Bax-dependent and -independent death of motoneurons after facial nerve injury in adult mice
Abstract
Nerve injury-induced neuronal death may occur after accidental trauma or nerve inflammation. Although the response to facial root avulsion has been examined in rodent models, there are conflicting results as to whether motoneuron (MN) death is mediated by apoptosis or necrosis. We examined the response of MNs and proximal nerves after facial nerve avulsion in adult mice. Following facial nerve avulsion in 4–5-week-old mice, we observed a progressive reduction of MNs such that by 4 weeks less than 10% of avulsed MNs remained compared with the control side. The profile of MN degeneration was distinct from axotomy-induced responses. For example, the onset of MN death was more rapid, and the extent of MN loss was greater compared with axotomy. Furthermore, the degeneration of oligodendrocytes and the activation of microglia were increased in the proximal nerve after avulsion. Ultrastructural observations suggested that root avulsion mainly induces non-apoptotic neuronal death, although a small subset of neurons appeared to die via apoptosis. To evaluate the contribution of apoptotic death, we evaluated MN responses in Bax-knockout (KO) mice in which neurons are rescued from apoptotic death. Surprisingly, although the majority of Bax-KO mice exhibited only a moderate MN loss after avulsion, a subset of Bax-KO mice (25%) exhibited extensive MN death and injury-induced changes in the nerve that were indistinguishable from events in wild-type littermates. These results suggest that both Bax-dependent and -independent forms of cell death are evoked by root avulsion, and that programmed cell death may be involved in triggering a robust necrotic response.
Introduction
It has long been known that the fate of injured neurons differs depending on the stage of development, the site of injury and animal species (Cowan, 1970; Lieberman, 1974; Crews & Wigston, 1990; Lowrie & Vrbova, 1992; Li et al., 1998; Dai et al., 2000; Lidman et al., 2002; Schweizer et al., 2002). Age-dependent alteration of the neuronal response to injury has been well characterized for motoneurons (MNs) following nerve injury in the rat. For instance, nerve axotomy rapidly triggers cell death in neonatal rats, whereas most injured neurons survive and undergo regenerative processes after a critical stage of postnatal development (P14; Crews & Wigston, 1990; Li et al., 1994). Similar to rats, injured neonatal mouse MNs undergo cell death via a Bax-dependent apoptotic cascade (Deckwerth et al., 1996; Chan et al., 2001, 2003; Kinugasa et al., 2002; Sun & Oppenheim, 2003). In contrast to rats, however, sciatic nerve axotomy in adult mice produces a substantial degree of neuronal death (Serpe et al., 2000), indicating that there are apparent species differences in the response to nerve injury. For example, adult rats but not mice exhibit robust upregulation of nitric oxide synthase (NOS) in MNs following nerve avulsion (Li et al., 1995).
Although the molecular mechanisms underlying such dramatic alteration of cellular responses (death vs survival/regeneration) are unclear, it has been proposed that other cell types such as Schwann cells in the peripheral nerve or the activation of microglial cells may be involved in these different cellular responses (Sendtner et al., 1997; Graeber et al., 1998; Hoke et al., 2002; Wu et al., 2003; Zhao et al., 2004). Because the survival of MNs is mainly dependent on target-derived retrograde trophic signals, the transition from target muscles in perinatal stages to nerve-associated cells, such as Schwann cells in the adult, as major sources of trophic signals may contribute to this marked alteration of the fate of injured neurons. The importance of nerve-associated cells has been proposed based on the fact that complete elimination of peripheral nerve by root avulsion induces massive cell death instead of a regenerative response in the adult rat (Mey & Thanos, 1996; Sakamoto et al., 2000, 2003). Assuming that avulsion-induced adult MN death is caused by an insufficiency of trophic signals, similar to neonatal MN death following axotomy, it has been proposed that apoptotic pathways may drive MN death following both axotomy and avulsion. Consistent with this idea, it has been reported that there was a complete absence of lumbar avulsion-induced MN death in Bax-knockout (KO) mice, which fail to execute apoptotic neuronal death (Martin & Liu, 2002). However, against these results, it has been reported that avulsion-induced MN death in wild-type (WT) mice is necrotic-like (Li et al., 1998). Furthermore, because root avulsion disrupts the brain–blood barrier and promotes traumatic responses such as the invasion of peripheral immune cells, it may induce a qualitatively distinct cell death pathway (Raivich et al., 1998; Piehl et al., 1999).
We have re-examined the issue of whether nerve injury in adult mice involves apoptotic or necrotic neuronal death using various facial nerve injury models in WT and Bax-KO mice. Our results indicate that both apoptotic-like and necrotic-like responses mediate injury-induced MN cell death, suggesting that multiple death pathways may be involved in MN death following nerve injury in adult mice.
Materials and methods
Animals and surgical procedures
Adult C57BL/6 male mice were anesthetized with pentobarbital sodium [50 mg/kg, intraperitoneal (i.p.)]. Bax-KO mice were maintained on a C57BL/6 genetic background, and heterozygous male and female mice were mated to obtain homozygous Bax-KO mice (Sun et al., 2003). For genotyping, polymerase chain reactions (PCR) of genomic DNA from the tail were performed as previously reported (Knudson et al., 1995). The sequences for the PCR used for genotyping are Bax exon 5 forward primer: 5′-TGA TCA GAA CCA TCA TG-3′; Bax intron 5 reverse primer: 5′-GTT GAC CAG AGT GGC GTA GG-3′; Neo reverse primer, 5′-CCG CTT CCA TTG CTC AGC GG-3′.
For facial root avulsion, animals were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and the right facial nerve was exposed at its exit from the stylomastoid foramen under a dissecting microscope. Using microhemostat forceps, the proximal facial nerve was avulsed by gentle traction and removed from the distal facial nerve. Only when a piece of nerve longer than 2 mm was observed was the surgery considered to be successful and the data included in the analyses. Following the surgery, the absence of the facial nerve from the brainstem was verified visually. Because facial nerves could have been detached during tissue sampling, we also evaluated the elimination of the facial nerve in serially cut sections. For proximal axotomy, the facial nerve was cut at the exit position and distal axotomy was performed 5 mm distal from the exit. Because the facial nerve is separated into three major branches, we dissected all three to injure equivalent numbers of MNs. Following surgery the skin incision was sutured, and animals were returned to the home cage. All experiments were carried out in accordance with the regulations and approval of the Animal Care and Use Committee of the Korea University.
Immunohistochemistry
Animals were deeply anesthetized and transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde in 0.1 m phosphate-buffered saline (PBS). The brains were removed, postfixed overnight in the same fixative, cryoprotected in 30% sucrose and sectioned (40 µm). Immunohistochemical staining was performed on free-floating sections using antibodies against SMI-32 (1 : 1000, Sternberger Monoclonals, Baltimore, MD, USA), glial fibrillary acidic protein (GFAP; 1 : 1000, Sigma, St Louis, MO, USA), CNPase (1 : 1000, Chemicon International, Temecula, CA, USA) and phosphorylation-specific c-jun (1 : 200, polyclonal, Cell Signalling, Boston, MA, USA). Briefly, sections were blocked with 3% bovine serum albumin and 0.2% Triton X-100 in PBS for 1 h, and primary antibodies were applied overnight. After several washes with PBS, Alexa-conjugated secondary antibodies (1 : 500, Molecular Probes, Eugene, OR, USA) were applied for 30 min. For isolectin B4 (IB4) histochemistry, Alexa594-conjugated IB4 (1 : 250, Molecular Probes) was incubated with Tris-buffered saline containing 2 mm CaCl2 overnight. Subsequently, sections were washed, counterstained nuclei with Hoechst33342, mounted and observed under a fluorescence microscope (Zeiss, Germany).
Electron microscopy
Animals were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde 10 days following surgery. After perfusion-fixation, brainstems were dissected and placed in the same fixative overnight. Tissues were coronally cut (500 µm) using a vibratome. Sections were rinsed in phosphate buffer, placed in 1% osmium tetroxide for 3 h, dehydrated and embedded in plastic. Semithin sections (1 µm) stained with 1% toluidine blue were screened for MNs of interest, and then thin sections were cut on an ultramicrotome, contrasted with uranyl acetate and lead citrate, and viewed in an Hitachi H-600 electron microscope.
Quantification of MNs
For quantification of facial MN numbers, brains were fixed in Bouin's solution, processed for paraffin embedding, sectioned serially (15 µm) and stained with thionin. Neurons localized in the facial nuclei were counted in every fifth section. We identified MNs based on the following criteria: (1) that the cells are localized in the confines of the facial nucleus; (2) that the cytoplasm in the soma is densely labeled by Nissl staining; and (3) that the cells have a distinct nucleus and a single discrete nucleolus. Because there is little chance that the nucleolus (< 1 µm) is split during the sectioning, this counting method has proven to be virtually independent of the size of objects or section thickness, and the values obtained have been shown to differ by only 1–2% from unbiased stereological methods (Clarke & Oppenheim, 1995; R.W. Oppenheim, unpublished data). In normal WT mice, all MNs in the facial nucleus are large and Nissl-positive with a distinct nucleus and nucleolus, whereas the sizes of injured MNs and a subpopulation of developmentally rescued Bax-KO MNs were smaller than normal WT MNs (Sun et al., 2003). Accordingly, all large and small MNs were included in the quantification of experimental animals. Even small MNs could be easily distinguished from non-neuronal cells. MN counts were multiplied by five for an estimation of the total number of MNs. For examination of cell size, every 10th section was captured by CCD camera and examined by NIH Image software. The same criteria described above for cell counts were also used for cells that were included for cell size measurements.
Results
Post-operative changes in the number of surviving facial MNs after nerve injury in adult mice
We compared the effects of three different types of nerve injury (facial root avulsion, distal and proximal axotomy) on MN death in adult mice (Fig. 1). Four weeks after injury, the number of surviving MNs was quantified following Nissl-staining (Fig. 1A–D). Root avulsion resulted in approximately a 90% loss of MNs during this period. In contrast, proximal or distal nerve axotomy resulted in a substantially smaller loss of MNs compared with root avulsion. It has been reported that nerve axotomy induces chromatolysis (loss of Nissl substrate) and atrophy of surviving MNs in some mouse strains, thereby making reliable MN counts difficult (McPhail et al., 2004). However, it is unlikely that this occurred in our experiments because all MNs visualized by retrograde labeling with DiI from the nerve end after proximal nerve axotomy (n = 3, data not shown) were Nissl-positive. Therefore, we conclude that the reduction in the number of Nissl-stained MNs was mainly attributable to the degeneration of neurons.
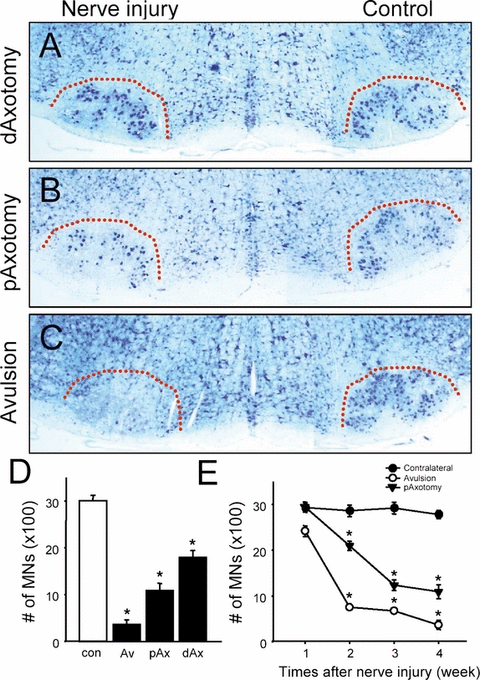
Differential motoneuron (MN) loss 4 weeks following distal facial nerve axotomy (A, dAxotomy, n = 4), proximal axotomy (B, pAxotomy, n = 4) and root avulsion (C, Avulsion, n = 6). Dotted lines indicate the boundary of the facial motor nucleus. (D) Quantification of the number of surviving MNs 4 weeks after avulsion (Av), proximal (pAx) or distal axotomy (dAx). The number of MNs on the contralateral control (con) side of the avulsion group is also included. Data are mean ± SEM. (E) Time-course changes in surviving MN numbers 1–4 weeks after proximal axotomy or root avulsion. Data are mean ± SEM. *P < 0.05 in Student's t-test comparisons.
To further characterize the degeneration response of MNs following the different types of nerve injury, we evaluated temporal changes in the number of surviving MNs after root avulsion or proximal axotomy (Fig. 1E). Although MN death was not evident 1 week after nerve axotomy, by 3 weeks the number of surviving MNs had been progressively reduced to 40% of control. In contrast, root avulsion induced significantly more rapid and greater MN loss. By 1 week, a substantial portion of avulsed MNs had degenerated, and only 25% remained at 2 weeks. Collectively, these results demonstrate that these two injuries induce a different degree and temporal-course of MN death.
Axonal degeneration and microglial activation in the injured proximal nerve
Because we found a significant difference in the extent of MN death following the two different types of nerve injury, we compared the response of the remaining proximal nerve 4 weeks following surgery. Following axotomy, substantial NF200-immunoreactivity (IR) remained in the proximal nerve (Fig. 2D), which is attributable to the axons of surviving MNs. In contrast, NF200-IR was virtually absent in the avulsed animals (Fig. 2G), suggesting that few surviving MNs remained and that those still present had already lost their proximal axon. Notably, we found that the avulsed nerve contained a large mass of cells revealed by fluorescent nuclear counterstaining or by dense background labeling in Nissl-stained sections, whereas control or proximal axotomized nerves contained few if any such cell accumulations. To characterize these compacted cells in the avulsed nerve, we performed immunolabeling with an astrocyte marker (GFAP), a microglial marker (IB4) and an oligodendrocyte marker (CNPase). In controls, there were only a few marginal IB4+ or GFAP+ glial cells (Fig. 2B and E), whereas we observed a significant accumulation of IB4+ cells but only marginal GFAP+ cells in the avulsed nerve (Fig. 2L). These results suggest that root avulsion promotes microglial activation in the injured proximal nerve, whereas very little microglial activation occurs in the nerve after axotomy. However, in contrast to the nerve, there was marked microglial activation in the facial motor nucleus following axotomy. Microglial activation was very evident 10 days after root avulsion when MNs were degenerating, suggesting that microglial activation in the proximal nerve is an early and specific event after root avulsion. Accordingly, we found that root avulsion induced the degeneration of oligodendrocytes in the proximal nerve. Following axotomy the morphology of oligodendrocytes appeared normal, as was expected because of the substantial survival of MNs (Fig. 2F). Following root avulsion, however, the columnar array of oligodendrocytes was disrupted and the number of surviving oligodendrocytes appeared diminished (Fig. 2I).
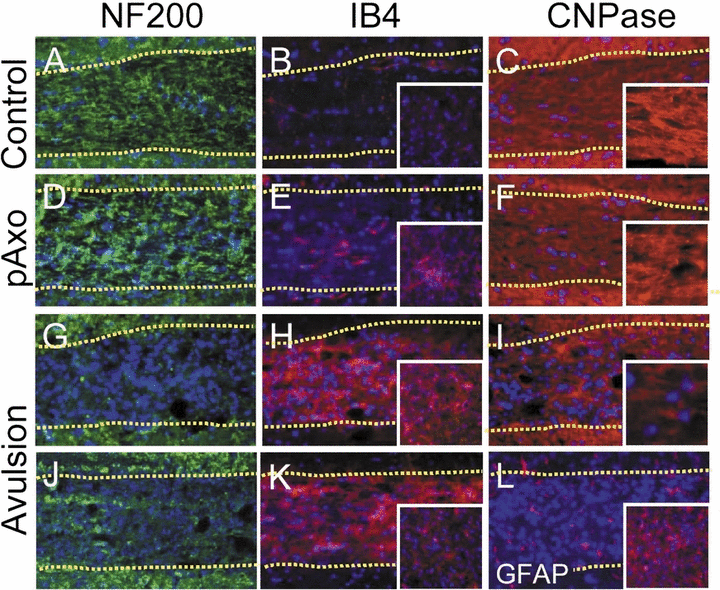
The response of the proximal nerve to the different types of injury. Four weeks (D–I, L) or 10 days (J, K) following proximal axotomy (pAxo; D–F) or avulsion (G–L) and in control (A–C), the proximal nerves were labeled with anti-neurofilament (NF200; A, D, G, J), isolectin B4 (IB4; B, E, H, K), CNPase (C, F, I) or glial fibrillary acidic protein (GFAP; L). The nucleus was counterstained with Hoechst33342 (blue). Boundaries of the nerve are indicated by dotted lines. Insets in (B, E, H, K, L) show labeling in the motor nucleus. Insets in (C, F, I) show higher-magnification images of CNPase-immunolabeled oligodendrocytes in the nerve.
Ultrastructural changes in the injured MNs
Electron microscopic examination of the ultrastructure of the dying MNs revealed that a small subpopulation of degenerating neurons following root avulsion exhibited morphological characteristics of apoptosis, such as condensation of the nucleus and shrinkage of the cytoplasm (Fig. 3A vs B). Supporting this, TUNEL+ cells were seen in the avulsed motor nucleus (Fig. 3F and G), indicating that at least a subset of avulsed MNs undergo apoptotic neuronal death. However, most dying avulsed MNs exhibited non-apoptotic morphology, including cells with numerous vacuoles containing membranous debris in the cytoplasm (Fig. 3C) and that were engulfed by phagocytic cells including microglia (Fig. 5). These cells also exhibited a dilated mitochondrial membrane, which is also indicative of necrotic death (Fig. 3E), and some avulsed cells contained cytoplasmic debris with chromatin clumps dispersed in the cytoplasm characteristic of cells at the end-stage of non-apoptotic cell death (Fig. 3D). These putative non-apoptotic dying cells were not observed following axotomy (data not shown).
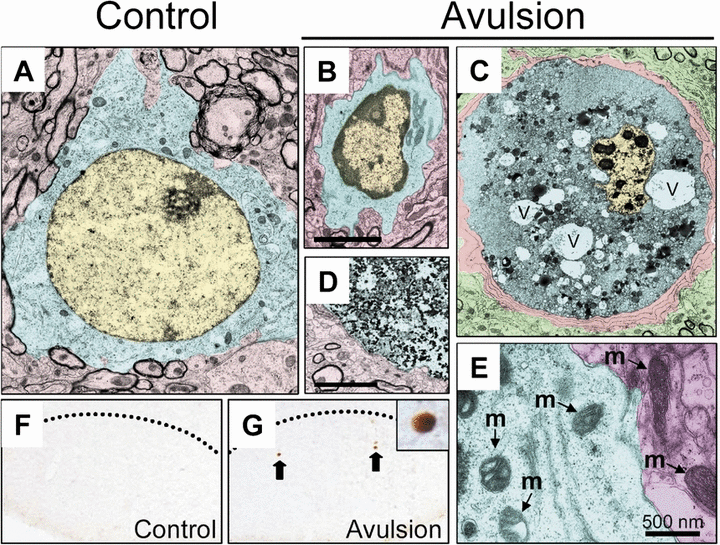
Ultrastructure of degenerating MNs following root avulsion. Note the initial modification of nuclear condensation in some cells (B, compared with intact cells in A). In some cells, nuclei were greatly condensed and vacuoles (v) were well developed (C). Note that microglial cells enwrap the degenerating MNs. Marked degeneration of intracellular materials was observed in an apparent end-phase degenerating cell (D). Images were pseudocolored in yellow (nuclei), blue (cytoplasm) and red (other intact structures or ensheathing microglia in C). In some degenerating MNs, mitochondrial swelling was evident compared with the mitochondria (m) in intact MNs (area pseudocolored in red, E). (F and G) TUNEL labeling of facial MNs indicates apparent apoptotic neuronal degeneration on the avulsed side (G) but not the control side (F).
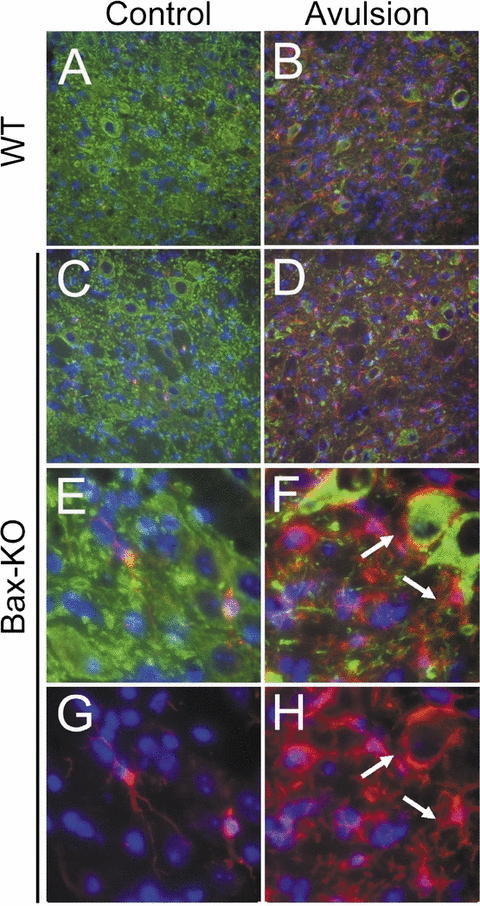
Microglial activation after root avulsion in Bax-knockout (KO) facial nuclei. Control (A, C, E, G) vs avulsed (B, D, F, H) side of facial nuclei in wild-type (WT; A, B) and Bax-KO (C–H) mice following double-immunofluorescence labeling with anti-SMI32 (MN marker, green) and IB4 (microglial marker, red). Nuclei were counterstained with Hoechst33342 (blue). (E–H) High-magnification views showing examples of quiescent and activated microglial cells found in Bax-KO mice. Arrows indicate microglial cells enwrapping injured/degenerating MNs.
Bax-dependent and -independent MN death following root avulsion
It has been previously reported that neonatal axotomy-induced MN death is mediated by the proapoptotic gene, Bax (Deckwerth et al., 1996; Sun & Oppenheim, 2003). However, because root avulsion appeared to induce both apoptotic and non-apoptotic MN death, we next asked whether avulsion-induced adult MN death is Bax-dependent. Four weeks after surgery when only 9% of MNs in WT littermates remained, almost 60% of MNs in Bax-KO mice were still viable (Fig. 4). Surprisingly, we found two distinct groups of mice that exhibited different amounts of MN survival in Bax-KO mice. One group (Group A, six out of the eight animals examined) exhibited a 30% loss of MNs, whereas the other subset of animals (Group B) exhibited an almost 80% loss of MNs, which is similar to the MN loss in WT littermates following avulsion. We failed to identify any substantial differences between these two groups in sex, age, body weight, housing condition or success of the surgery. Interestingly, however, we found that all the animals in Group B exhibited cell accumulations in the proximal nerve as revealed by Nissl staining, whereas Group A lacked such cell accumulations (Inset in Fig. 4, Db). Collectively, these results suggest that although a subset of avulsion-induced MN death is Bax dependent, a substantial amount of MN death is Bax independent and appears to involve a non-apoptotic mode of degeneration.
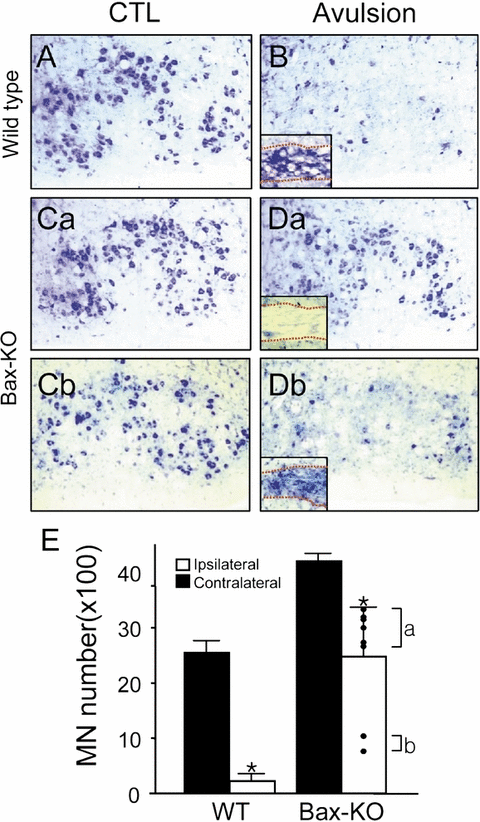
Reduction of avulsion-induced motoneuron (MN) death in Bax-knockout (KO) mice. Typical Nissl-stained images of facial motor nuclei on control (CTL; A, C) and avulsed sides (4 weeks; B, D) of wild-type (WT; A, B) and Bax-KO (C, D) mice. Note that two contrasting responses were seen in Bax-KO animals (Da vs Db). Insets in (B) and (D) show the Nissl-stained nerve from the same animals. Dotted lines indicate the boundaries of nerve. (E) Quantification of surviving MN numbers. MN numbers in individual avulsed Bax-KO mice are indicated by dots. Data are mean ± SEM, n = 8. *P < 0.05 in Student's t-test comparisons.
Because microglial activation is indicative of neuronal degeneration, we examined microglial activation in the WT and Bax-KO facial nucleus 2 weeks after root avulsion, which is the peak period of MN loss (n = 3, Fig. 5). On the contralateral side, only a limited number of quiescent microglia was observed in both WT and Bax-KO mice, whereas microglial cells were markedly increased on the avulsed sides in both WT and Bax-KO mice, and they exhibited ameboidal morphology, which is a hallmark of activated microglia. Many injured MNs were enwrapped by microglial processes, which is inconsistent with our ultrastructural observations (Fig. 3C). The extent of microglial activation was quite variable in both WT and Bax-KO mice; a more comprehensive quantitative analysis will be needed to evaluate that microglial activation is related to the extent of MN death in WT and Bax-KO mice. However, the existence of marked microglial activation in the avulsed Bax-KO facial nucleus supports the idea that root avulsion evokes Bax-independent MN death.
Differential atrophic responses of WT and Bax-KO MNs after root avulsion
It is well known that injured surviving MNs are atrophied because they fail to receive appropriate trophic supports from efferent targets. Compared with WT, Bax-KO mice exhibited significantly reduced MN sizes (Fig. 6), which is consistent with our previous observation of spinal MNs in Bax-KO mice (Buss et al., 2006). We observed that a subset of Bax-KO MNs that were < 200 µm2, that were absent in WT littermates, and that are likely developmentally rescued, atrophied MNs in Bax-KO mice (Sun et al., 2003). Following root avulsion, the size of WT MNs was substantially reduced, and MNs that were less than 100 µm2 or between 100 and 200 µm2 were observed. A similar reduction in cell size was also seen in avulsed Bax-KO MNs. However, the responses of Group A and B in the Bax-KO were different. In Group A (with mild MN loss), MN sizes were only moderately reduced and the size histograms of avulsed MNs peaked at 100–200 µm2, whereas the MNs smaller than 100 µm2 were rare. By contrast, MNs in Group B (with robust MN loss) exhibited more striking size reductions such that > 40% MNs were in the > 100 µm2 size range.
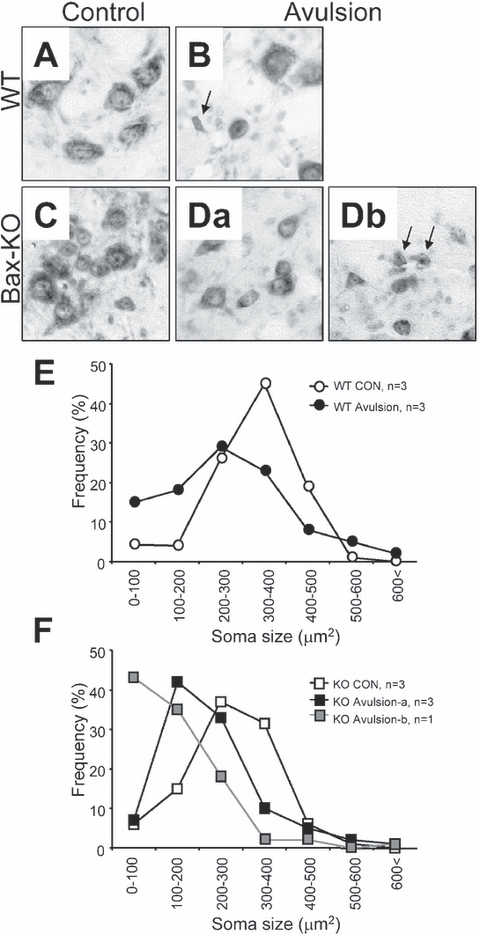
Morphology of MNs in (A) control wild-type (WT), (B) avulsed WT, (C) control Bax-knockout (KO), (Da) Group A avulsed Bax-KO and (Db) Group B avulsed Bax-KO mice. Arrows indicate the MNs smaller than 100 µm2. (E and F) Frequency-fractionation analyses of the soma size changes after root avulsion in (E) WT and (F) Bax-KO mice. Mean values from three animals (except the Bax-KO, Group B, n = 1) are shown.
Deletion of Bax reduces the death of oligodendrocytes and increases the activation of microglia following avulsion
Differential changes in the proximal nerve of Bax-KO mice following avulsion (Group A vs Group B) appears to be associated with the extent of MN death. To further examine this possibility, an additional group of four Bax-KO animals were analysed; similar to the animals described above one of these had MN loss similar to Group B whereas the other three were comparable to Group A animals (data not shown).
On the contralateral control side of both WT and Bax-KO mice, the appearance and density of myelinated oligodendrocytes were similar (Fig. 7A and E). Following root avulsion in WT mice, the number and appearance of oligodendrocytes in the ipsilateral nerve appeared to be reduced and disorganized (Fig. 7B), and there was increased microglial activation (Fig. 7D). By contrast, in the three Group A-like Bax-KO mice (Fig. 7, Fa), oligodendrocytes appeared normal and there was little microglial activation (Fig. 7, Ha), whereas the Group B-like Bax-KO nerve exhibited a marked reduction and severe disorganization of oligodendrocytes and reduced microglial activation, which were indistinguishable from avulsed WT nerves (Fig. 7, Fb and Hb). These results are consistent with the suggestion that microglial activation in the nerve may be related to a Bax-independent mode of MN death.
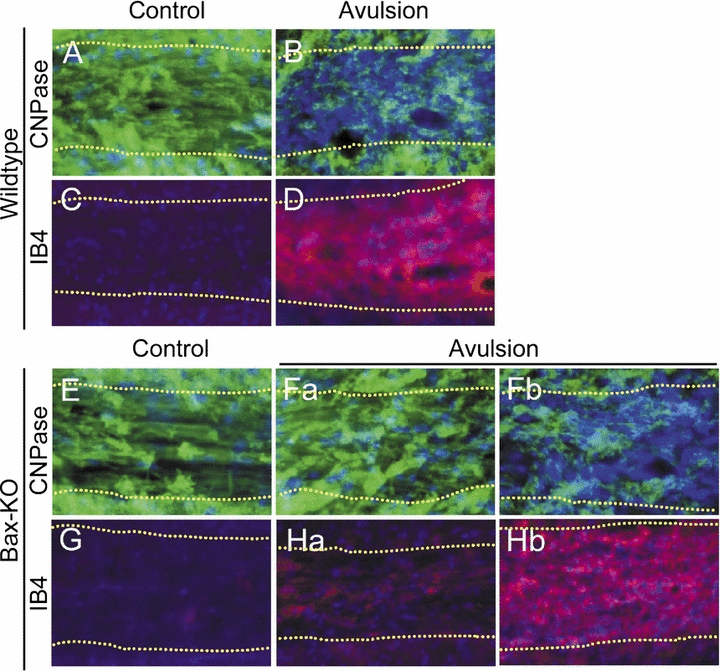
Differential response of Bax-knockout (KO) proximal nerves is related to the degree of MN death. Oligodendrocytes in the wild-type (WT; A, B) or Bax-KO (E, F) were immunolabeled with anti-CNPase (green), and microglial cells in the WT (C, D) or Bax-KO (G, H) were labeled with isolectin B4 (IB4; red) in control (A, C, E, G) or avulsed proximal nerves (B, D, F, H). Nuclei were counterstained with Hoechst33342 (blue). Boundaries of the nerve are indicated by dotted lines. Note the two contrasting responses of oligodendrocyte degeneration (Fa vs Fb) and microglial activation (Ha vs Hb) in the two subgroups (A and B) of Bax-KO mice.
Discussion
Characterization of the facial nerve injury models in adult mice
Nerve injury has been widely used as a model to study trauma-induced responses in adult animals. Because traumatic nerve injury occurs following accidents, tumors, infections, diseases and surgical manipulations in humans, these models may be useful for understanding the biological basis of the resulting pathologies and for developing therapeutic strategies. Among the many available nerve injury models, root avulsion is considered to be one of the most radical procedures that induces massive MN death in the adult (Koliatsos et al., 1994; Li et al., 1995, 1998; Sakamoto et al., 2000, 2003). However, the response to facial root avulsion in adult mice has received little attention (Moran & Graeber, 2004). Because the mouse is the species of choice for gene modification, the characterization of the genes involved in the response of adult mice to avulsion injury is of increasing interest. Our results show that avulsion of the facial nerve induces a massive and progressive MN death (4 weeks: 90% loss) in mice, which is comparable to the response of adult rats (4 weeks: 70% loss; Sakamoto et al., 2000). However, we cannot exclude the possibility that the underlying molecular events in mice vs rats may differ. For example, NOS is induced in MNs after cervical root avulsion in adult rats but not in mice (Li et al., 1995, 1998; Martin et al., 1999). Furthermore, it is known that root avulsion in mice can promote infiltration of activated T-cells to the injured site, whereas a similar response is reduced in the rat (Graeber et al., 1990; Raivich et al., 1998).
In comparison to facial nerve axotomy, we observed substantially more MN death following root avulsion. In agreement with previous studies in rats, the extent of MN death in mice was related to the length of the remaining nerve. In addition, comparison of the temporal-course of MN loss following root avulsion vs proximal axotomy revealed that root avulsion induced a more rapid MN death. Following axotomy, strong microglial activation was seen in the motor nucleus where some MNs are degenerating, whereas very little microglial activation occurred in the nerve. By contrast, root avulsion induced a marked microglial activation in both the injured nerve and the motor nucleus. At time points when similar numbers of surviving MNs were observed in avulsed (10 days) and axotomized (4 weeks) animals, a marked loss of axons accompanied by microglial activation was evident only in the avulsed nerve, suggesting that the different responses to the two injuries cannot be explained by differential MN loss. We argue that the different response of the nerve may be related to the marked loss of injured nerve segments and the associated death of oligodendrocytes in proximal nerve following nerve avulsion. Because the survival of oligodendrocytes is supported by factors released from axons (Sendtner et al., 1997; Hoke et al., 2002), the combination of rapid degeneration of axons by root avulsion and the traumatic injury of oligodendrocytes may trigger a massive loss of oligodendrocytes and microglial activation. Collectively, these results suggest that root avulsion and nerve axotomy may produce not only quantitative differences in MN death, but the resulting MN death may occur by qualitatively different cell death pathways.
Apoptotic and necrotic death of root avulsed MNs
Although it is well known that neonatal nerve injury induces apoptotic neuron death (Deckwerth et al., 1996; Sun & Oppenheim, 2003), the mechanism of adult MN death after root avulsion remains unclear. Some investigators have reported that the degeneration of MNs following avulsion is due to apoptosis (Martin et al., 1999, 2001; Martin & Liu, 2002), whereas others indicate that it more closely resembles necrosis (Li et al., 1998). These discrepancies may be attributable to differences in the region of injury (cervical vs lumbar) or species of animals examined (rat vs mouse). The present results suggest that the mechanism of adult MN degeneration after root avulsion is rather complex. Although we found a few degenerating MNs exhibiting apoptotic morphology, other dying MNs exhibited morphological characteristics of necrosis, as revealed by ultrastructural examination.
In this context, the observations from Bax-KO mice are especially interesting. Because it is well known that the pro-apoptotic gene bax is essential for postmitotic neuronal apoptosis but not for necrosis, the extent of neuronal death after root avulsion in Bax-KO mice may implicate the contribution of apoptosis. In attempts to quantify the number of surviving MNs in Bax-KO mice, there are a few methodological considerations. First, although all facial MNs are rescued from developmental programmed cell death (Deckwerth et al., 1996; White et al., 1998), the excess MNs became atrophied and may fail to maintain the expression of some MN-specific markers (Sun et al., 2003). Thus, we applied modified criteria for MN counts and we counted all neurons within the facial nucleus regardless of soma size or the extent of Nissl label. Because the facial motor nucleus is anatomically separated from other neuronal types and few if any interneurons are found in the facial motor nucleus, we are confident that these counts include all normal and atrophied MNs. The number of facial MNs in the adult Bax-KO is equivalent to the number present in the embryo on embryonic day (E)16 prior to the onset of normal programmed cell death (Sun et al., 2003), and the number of axons in the proximal facial nerve closely matches the number of neurons in the facial motor nucleus in both WT and Bax-KO mice (Buss et al., 2006).
Our results indicate that there is approximately 30–80% MN loss in adult Bax-KO mice following avulsion. Although there is significantly more surviving MNs in Bax-KO mice compared with WT, these data indicate that a subpopulation of injured MNs may die by a Bax-independent mechanism. Contrary to our results, Martin et al. (2002) reported that Bax-KO mice are resistant to sciatic nerve avulsion, and that all spinal MNs survive following injury. It seems likely that this discrepancy reflects the differential role of Bax in spinal vs facial avulsion-induced death. It would be interesting to determine whether sciatic nerve avulsion evokes microglial activation in the remaining proximal nerve, similar to what we have observed in the proximal facial nerve.
Although we cannot entirely rule out the possibility that a subset of small atrophied MNs were excluded from our facial MN counts, we consider it unlikely that the reduction in MN counts in Bax-KO mice can be attributed to MN atrophy. Because we were able to recognize and include small MNs in our cell counts, it is unlikely that the reduction in MN number in Group A is due to the exclusion of small unrecognizable MNs.
The possible interaction of Bax-dependent and -independent responses to root avulsion
Although the majority of Bax-KO mice exhibited only a moderate loss (29%) of MNs after avulsion injury, a substantial proportion of Bax-KO mice (25%) exhibited much more extensive MN death (80%), similar to the response of WT littermates. Although it is unclear what is responsible for this striking difference in animals of the same genotype, our data suggest that non-cell (MN) autonomous events are involved. For example, these two groups also exhibited distinct glial responses in the remaining proximal nerve. Microglial activation in the nerve occurred earlier than the major period of MN death following root avulsion, and this microglial activation appeared to be associated with the degeneration of axons and oligodendrocytes. Microglial activation is believed to be part of the response to traumatic injury and acts to enhance neuronal death (Serpe et al., 1999; Jones et al., 2005), although a moderate activation of microglial cells may actually protect neurons from death (Raivich et al., 1998). Because microglial activation can be evoked by apoptosis, reduced cell death in Bax-KO mice might reduce microglial activation and attenuate further MN loss. In fact, it has been reported that injury-induced oligodendrocyte death is also dependent on Bax, and that oligodendrocytes degenerate in Bax-KO mice following injury (Dong et al., 2003). Thus, reduced axonal and oligodendrocyte loss in the Bax-KO nerve may inhibit or reduce microglial activation, and thereby reduce the role of microglial-derived death signals. In this context, our observations suggest that Bax-dependent apoptosis may trigger Bax-independent necrosis by a non-cell autonomous pathway.
Acknowledgements
This study was supported by the Korean Ministry of Science, Technology Grants M10412000078-04N1200-07810 (W.S.), and NIH grants NS20402 and NS048982 and a grant from the Robert Packard ALS centre at Johns Hopkins University (R.W.O.). Some of the experiments were technically supported by the 21C Frontiers BRC program.
Abbreviations
-
- GFAP
-
- glial fibrillary acidic protein
-
- IB4
-
- isolectin B4
-
- i.p.
-
- intraperitoneal
-
- IR
-
- immunoreactivity
-
- KO
-
- knockout
-
- MN
-
- motoneuron
-
- NOS
-
- nitric oxide synthase
-
- PBS
-
- phosphate-buffered saline
-
- PCR
-
- polymerase chain reaction
-
- WT
-
- wild-type.