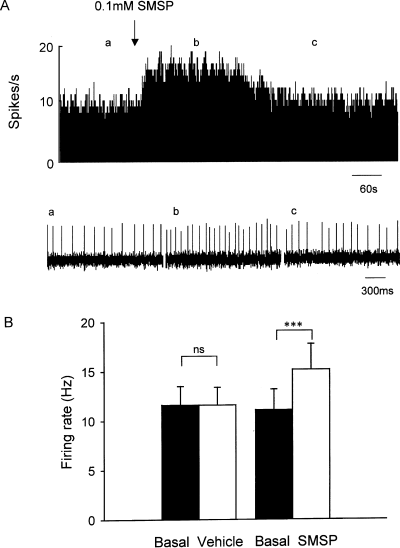
Further studies were performed to observe the effects of different concentrations of SMSP on pallidal neurons. SMSP at 0.01 mm caused a 27.3 ± 3.4% increase in seven out of 18 neurons (from seven rats) and 0.03 mm SMSP caused a 33.4 ± 5.2% increase in seven out of 22 neurons (from five rats). Furthermore, SMSP at 0.3 mm increased the firing rate by 38.4 ± 4.8% (11 out of 27 neurons, six rats). At the higher concentration of 1 mm, the frequency was increased by 36.4 ± 6.7% in seven out of 22 neurons (from six rats). As shown in Fig. 3, within the range of concentration from 0.01 to 1 mm, SMSP produced an approximate bell-shaped concentration-dependent effect in increasing the firing rate of pallidal neurons.
Concentration-dependent response induced by SMSP in globus pallidus. The increase in firing rate induced by SMSP is in a concentration-dependent manner within the range from 0.01 to 1 mm.
Neurokinin-1 receptor was involved in SMSP-induced excitation
To identify the subtype of neurokinin receptor that mediated the excitatory response, the effect of SR140333B, a selective neurokinin-1 receptor antagonist, was investigated in eight rats. In three out of 16 pallidal neurons, administration of SR140333B (0.5 mm) alone inhibited the firing rate from 13.6 ± 2.7 to 10.3 ± 2.0 Hz (P < 0.05; Fig. 4A). The average decrease was 23.8 ± 1.8%. No significant change in firing rate was observed following SR140333B administration in another 13 neurons. Co-ejection of SR140333B and SMSP completely blocked SMSP-induced excitatory responses in 13 out of 15 pallidal neurons. The average change was 4.0 ± 1.8% with regard to basal firing (n = 13; Fig. 4B). The increases in firing rate in the other two neurons were 24.7%.
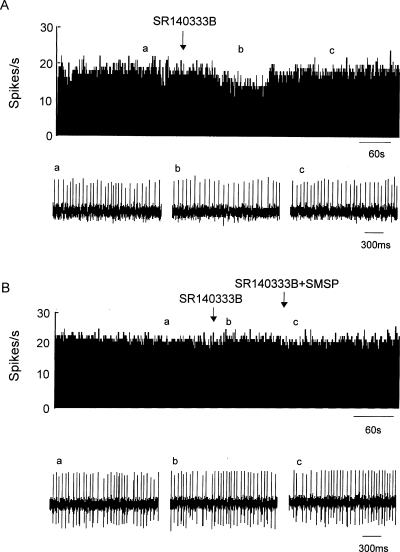
Effects of neurokinin-1 receptor antagonists on the SMSP-induced increase in firing rate. (A) In this cell, the neurokinin-1 receptor antagonist SR140333B alone decreased the firing rate by 23.7%. (B) In this cell, SR140333B alone had no significant effect on the spontaneous firing rate. However, the presence of SR140333B completely blocked the increase in firing rate induced by SMSP.
Effects of SMSP on postural behaviour
To study whether the pallidal substance P system modulates movement, the behavioural effects of SMSP were studied in awake animals. Unilateral microinjection of SMSP (0.1 mm, 0.5 µL) into globus pallidus did not produce a clear spontaneous turning behaviour (2.20 ± 1.32 contralateral turns in 30 min; n = 5), which was not different from that of vehicle microinjection (1.40 ± 1.17 contralateral turns in 30 min, n = 5, P > 0.05). However, in the presence of systemic administration of haloperidol, SMSP induced significant contralateral dystonic posture (n = 7). The deviant posture began almost immediately and lasted for nearly 1 h whereas, in the control group, vehicle injection did not induce any fixed deviant posture in rats under identical conditions (n = 5). The difference between vehicle and SMSP application was statistically significant (P < 0.05). To determine whether the postural effect produced by SMSP was mediated via neurokinin-1 receptors, the role of SR140333B was studied. Microinjection of SR140333B (0.5 mm, 0.5 µL) alone into globus pallidus unilaterally did not produce any dystonic posture under conditions of haloperidol administration (n = 5). Co-injection of SR140333B and SMSP (0.5 µL in total volume) completely prevented SMSP-induced contralateral dystonic posture (n = 5, P < 0.001 compared with SMSP alone, P > 0.05 compared with SR140333B alone). These data are summarized in Fig. 5A. Only data obtained from animals with correct placement of the cannulae were included for statistical analysis (Fig. 5B).
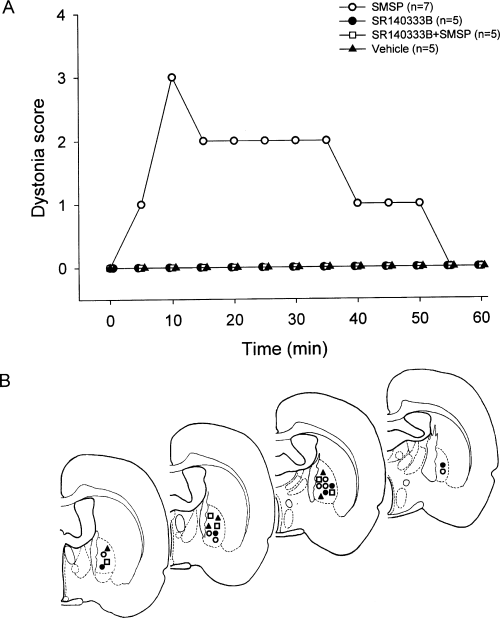
Postural behaviour induced by intrapallidal microinjection of SMSP in rats receiving systemic administration of haloperidol. (A) Scores of deviant posture in rats recorded in the 60 min after unilateral microinjection of vehicle, SMSP alone, SR140333B alone and SR140333B with SMSP. Data represent median values. (B) Confirmation of the sites of microinjection into the globus pallidus. The symbols represent different drug injections: control (, n = 5), SMSP (^, n = 7), SR140333B (, n = 5) and SR140333B + SMSP (, n = 5).
To provide further evidence for the specificity of the effect of SMSP on the globus pallidus, we microinjected 0.5 µL of SMSP into the striatum, which is located adjacent to the globus pallidus. We found that 0.1 mm SMSP induced ipsilateral dystonic posture (n = 3), which was opposite to the dystonic posture induced by SMSP in globus pallidus. In another two rats, 0.25 µL of SMSP was microinjected into the ventral pallidum, a structure located ventrally to the globus pallidus. SMSP (0.1 mm) microinjected into the ventral pallidum induced weak contralateral dystonic posture.
SMSP increased the pallidal firing of rats with haloperidol administration
To elucidate the electrophysiological basis of SMSP-induced contralateral dystonic posture, the effects of intrapallidal SMSP on firing rate were observed in 10 rats with haloperidol administration. Intraperitoneal administration of haloperidol reduced the basal firing rate in three out of 10 pallidal neurons (by 29.7 ± 2.7%) and had no significant effect in the remaining seven neurons. As shown in Fig. 6, in the presence of haloperidol, 0.1 mm SMSP increased the firing rate from 14.4 ± 3.0 to 19.9 ± 3.7 Hz (P < 0.001) in 12 out of 25 pallidal neurons recorded. The average increase was 49.0 ± 8.4%, which was not significantly different from that obtained without haloperidol administration (P > 0.05). In the control group, normal saline ejection did not change the pallidal firing in the presence of haloperidol (basal, 14.4 ± 2.3 Hz; vehicle, 14.6 ± 2.3 Hz; n = 14, P > 0.05; Fig. 6B). The average increase was 2.3 ± 1.1%.
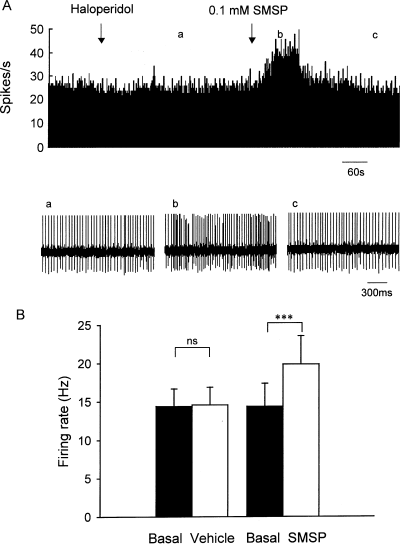
Effects of SMSP on the spontaneous pallidal firing of rats with haloperidol administration. (A) Typical frequency histograms showing that intraperitoneal administration of haloperidol had no effect on the basal firing rate of globus pallidus neurons. In the presence of haloperidol, intrapallidal micropressure ejection of 0.1 mm SMSP increased the firing rate by 46.4%. Lower trace shows the firing of the neuron displayed at a faster time base (a, b and c). (B) Pooled data summarizing the effects of SMSP and vehicle on the firing rate of globus pallidus neurons. ***P < 0.001; ns, not significant.
Discussion
In the present study we showed that neurokinin-1 receptor activation increased the spontaneous electrical activity of pallidal neurons. The excitatory effect induced by neurokinin-1 receptor activation was approximately bell-shaped and concentration-dependent; this is similar to findings in other studies. For instance, dose–response curves produced by substance P fragments on endogenous dopamine outflow are bell-shaped in rat striatal slices (Khan et al., 1995, 1996). Substance P and its C-terminal fragment Substance P (7–11)-increased locomotor activity displayed a similar pattern (de Araujo et al., 1998).
It has been reported that the cholinergic neurons of many nuclei, such as striatum, are densely occupied by substance P receptors (Gerfen, 1991; Chen et al., 2001). It is possible that the excitation recorded reflects a long-loop multisynaptic response. In other words, activation of corticopetal cholinergic neurons in the globus pallidus projects to and activates the cortex which, in turn, projects back to the globus pallidus via corticopallidal connections. To study this possibility, we performed control experiment to selectively lesion pallidal cholinergic neurons. The fact that SMSP-induced increase in firing rate after lesion of cholinergic neurons was not significantly different from that observed in intact rats suggests that substance P may directly excite GABAergic neurons in globus pallidus.
It has been reported that substance P receptors, i.e. neurokinin-1 receptors, were localized in the globus pallidus of rats (Mantyh et al., 1984). By using single and double immunolabelling, numerous neurokinin-1 receptor-expressing neurons were observed in both the external and internal segments of human globus pallidus (Mounir & Parent, 2002). Furthermore, subcellular localization revealed that neurokinin-1 receptor is mainly associated with intracellular sites or situated at extrasynaptic positions on the plasma membrane of pallidal neurons (Levesque et al., 2006). In addition, presynaptic axon terminals forming symmetric and asymmetric synapses occasionally contained neurokinin-1 receptors. The present finding that SR140333B, a selective neurokinin-1 receptor antagonist, blocked SMSP-induced excitation provides further functional evidence for the existence of neurokinin-1 receptors in globus pallidus. Morphological studies demonstrated that the globus pallidus receives a substance P-ergic projection from the striatum. The present observations that a neurokinin-1 receptor antagonist decreased the spontaneous spiking frequency in some pallidal neurons indicates an involvement of the endogenous substance P system in modulating electrical activity of pallidal neurons.
Several lines of evidence reveal that the globus pallidus is involved in motor control under both normal and pathophysiological states. As SMSP increased the spontaneous firing rate and thus excited pallidal neurons, we further studied whether intrapallidal SMSP is involved in motor regulation. It has been suggested that the basis of turning behaviour is the imbalance of bilateral basal ganglia output activity (Pycock, 1980). The overactivity of unilateral globus pallidus would lead to contralateral dystonic posture as a result of disinhibition from the indirect pathway of the basal ganglia to the ipsilateral cortex. Thus, our findings that unilateral microinjection of SMSP induced contralateral dystonic behaviour suggested that the pallidal substance P system is involved in motor regulation. Intrapallidal application of SMSP probably compensated for the hypoactivity of the globus pallidus induced by haloperidol, and induced a marked difference in the excitability of globus pallidus between the intact side and the SMSP-injection side, thereby causing contralateral dystonic posture.
There is much evidence indicating that substance P is involved in the pathophysiology of Parkinson's disease. Firstly, as mentioned above, substance P and its receptor are densely and widely distributed in the basal ganglia. Secondly, substance P can not only excite dopaminergic neurons in substantia nigra but also increase dopamine levels in the striatum under parkinsonian conditions (Arenas et al., 1991; Humpel et al., 1991; Overton et al., 1992; Glowinski et al., 1993; Minabe et al., 1996; Nalivaiko et al., 1997). In addition, the substance P system in globus pallidus exhibits pronounced variations under parkinsonian conditions, with substance P immunoreactivity increasing markedly while the expression of substance P receptor decreases significantly (Fernandez et al., 1994; Martorana et al., 2003). Furthermore, previous studies reported that pre- or post-treatment with substance P or its C-terminal fragments can promote functional recovery with partial nigrostriatal 6-OHDA lesions (Mattioli et al., 1992; Nikolaus et al., 1997, 1999). However, the mechanism of the substance P-induced neuroprotective effect is unknown. It has been reported that substance P could exert neuroprotection on some types of neurons through its role against glutamate excitotoxicity (Yankner et al., 1990; Kowall et al., 1991; Sanberg et al., 1993; Calvo et al., 1996). Besides, neuropeptides, including substance P, contribute to cell growth, regeneration and recovery from impairment (Spoerri et al., 1992). These actions may underlie therapeutic effects of substance P in Parkinson's disease.
It is well known that the hypoactivity of globus pallidus is directly related to the hypokinesia observed in Parkinson's disease (Albin et al., 1989; Wichmann & DeLong, 1996). As the present electrophysiological and behavioural findings indicated that substance P can excite pallidal neurons, it is worth considering the possible therapeutic effects of striatopallidal substance P system in Parkinson's disease. In line with our hypothesis, the increased striatopallidal substance P immunoreactivity in Parkinson's disease may reflect a compensatory mechanism. However, morphological (Jakab et al., 1996; Futami et al., 1998; Chen et al., 2001) and electrophysiological (Aosaki & Kawaguchi, 1996; Minabe et al., 1996; Nalivaiko et al., 1997; Galarraga et al., 1999) studies have revealed the expression of substance P receptors in other basal ganglia nuclei, including striatum and substantia nigra. Therefore, the therapeutic effects of substance P in Parkinson's disease, if any, would depend on its mutual actions on these nuclei. Thus, more experiments and evidence are needed before we can fully understand the functions of substance P in the whole basal ganglia circuit under normal and dysfunctional conditions. In conclusion, our results extend previous studies by providing evidence for a direct excitatory effect of neurokinin-1 receptor activation on pallidal neurons, which suggests a role for the substance P system in the aetiology and treatment of Parkinson's disease.
Acknowledgements
The authors wish to thank Sanofi–Aventis–Chilly Mazarin for kindly providing SR140333B. This work was supported by the grants from National Natural Science Foundation of China (30670664 to L.C.) and Natural Science Foundation of Shandong Province (Y2006C18 to L.C.).
Abbreviations
-
- 6-OHDA
-
- 6-hydroxydopamine
-
- ChAT
-
- choline acetyltransferase
-
- GABA
-
- γ-aminobutyric acid
-
- SMSP
-
- [Sar9,Met(O2)11]substance P
-
- SR140333B
-
- (S)1-(2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidin-3-yl]ethyl)-4-phenyl-1-azoniabicyclo[2.2.2]octane chloride.