Interleukin-1 inhibits firing of serotonergic neurons in the dorsal raphe nucleus and enhances GABAergic inhibitory post-synaptic potentials
Abstract
In vitro electrophysiological data suggest that interleukin-1 may promote non-rapid eye movement sleep by inhibiting spontaneous firing of wake-active serotonergic neurons in the dorsal raphe nucleus (DRN). Interleukin-1 enhances GABA inhibitory effects. DRN neurons are under an inhibitory GABAergic control. This study aimed to test the hypothesis that interleukin-1 inhibits DRN serotonergic neurons by potentiating GABAergic inhibitory effects. In vitro intracellular recordings were performed to assess the responses of physiologically and pharmacologically identified DRN serotonergic neurons to rat recombinant interleukin-1β. Coronal slices containing DRN were obtained from male Sprague–Dawley rats. The impact of interleukin-1 on firing rate and on evoked post-synaptic potentials was determined. Evoked post-synaptic potentials were induced by stimulation with a bipolar electrode placed on the surface of the slice ventrolateral to DRN. Addition of interleukin-1 (25 ng/mL) to the bath perfusate significantly decreased firing rates of DRN serotonergic neurons from 1.3 ± 0.2 Hz (before administration) to 0.7 ± 0.2 Hz. Electrical stimulation induced depolarizing evoked post-synaptic potentials in DRN serotonergic neurons. The application of glutamatergic and GABAergic antagonists unmasked two different post-synaptic potential components: a GABAergic evoked inhibitory post-synaptic potentials and a glutamatergic evoked excitatory post-synaptic potentials, respectively. Interleukin-1 increased GABAergic evoked inhibitory post-synaptic potentials amplitudes by 30.3 ± 3.8% (n = 6) without affecting glutamatergic evoked excitatory post-synaptic potentials. These results support the hypothesis that interleukin-1 inhibitory effects on DRN serotonergic neurons are mediated by an interleukin-1-induced potentiation of evoked GABAergic inhibitory responses.
Introduction
Although interleukin-1 (IL-1) was originally described as a product of the peripheral immune system, there is now ample evidence that IL-1, IL-1 receptors and the IL-1 receptor antagonist are constitutively expressed in normal brain (Vitkovic et al., 2000). IL-1 modulates behaviors such as feeding, sexual behavior, social exploration, locomotor activity and sleep (Opp, 2005). IL-1 consistently has been shown in several animal species to enhance non-rapid eye movement (NREM) sleep and inhibit rapid eye movement (REM) sleep (Krueger et al., 1984; reviewed in Opp, 2005). Moreover, central administration of the IL-1 receptor antagonist (Opp & Krueger, 1991), of antibodies directed against IL-1 or inhibition of cleavage of biologically active IL-1 from its inactive precursor reduces spontaneous NREM sleep in normal animals and inhibits the physiological NREM sleep rebound that follows sleep deprivation (Opp & Krueger, 1994a, b; Imeri et al., 2006). IL-1 mRNA expression in rat brain exhibits diurnal variation with greater levels during the light (rest/sleep) period than during the dark (active) period (Taishi et al., 1997) and increases during sleep deprivation (Mackiewicz et al., 1996). IL-1 is detected more frequently in plasma samples taken from humans during sleep than during waking (Gudewill et al., 1992). IL-1-like activity in cerebrospinal fluid of cats varies in phase with the sleep–wake cycle (Lue et al., 1988) and IL-1 plasma levels in humans peak at sleep onset (Moldofsky et al., 1986).
Interleukin-1 receptors have been described in the dorsal raphe nucleus (DRN) (Cunningham. & De Souza, 1993; Schobitz et al., 1994), which contains the cell bodies of serotonergic neurons innervating the entire central nervous system (Jacobs & Azmitia, 1992). Extensive experimental data and clinical observations indicate that brain serotonin (5-hydroxytryptamine; 5-HT) plays a pivotal role in the regulation of many physiological processes and complex behaviors (Jacobs & Azmitia, 1992), including waking and sleep (Jouvet, 1999; Pace-Schott & Hobson, 2002). Because IL-1 enhances NREM sleep when microinjected into the rat DRN and inhibits firing rates of electrophysiologically and pharmacologically identified DRN serotonergic neurons in a guinea-pig slice preparation (Manfridi et al., 2003), it has been proposed that IL-1-induced NREM sleep enhancement may result, in part, from the inhibition of wake-active serotonergic DRN neurons.
Serotonergic DRN neurons are inhibited by γ-aminobutyric acid (GABA) (Gallager & Aghajanian, 1976; Becquet et al., 1990; Becquet et al., 1993). GABA plays a crucial role in shaping the state-dependent firing and neurotransmitter release of serotonergic neurons, which are highly active during wakefulness, reduce their activity during NREM sleep and are almost silent during REM sleep (McGinty & Harper, 1976; Trulson & Jacobs, 1979; Cespuglio et al., 1981, 1990; Lydic et al., 1987; Wilkinson et al., 1991; Levine & Jacobs, 1992; Imeri et al., 1994, 1999; Portas & McCarley, 1994; Gervasoni et al., 2000). IL-1 enhances GABA inhibitory effects acting at both pre- and post-synaptic levels. IL-1 enhances GABA release (Feleder et al., 2000; Tabarean et al., 2006) and it also enhances GABA-induced post-synaptic responses in different in vivo and in vitro experimental models (Miller et al., 1991; Luk et al., 1999; Serantes et al., 2006). The present study was designed to test the hypothesis that IL-1 inhibits DRN serotonergic neurons by potentiating GABAergic inhibition.
Materials and methods
Substances
IL-1 (rat recombinant IL-1β expressed in E. coli) was purchased from Euroclone (Devon, UK). Lyophilized IL-1 was dissolved in phosphate-buffered solution containing 0.1% bovine serum albumin, aliquoted, frozen and stored at −20 °C until used. The drugs used in these experiments were dl-noradrenaline hydrochloride (NA), 5-HT (5-hydroxytriptamine, serotonin) hydrochloride, dl-2-amino-5-phosphonopentanoic acid (APV), (–)-bicuculline methiodide (BMI) and 6,7-dinitroquinoxaline-2,3(1H,4H)-dione (DNQX), all obtained from Sigma and phenylephrine (PE), obtained from RBI (Natick, MA, USA). Drugs were aliquoted, stored at −20 °C and dissolved just prior to use in artificial cerebrospinal fluid (ACSF) of the following composition: 124 mm NaCl, 2 mm KCl, 3 mm KH2PO4, 26 mm NaHCO3, 1.3 mm MgCl2, 2.5 mm CaCl2, 10 mm glucose (final pH 7.4) and applied via bath perfusion. The stock of DNQX was dissolved in dimethyl sulfoxide (DMSO) before being added to ACSF (final concentration of DMSO < 0.1%).
Preparation of brainstem slices
Juvenile male Sprague–Dawley rats (25–50 g at time of the experiment; Charles River, Italy) were anesthetized with isofluorane and decapitated. The brain then was removed rapidly and placed in ice-cold (4 °C) ACSF, continuously bubbled with an O2–CO2 mixture (95 : 5%). Coronal sections from a block of tissue containing the DRN and kept in ice-cold carbogenated ACSF were then cut (400 µm thick) with a vibratome (752 Vibroslice, Campden Instruments Ltd, Loughborough, UK). Three slices were taken from each animal for subsequent recording. The DRN was located in the midline of the slices, between the medial longitudinal fasciculi extending dorsally towards the aqueduct. Only sections containing the midline decussating fibers of the superior cerebellar peduncle were selected for use. The slices were then incubated at room temperature in carbogenated ACSF and were left to recover for at least 1 h. After recovery, slices were individually transferred to a warmed (32 °C) submersion-type slice recording chamber, through which carbogenated ACSF was continuously superfused at a rate of 2.5 mL/min.
All procedures involving animals and their care were conducted in conformity with institutional guidelines that are in compliance with European Union (EEC Council Directive 86/609, OJ L 358,1; 12 December 1987) and Italian (D.L. n.116, G.U. suppl. 40, 18 February 1992) laws and policies, as well as with the United States Department of Agriculture Animal Welfare Act and the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Recording methods
Intracellular recordings were made throughout the midline and paramidline DRN. DRN neurons were impaled with glass microelectrodes pulled from 1.5-mm filament-containing tubing (Clark Electromedical Instruments, Pangbourne, UK) filled with 2 m potassium-acetate (impedance 60–90 MΩ). Conventional intracellular recordings were made from DRN neurons using the bridge balance mode. The bridge was frequently checked to ensure that it was balanced throughout experiments. The impact of IL-1 on spontaneous firing rates and the amplitude of evoked post-synaptic potentials (evPSPs) was determined. Changes in DRN neuron discharge rate due to IL-1 administration were evaluated using a continuous gap-free recording (gap-free protocol). evPSPs were elicited using a bipolar stimulating electrode placed on the surface of the slice ventro-lateral to DRN (Pan et al., 1989; Pinnock, 1992), connected to an isolation unit (SC-100; Winston Electronics, Millbrae, CA, USA) delivering single square wave current pulses (0.8–5 mA; 200-µs duration: 0.2 Hz). All evoked responses, both excitatory and inhibitory (see below the Results section), were recorded at membrane potential of −70 mV. In current-clamp mode, putative serotonergic neurons were recognized as described previously (Manfridi et al., 2003; see also below the Results section). The firing activity of serotonergic DRN neurons in anesthetized rats in vivo is related to an undamaged and tonically active noradrenergic system (Gallager & Aghajanian, 1976; Baraban et al., 1978). In brain slices, noradrenergic inputs are severed and serotonergic neurons are often quiescent. The firing of serotonergic neurons that are not spontaneously active can be restored by injecting depolarizing current or by adding the α1-adrenergic agonist PE (3 µm) (Vandermaelen & Aghajanian, 1983) to the perfusion medium.
Drug application
Test substances, dissolved in carbogenated ACSF just prior to use and applied via bath perfusion, arrived to the recording chamber 3 min after application. 5-HT (40 µm), NA (30 µm), PE (3 µm) and IL-1β (25 ng/mL) were applied for 3 min. The effects of the different drugs were evaluated during intracellular recording by comparing the mean neuronal discharge frequency, the membrane potential and the average amplitude of ten evPSPs before any treatment and 3 min after their addition to the superfusing ACSF. The effect of IL-1 on evEPSPs or on evoked inhibitory post-synaptic potentials (evIPSPs) was evaluated only after the effects of GABAergic antagonist (BMI) or glutamatergic antagonists (APV and DNQX), respectively, were established (from 5 to 10 min).
Data acquisition and analysis
Signals were conditioned using a high-impedance amplifier (Axoclamp-2B, Axon Instruments, Union City, CA, USA) connected to a digitizer (Digidata 1322A, Axon Instruments), interfaced to a computer. Axon Instruments software (pClamp 8.2) was used for the on- and off-line data acquisition and analysis.
All data are expressed as means ± SE. Data were statistically analysed by the non-parametric Kolmogorov–Smirnov test (K-S test), one-way anova and Tukey or t-tests, as appropriate.
An α level of P < 0.05 was taken as indicating a statistically significant difference between experimental conditions.
Results
One hundred and fifty-one neurons were recorded. Ninety-one of these neurons were identified as serotonergic (see below), whereas 60 neurons were identified as non-serotonergic on the basis of either their electrophysiological properties (n = 16) or their pharmacological response (n = 44). Forty-eight of the 91 serotonergic neurons were used in the study: in 25 neurons IL-1 effects on firing rate were tested, while in the other 23 neurons IL-1 effects on the amplitude of evPSP were evaluated.
Electrophysiological and pharmacological identification of DRN serotonergic neurons
Recorded neurons were characterized as serotonergic on the basis of their distinctive electrophysiological and pharmacological properties (Baraban et al., 1978; Vandermaelen & Aghajanian, 1983; Yoshimura & Higashi, 1985; Sprouse & Aghajanian, 1987; Williams et al., 1988; Pan et al., 1994; Stezhka & Lovick, 1997; Li et al., 2001; Liu et al., 2002; Kirby et al., 2003; Manfridi et al., 2003; Marinelli et al., 2004). DRN serotonergic neurons were characterized by (1) high-input resistance (210 ± 6 MΩ), (2) a gradual initial phase of afterhyperpolarization potential (tau = 5.0 ± 0.6 ms) and (3) a long spike width at half height (1.44 ± 0.04 ms; Fig. 1A). Neurons that were identified as non-serotonergic for their electrophysiological properties were characterized by lower input resistance (136 ± 4 MΩ) and shorter spike width at half height (0.90 ± 0.04 ms). Both these values were significantly different from those recorded in serotonergic neurons (P < 0.001).
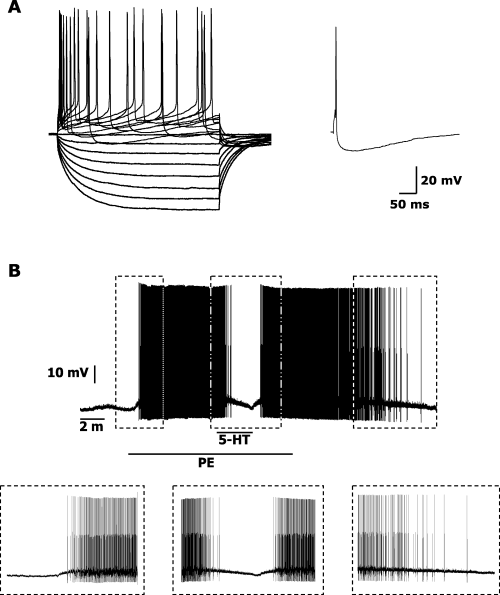
Electrophysiological (A) and pharmacological (B) identification of dorsal raphe nucleus (DRN) serotonergic neurons. (A, left panel) Response of a serotonergic-type neuron to depolarizing and hyperpolarizing current steps (40 pA; 500 ms). (A, right panel) Single evoked action potential recorded from the same neuron showing a gradual initial phase of afterhyperpolarization potential (AHP). The AHP onset was observed to be more gradual in serotonergic neurons, than in non-serotonergic neurons (Kirby et al., 2003). The time constant (tau) is calculated fitting a single exponential function to the initial phase of the AHP (from the onset of the AHP following an action potential to its maximal level) and is defined as the time required to reach 63% of the maximum AHP amplitude. (B) Representative DRN neuron, previously identified as putatively serotonergic on the basis of its electrophysiological properties (see Material and methods for details), responded to phenylephrine (PE, 3 µm) administration with depolarization and firing (left box). Serotonin (5-hydroxytriptamine, 5-HT, 40 µm), administrated during PE application, reversibly hyperpolarized and inhibited the PE-induced activity (middle box). Both PE and 5-HT effects were completely reversible after wash-out (right box).
All 91 serotonergic neurons were inhibited by 5-HT (40 µm) and, when tested, excited by adrenergic activation (Fig. 1B). Mean resting membrane potential in all the 91 recorded neurons was −54.1 ± 0.6 mV. As at resting potential only three out of 91 recorded neurons fired spontaneously (see Materials and methods), in order to facilitate pharmacological identification and to allow evaluation of IL-1 effects on firing, firing was induced in 88 neurons by either injecting a depolarizing current (0.15 ± 0.02 nA, bringing membrane potential to −44.1 ± 1.2 mV; n = 21) or adding PE (3 µm, bringing membrane potential to −48.2 ± 0.6 mV; n = 67) to the perfusion bath. Mean firing rate in the 91 recorded neurons was 1.7 ± 0.2 Hz. All neurons (n = 91) responded to 5-HT administration with a significant membrane hyperpolarization of 2.2 ± 0.3 mV and a significant decrease in firing rate of 1.2 ± 0.1 Hz. Adrenergic stimulation (obtained by either PE or NA administration) excited tested neurons, significantly depolarizing membrane potential and bringing it to −48.3 ± 0.6 mV. This depolarization was associated with a significant increase in firing rate of 1.7 ± 0.3 Hz. All these pharmacological effects were reversible after wash-out.
IL-1 administration specifically decreased spontaneous firing rates of DRN serotonergic neurons
Seventeen (68%) out of the 25 identified serotonergic DRN neurons used in this part of the study responded to bath application of rat recombinant IL-1 (25 ng/mL; K–S test, P < 0.05). All responding neurons were inhibited by bath application of rat recombinant IL-1 (Fig. 2A). As a group (Fig. 2B), the firing rate of the 17 responding neurons was reduced from 1.3 ± 0.2 Hz prior to IL-1 administration to 0.7 ± 0.2 Hz following IL-1 and recovered to 1.0 ± 0.2 Hz after the wash-out (one-way anova, F2,32 = 11.3, P < 0.001), indicating IL-1 effects were reversible. The IL-1-induced reduction in firing rate was associated with a non-significant membrane hyperpolarization.
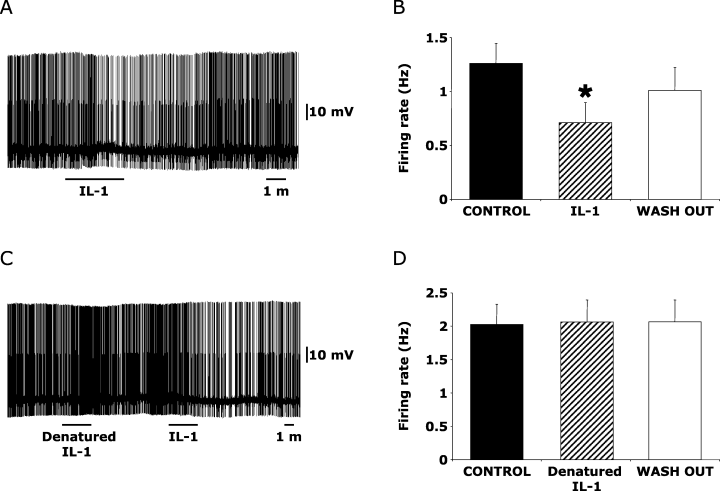
Interleukin-1 (IL-1) specifically inhibits spontaneous firing rates of DRN serotonergic neurons. (A) Inhibitory effects of IL-1 (25 ng/mL) administration on the spontaneous firing rate of a representative dorsal raphe nucleus (DRN) neuron, which was electrophysiologically and pharmacologically identified as serotonergic. (B) Mean (± SE) effects of IL-1 administration on the population of responding DRN serotonergic neurons (17 out of 25 recorded neurons). The application of denatured IL-1 did not affect the spontaneous activity of a representative serotonergic DRN neuron (C), did not alter the mean population discharge rate of tested neurons (D; n = 9). Five out of the nine recorded neurons that did not respond to denatured IL-1 (including the representative neuron depicted in C) were significantly inhibited by IL-1 administration. *P < 0.05 vs control and wash-out conditions.
In order to establish the specificity of IL-1 effect, in nine out of the 25 identified serotonergic neurons, denatured IL-1 (heat-inactivated IL-1; Opp et al., 1995) was tested prior to IL-1 administration. Firing rate was never modified by denatured IL-1 (Fig. 2C and D; one-way anova, F2,16 = 0.1, P = 0.9) in both neurons that responded (n = 5; Fig. 2C) and did not respond (n = 4) to the subsequent IL-1 administration.
IL-1β decreased depolarizing evPSP amplitude in DRN serotonergic neurons
Electrical stimulation induced depolarizing evPSPs in the 23 DRN serotonergic neurons used in this part of the study. In four out of five DRN serotonergic neurons, IL-1 administration reversibly decreased the evPSP amplitudes by 30.4 ± 5.8% (P = 0.014, paired t-test; Fig. 3A). This effect was reversible upon wash out.
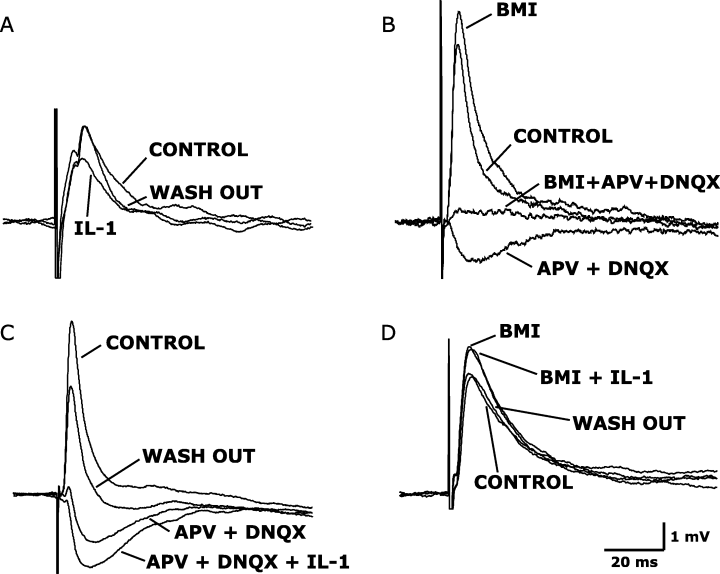
Interleukin-1 (IL-1) administration enhances the amplitude of evoked GABAergic inhibitory post-synaptic potentials in dorsal raphe nucleus (DRN) serotonergic neurons. (A) Electrical stimulation evoked a depolarizing post-synaptic potential (evPSP), the amplitude of which was reduced by IL-1 administration (25 ng/mL). (B) Depolarizing evPSP in DRN serotonergic neurons has two components: the depolarizing and excitatory component (evEPSP) can be unmasked by blocking GABAA receptors [by means of the administration of 10 µm BMI, (–)-bicuculline methiodide], whereas the hyperpolarizing and inhibitory component (evIPSP) can be unmasked by blocking NMDA and non-NMDA glutamate receptors [by means of the administration of 50 µm APV, dl-2-amino-5-phosphonopentanoic acid and 20 µm DNQX, 6,7-dinitroquinoxaline-2,3(1H,4H)-dione]. (C) IL-1 enhanced the amplitude of the GABAergic evIPSP, previously isolated by APV and DNQX administration. (D) IL-1β did not affect the amplitude of the glutamatergic evEPSP, previously isolated by BMI administration. All changes induced by the tested substances were reversible upon wash-out.
Recorded evPSPs consisted of two components (Fig. 3B). N-methyl-d-aspartate (NMDA) and non-NMDA antagonists APV and DNQX were used to unmask the hyperpolarizing and inhibitory, GABAergic component of the evPSP. BMI was used to unmask the depolarizing, excitatory and glutamatergic component of the evPSP. Administration of the three antagonists together abolished the evPSPs (Fig. 3B).
IL-1 increased the GABAergic evIPSP amplitude without affecting glutamatergic evEPSPs
To determine whether IL-1 inhibitory effect on the evPSPs was due to an increase in GABAergic inhibition or to a decrease of glutamatergic excitation, IL-1 was tested on the two single and pharmacologically isolated components of the evPSPs. The administration of IL-1 increased the amplitude of GABAergic evIPSPs. The response in a representative neuron is depicted in Fig. 3C. In responding neurons (eight out of 13 recorded neurons) the amplitude of the evIPSPs was increased by 30.3 ± 3.8% (P = 0.0001; Fig. 4). The effect of IL-1 on evIPSP amplitude was reversible upon wash-out (Fig. 3C). IL-1 application did not change the amplitude of the glutamatergic evEPSP (3, 4; n = 5). The observation that the IL-1-induced decrease in the amplitude of the evPSPs is of similar magnitude to the IL-1-induced increase in the amplitude of the evIPSPs (about 30% in both cases, Fig. 4) suggests that the former effect can be fully attributed to the action of IL-1 on the evoked GABAergic response.
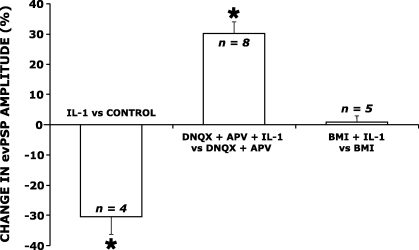
Effects of interleukin-1 (IL-1) administration on the mean amplitude of evoked post-synaptic potentials (evPSPs) in dorsal raphe nucleus (DRN) serotonergic neurons. Bars represent mean (± SE) values (obtained from n = 4–8 neurons) and are expressed as change from the control condition represented by the zero line (see text and Fig. 3 for details). APV, dl-2-amino-5-phosphonopentanoic acid; BMI, (–)-bicuculline methiodide; DNQX, 6,7-dinitroquinoxaline-2,3(1H,4H)-dione. IL-1 administration (25 ng/mL) decreased the amplitude of evPSPs (see Fig. 3A), increased the amplitude of the evoked GABAergic inhibitory post-synaptic potential (see Fig. 3C), without affecting the amplitude of the evoked glutamatergic excitatory post-synaptic potential (see Fig. 3D). *P < 0.05 vs. control condition.
Discussion
Results of the present study show that IL-1 (1) inhibits the firing rate of electrophysiologically and pharmacologically identified serotonergic DRN neurons, and (2) enhances evoked inhibitory post-synaptic GABAergic responses, without affecting evoked glutamatergic post-synaptic responses. As such, these results confirm previous findings that IL-1 inhibits the electrophysiological activity of serotonergic neurons and extend previous findings by indicating that this IL-1 effect is due to the IL-1-induced potentiation of GABAergic inhibition of serotonergic DRN neurons.
It has been consistently shown that IL-1 promotes NREM sleep (Krueger et al., 1984; reviewed in Opp, 2005). Recent observations suggest that IL-1-induced enhancement of NREM sleep may be due to direct IL-1 inhibitory effects on brain wake-promoting neuronal systems.
After extensive investigation during the last 50 years, it is now thought that the direct actions of 5-HT per se are to promote wakefulness (Jouvet, 1999; Pace-Schott & Hobson, 2002), whereas 5-HT may induce other factors that promote subsequent sleep. Experimental manipulations that increase 5-HT release and synaptic availability, such as the electrical stimulation of dorsal raphe nuclei, enhance waking (Cespuglio et al., 1979). In agreement with the interpretation of 5-HT as a wake-inducing substance, blockade of 5-HT2 receptors increases NREM sleep in both rats and humans (Dugovic, 1992). Moreover, neurophysiological (McGinty & Harper, 1976; Trulson & Jacobs, 1979; Cespuglio et al., 1981; Jacobs & Azmitia, 1992) and neurochemical (Cespuglio et al., 1990; Wilkinson et al., 1991; Imeri et al., 1994, 1999; Portas & McCarley, 1994) activity of the serotonergic system increases during wakefulness and decreases during sleep.
As such, results of the present study suggest that IL-1-induced enhancement of NREM sleep may be due to the inhibition of DRN serotonergic neurons, which promotes wakefulness. Such a conclusion supports and extends previous observations that IL-1 electrophysiologically inhibits DRN serotonergic neurons and, when microinjected into the DRN, enhances NREM sleep (Manfridi et al., 2003). Results of the present study, by showing that IL-1 enhances GABAergic evIPSP (without affecting glutamatergic evEPSP), elucidate potential mechanisms by which IL-1 inhibits serotonergic DRN neurons and may thus affect sleep–wake behavior. These results are in agreement with observations showing that IL-1 enhances GABA inhibitory effects acting at both pre- and post-synaptic levels. Interleukin-1 increases GABA release (Feleder et al., 2000; Tabarean et al., 2006), Cl– uptake in synaptosomes (Miller et al., 1991) and GABA-elicited Cl– current in voltage-clamped Xenopus oocytes, GABAergic inhibitory post-synaptic potentials in hippocampal neurons (Luk et al., 1999), recruitment of GABAA receptors to the cell surface in rat cultured hippocampal neurons, as well as in Xenopus oocytes (Serantes et al., 2006). IL-1 also increases cytosolic Ca2+ in 4% of cultured hypothalamic neurons, which were mostly GABAergic (De et al., 2002).
GABAergic neurons that may inhibit DRN serotonergic neurons, as well as other brainstem aminergic wake-active neurons, are distributed throughout the brainstem. The locus coeruleus and DRN itself contain GABAergic local interneurons, which are active during REM sleep, when brainstem aminergic neurons are almost silent (Maloney et al., 1999). Other REM-active GABAergic neurons have been described in the ventrolateral periaqueductal grey (Peyron et al., 1995; Gervasoni et al., 1998, 2000). In addition to cholinergic neurons, the laterodorsal and peduncolopontine nuclei also contain a population of GABAergic neurons that are active during spontaneous REM sleep and during the REM sleep rebound that follows sleep deprivation (Maloney et al., 1999). GABAergic neurons projecting to the brainstem circuits involved in sleep regulation are also present in the hypothalamus (reviewed by Saper et al., 2001).
Although IL-1 receptors have been described in the DRN (Cunningham & De Souza, 1993; Schobitz et al., 1994), their cellular localization (i.e. whether they reside on the serotonergic cell bodies) is not known. As such, whether IL-1 potentiation of GABAergic inhibition of DRN serotonergic neurons results from a pre- or direct post-synaptic action remains to be determined.
Beside DRN serotonergic neurons, IL-1 also inhibits other brain neuronal systems that promotes wakefulness. IL-1 inhibits firing of wake-active neurons in the preoptic area and basal forebrain (Alam et al., 2004). IL-1 also increases the number of c-fos-immunoreactive neurons in the preoptic area and basal forebrain (Baker et al., 2005). The number of these c-fos-immunoreactive neurons positively correlates with the amount of NREM sleep prior to the animals being killed (Baker et al., 2005).
While acting in the DRN and in the preoptic area/basal forebrain, IL-1 can promote NREM sleep and cortical synchronization affecting other brain areas. For instance, IL-1 increases NREM sleep when infused into the subarachnoid space underlying the ventral surface of the rostral basal forebrain (Terao et al., 1998). Unilateral local application of IL-1 onto the somatosensory cortex of rats increases electroencephalographic slow frequencies (Yasuda et al., 2005) and local Fos expression at cortical level, as well as in some subcortical structures such as the thalamic reticular nucleus (which receives a main cortical projection) and hypothalamic regions implicated in sleep regulation (Yasuda et al., 2007).
Beside these direct neuronal actions, IL-1 enhances NREM sleep through several brain systems: (1) growth hormone releasing hormone (GHRH), (2) prostaglandins (PG), such as PGD2, (3) adenosine and (4) nitric oxide (NO). Of note are that (1) IL-1 stimulates the production of GHRH, PGD2, NO and adenosine, (2) it has consistently been shown that GHRH, PGD2 and adenosine enhance NREM sleep, and (3) antagonism (by means of different experimental tools) of these same substances decreases NREM sleep and IL-1-induced NREM sleep enhancement (Obal & Krueger, 2003). Furthermore, IL-1 induces tumour necrosis factor (TNF), a cytokine that has been shown to regulate sleep in both human and non-human animals (Obal & Krueger, 2003).
The precise effects of IL-1 on vigilance states are complex, and depend on dose and timing of administration. IL-1 administered intracerebroventricularly into rats increases NREM sleep across an effective dose range of about 2.5–10 ng (Opp et al., 1991; Gemma et al., 1997). However, doses of IL-1 greater than 10 ng (i.e. 25 ng) when injected intracerebroventricularly disrupt NREM sleep and promote arousal (Opp et al., 1991; Gemma et al., 1997). Although definitive experiments remain to be conducted, the disruptive effects of high doses of IL-1 on sleep are probably due to stimulation of the hypothalamic–pituitary–adrenal (HPA) axis. The HPA axis is the major regulator of the action of IL-1 in the brain, IL-1 is a powerful inducer of HPA axis activity, and components of the HPA axis, notably corticotropin-releasing factor, induce wakefulness (reviewed by Turnbull & Rivier, 1995; Chang & Opp, 2001).
Finally, the present study confirms in a different animal species (i.e. the rat), and using species-specific recombinant IL-1, data previously obtained in guinea-pigs, using human recombinant IL-1 (Manfridi et al., 2003).
Abbreviations
-
- AHP
-
- afterhyperpolarization potential
-
- ACSF
-
- artificial cerebrospinal fluid
-
- APV
-
- dl-2-amino-5-phosphonopentanoic acid
-
- BMI
-
- (–)-bicuculline methiodide
-
- DMSO
-
- dimethyl sulfoxide
-
- DNQX
-
- 6,7-dinitroquinoxaline-2,3(1H,4H)-dione
-
- DRN
-
- dorsal raphe nucleus
-
- evEPSPs
-
- evoked excitatory post-synaptic potentials
-
- evIPSPs
-
- evoked inhibitory post-synaptic potentials
-
- evPSPs
-
- evoked post-synaptic potentials
-
- GABA
-
- γ-aminobutyric acid
-
- GHRH
-
- growth hormone releasing hormone
-
- HPA axis
-
- hypothalamic–pituitary–adrenal axis
-
- 5-HT
-
- 5-hydroxytryptamine
-
- IL-1
-
- interleukin-1
-
- NA
-
- noradrenaline
-
- NO
-
- nitric oxide
-
- NREM sleep
-
- non-rapid eye movement sleep
-
- PE
-
- phenylephrine
-
- PG
-
- prostaglandins
-
- REM sleep
-
- rapid eye movement sleep.