Cannabinoids modulate Olig2 and polysialylated neural cell adhesion molecule expression in the subventricular zone of post-natal rats through cannabinoid receptor 1 and cannabinoid receptor 2
Abstract
The subventricular zone (SVZ) is a source of post-natal glial precursors that can migrate to the overlying white matter, where they may differentiate into oligodendrocytes. We showed that, in the post-natal SVZ ependymocytes, radial glia and astrocyte-like cells express cannabinoid receptor 1 (CB1), whereas cannabinoid receptor 2 (CB2) is found in cells expressing the polysialylated neural cell adhesion molecule. To study CB1 and CB2 function, post-natal rats were exposed to selective CB1 or CB2 agonists (arachidonyl-2-chloroethylamide and JWH-056, respectively) for 15 days. Accordingly, we found that CB1 activation increases the number of Olig2-positive cells in the dorsolateral SVZ, whereas CB2 activation increases polysialylated neural cell adhesion molecule expression in this region. As intense myelination occurs during the first weeks of post-natal development, we examined how modulating these factors affected the expression of myelin basic protein. Pharmacological administration of agonists and antagonists of CB1 and CB2 showed that the activation of both receptors is needed to augment the expression of myelin basic protein in the subcortical white matter.
Introduction
The subventricular zone (SVZ) is an adult neurogenic area that develops from the embryonic and early post-natal ventricular zone and SVZ (Alvarez-Buylla & Lim, 2004). In these early post-natal periods, radial glia are the neural stem cells that mature into ependymocytes and adult SVZ stem cells (astrocyte-like cells) (Tramontin et al., 2003; Spassky et al., 2005). In the adult, astrocyte-like cells or type B cells give rise to type C cells or transit-amplifying precursors, which divide very actively and mature into type A cells or neuroblasts. These cells migrate through the rostral migratory stream into the olfactory bulb where they become neurones (Doetsch et al., 1999). However, SVZ precursors can also migrate radially through the dorsolateral aspect to the overlying white matter, a site where they can differentiate into astrocytes and oligodendrocytes during post-natal myelination (Levison & Goldman, 1993; Kakita & Goldman, 1999). Post-natal myelination progresses in a caudo-rostral and latero-medial fashion and, by post-natal day (P)15, a peak of gliogenic activity in the SVZ is observed in conjunction with intense myelination in subcortical white matter tracts (Bjelke & Seiger, 1989; Hamano et al., 1996). This gliogenic activity decreases as development progresses into adulthood, a period when neurogenesis predominates in the SVZ (Suzuki & Goldman, 2003). However, a subpopulation of B- and C-cells that express the Olig2 transcription factor and the polysialylated neural cell adhesion molecule (PSA-NCAM) migrates into areas of the white matter and differentiates into oligodendroglial cells (Menn et al., 2006). Interestingly, adult oligodendrogenesis from the SVZ increases after toxic and autoimmune demyelinating lesions, when stem/precursor cells are recruited from this zone towards the injured area and give rise to oligodendrocytes (Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002; Menn et al., 2006). Therefore, the SVZ is a potential source of glial precursors that can migrate and differentiate along the oligodendrocyte lineage on demand. However, the molecular orchestra that drives the immature precursors to become myelinating oligodendrocytes remains unknown, like that which controls their migration towards de- or unmyelinated areas. Cannabinoids could contribute to the signals that control this process as they promote remyelination in a murine model of multiple sclerosis and oligodendrocyte progenitor survival in vitro (Molina-Holgado et al., 2002; Arevalo-Martin et al., 2003).
Cannabinoids bind to and activate cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) G-protein-coupled receptors (Matsuda et al., 1990; Munro et al., 1993). CB1 is primarily expressed in areas of the brain involved in the control of motor coordination, memory and cognitive processes (Herkenham et al., 1990). It has also been localized in the SVZ and, although its cellular distribution has not been fully resolved, it appears to increase the proliferation of neural precursor cells in this tissue (Berrendero et al., 1999; Jin et al., 2004). However, CB2 is predominantly found in the immune system where it modulates inflammatory processes, whereas in the brain it is thought to be restricted to microglial cells (for a review see Correa et al., 2005). However, it was recently shown that CB2 is also functionally expressed by neurones in the brainstem and other brain structures (Van Sickle et al., 2005; Gong et al., 2006).
We have studied the cellular localization of CB1 and CB2 in the post-natal SVZ and have evaluated the effects of cannabinoid treatments on gliogenesis in the SVZ, and on the myelination of subcortical white matter tracts.
Materials and methods
Reagents
The selective agonist of CB1, arachidonyl-2-chloroethylamide (ACEA), was purchased from Tocris (Bristol, UK), as was the selective CB2 agonist, JWH-056 (JWH). The non-selective CB1/CB2 agonist (+)WIN 55,212-2 (WIN) was obtained from Sigma (Madrid, Spain). The selective antagonists of CB1, SR141716A (SR1), and CB2, SR144528 (SR2), were kindly provided by Sanofi-Aventis (Montpellier, France). All other reagents were obtained from standard suppliers.
Animals
Pregnant Wistar rats were obtained from Harlan-Interfauna Ibérica (Barcelona, Spain). For the localization of CB1 and CB2 in the post-natal SVZ, non-treated male progeny were killed at P7 and P15. For the experiments with cannabinoids, male pups were exposed to agonists or agonists and antagonists from P1 to P14 and the animals were killed at P15. The pups were maintained with their dams on a 12 h light/dark cycle in our in-house colony (Hospital Nacional de Parapléjicos, Toledo, Spain). Male littermates were included as control animals for each treatment and all litters were reduced to a fixed number of 10 pups.
Cannabinoid treatment of animals
Animals received a daily subcutaneous injection of cannabinoids in the neck from P1 to P14. Cannabinoids were always injected at noon with a G30 non-pyrogenic and sterile needle. Each treatment was performed in a separate litter and control littermates were injected with vehicle alone [either 1% bovine serum albumin and 0.2% dimethylsulphoxide in saline solution for WIN and WIN plus SR1 or SR2, or 1% bovine serum albumin and 0.2% ethanol in saline solution for ACEA]. Four neonatal males were injected with WIN, ACEA or WIN plus SR2 and five were treated with WIN plus SR1 or JWH. The doses of cannabinoids administered were based on those previously reported to effectively promote remyelination in a murine model of multiple sclerosis (Arevalo-Martin et al., 2003). To avoid potential habituation, the doses of the drugs administered increased as the treatment progressed, in accordance with the following regimes: 2.5 mg/kg of WIN up to P5, 3.75 mg/kg on P6–10 and 5 mg/kg on P11–14; the doses of ACEA were maintained at 1.25 mg/kg until P5 and rose to 1.9 mg/kg on P6–10 and 2.5 mg/kg on P11–14; SR1 or SR2 was administered at 2 mg/kg until P5, 3 mg/kg on P6–10 and 4 mg/kg on P11–14; JWH was injected at 2.5 mg/kg until P5, 3.75 mg/kg on P6–10 and 5 mg/kg on P11–14. Animals were handled in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals, the principles presented in the Guidelines for the Use of Animals in Neuroscience Research by the Society for Neuroscience and following the European Union guidelines (Council Directive 86/609/EEC). Experimental procedures were approved by our institutional animal use and care committee, the Ethical Committee for Animal Welfare at the National Paraplegics Hospital (CEBA). Special care was taken to reduce the number of animals used to the minimum required for statistical accuracy.
Immunohistochemistry
Rats were deeply anaesthetized with pentobarbital (100 mg/kg, Normon Veterinary Division, Madrid, Spain) and perfused transcardially with 0.1 m phosphate buffer and then with 4% paraformaldehyde in 0.1 m phosphate buffer or with a mixture of 4% paraformaldehyde and 1% glutaraldehyde in 0.1 m phosphate buffer for electron microscopy. The isolated brains were post-fixed in the same fixative for 4 h and stored in non-freezing Olmos solution at −18 °C until use. Serial coronal vibratome sections (50 µm) were obtained and subjected to immunohistochemistry using antibodies against: CB1 (1 : 300; 216401, Calbiochem, Barcelona, Spain or AB5636P, Chemicon, Madrid, Spain); the C-terminal region of CB2 (1 : 500; AB5642P, Chemicon); the N-terminal region of CB2 (1 : 500; PA1-746, Affinity Bioreagents, Madrid, Spain); glial fibrillary acidic protein (GFAP) (1 : 1000; Sigma, clone GA5); nestin (1 : 100; 556309, BD Biosciences, Madrid, Spain); phospho-histone H3 to specifically label mitotic nuclei (1 : 1000; 06-570, Upstate, Barcelona, Spain); PSA-NCAM (1 : 500, ABC0019, Abcys, Paris, France); anti-olig2 (1 : 30 000; from Dr Stiles' laboratory, a generous gift from Dr Rowitch); myelin basic protein (MBP) (1 : 1000; Sternberger Monoclonals); anti-vimentin phosphorylated at Ser55, clone 4A4 (1 : 1000; a generous gift from Dr Martínez-Cerdeño); the radial glia marker anti-3CB2 (1 : 300; a generous gift from Dr de la Rosa; Prada et al., 1995); or anti-aldolase C/zebrin II (1 : 1000; a generous gift from Dr Hawkes). For double-labelling experiments and optical density measurements, primary antibodies were detected with Alexa-conjugated anti-mouse and anti-rabbit secondary antibodies (Molecular Probes, Eugene, OR, USA), which were visualized by confocal microscopy (TCS 4D; Leica, Madrid, Spain). To estimate cell number, the primary antibodies were detected with secondary peroxidase-conjugated (Bio-Rad, Hercules, CA, USA) or biotinylated (Jackson Immunoresearch Laboratories, West Grove, PA, USA) antibodies that were then incubated with avidin-peroxidase (Sigma). After developing with diaminobenzidine (Sigma), sections were mildly counterstained with toluidine blue to visualize the SVZ. Sections processed for phospho-histone H3 and phospho-vimentin 4A4 were developed using nickel-intensified diaminobenzidine staining as described previously (DonCarlos et al., 2003). Negative controls were performed by omitting the primary antibody or by pre-absorbing the CB1 antibody with the immunogenic peptide derived from residues 1–14 of the human, mouse and rat CB1 protein (Cayman Chemical, Madrid, Spain).
Electron microscopy
For electron microscopy, brains were post-fixed overnight in 4% paraformaldehyde and 1% glutaraldehyde, rinsed in phosphate buffer and coronal sections (50 µm) were obtained using a vibrating microtome. CB1 was visualized by immunocytochemistry carried out in the absence of detergent and following diaminobenzidine staining. The dorsolateral SVZ was dissected out, post-fixed in 1% osmium tetroxide in 0.1 m phosphate buffer with 5% glucose, dehydrated in acetone and embedded in Spurr resin (TAAB, UK). Semithin sections (3 µm) were stained with 1% toluidine blue, re-embedded in Spurr and 60 nm sections were mounted onto grids and examined under a Jeol 1200EXII electron microscope.
Quantification of the cell number in the subventricular zone
The complex mixture and distribution of cells in the SVZ make it difficult to count cells other than by nuclear staining. In a thick section of the SVZ, it was not easy to identify the number of cells with cytoplasmic or membrane immunoreactivity in an unbiased manner. Therefore, we only quantified the number of cells when the immunostainings were directed against nuclear markers except in the case of 4A4, in which the cytoplasmic staining was easy to distinguish and was restricted to a small number of cells.
In any type of quantification, at least three histological sections lying between the coordinates +1.3 and 0 mm from Bregma (Paxinos & Watson, 2005) were assessed per rat. These coordinates were macroscopically distinguishable as a region immediately posterior to the disappearance of the ventral interhemispheric fissure and the appearance of the medial septum (approximately +1.3 cm from Bregma) and immediately anterior to the crossing of the anterior commissure (approximately 0 cm from Bregma). The SVZ was defined as the area of high nuclear density between the lateral ventricle, striatum and floor of the corpus callossum. This area was delimitated using a slight counterstaining of cell nuclei with toluidine blue or Hoescht.
Number of proliferating cells (phospho-histone H3 or 4A4)
The number of proliferating cells (phospho-histone H3- or 4A4-immunoreactive cells) was estimated by the optical disector method, using the total section thickness as the disector height (Hatton & von Bartheld, 1999). We counted all phospho-histone H3- or 4A4-positive cells within the upper lateral and dorsolateral SVZ. At least three histological sections that lay approximately between the coordinates +1.3 and 0 mm from Bregma were assessed per rat (Paxinos & Watson, 2005). Toluidine blue counterstaining allowed us to measure the cross-sectional area of the SVZ in each section from digital photographs using the NIH image-analysis program (imagej, NIH, Bethesda, MD, USA). To calculate the number of cells/mm3, the section thickness was measured using a digital length gauge device attached to the stage of a Leitz microscope (MT 12/ND221, Heidenhain-Metro, Germany). As certain variability was observed between litters, the number of cells and other quantitative measurements were considered in relation to control littermates to enable different treatments to be compared.
Number of Olig2-positive cells in the subventricular zone
The number of Olig2-positive cells was also estimated using the optical disector method in the upper lateral SVZ, the dorsolateral SVZ. We used the total section thickness as the disector height and a counting frame of 21 × 21 µm. In each section, six counting frames were assessed per region and at least three histological sections were included per region and rat. The reference space always fell within the coordinates described above. The section thickness was measured and the number of cells/mm3 was determined and compared with the controls as the relative percentage.
Optical density measurements
3CB2
In order to assess the number of radial glia present in the SVZ with the optical disector, we were unable to count the cells directly due to the cytoplasmic 3CB2 staining, which made it difficult to determine the number of nuclei belonging to radial glia cells, and the high density of nuclei in this region. Therefore, we measured the proportion of the SVZ covered by radial glia (3CB2 immunoreactivity) from digital pictures of the dorsolateral SVZ captured on a Leica DMR microscope and with a DC480 camera. At least three sections per animal were processed in parallel and developed at the same time. The images were acquired in the same session at the same magnification, maintaining the same light intensity and exposure time. Using the NIH image-analysis software (imagej), we transformed pictures to grey scale on which the SVZ was drawn and a threshold was determined by the observer so that only positive staining was recognized. The area of the SVZ stained for 3CB2 was measured and the data expressed as the variation with respect to the control levels.
Myelin basic protein
We measured the optical density of MBP staining in digital pictures of the subcortical white matter tract at the level of the external capsule. At least three sections per animal were processed in parallel and they were developed at exactly the same time. We acquired the images in the same session with the same intensity and amplification of fluorescent signal. The levels of intensity were fixed to those at which the control sections without primary antibody gave no signal. Using NIH image-analysis software (imagej), we transformed the pictures to grey scale and the mean grey values were measured.
Polysialylated neural cell adhesion molecule
We measured the optical density of PSA-NCAM staining in the dorsolateral SVZ with the same protocol as that used for MBP.
Cannabinoid receptor 2 expression in the post-natal subventricular zone
We studied the expression of CB2 in the neonatal rat SVZ by reverse transcriptase-polymerase chain reaction and immunohistochemistry.
Reverse transcriptase-polymerase chain reaction
The SVZ from two P7 and two P15 rats was dissected out under a Leica MS5 stereomicroscope and RNA was extracted from one hemisphere with TRIzol according to the manufacturer's instructions (Invitrogen, Barcelona, Spain). The other hemisphere was used for protein purification (see below). The purified RNA was quantified spectrophotometrically, treated with DNase I (Invitrogen) to digest any contaminating genomic DNA and its integrity was confirmed by electrophoresis in a 1% agarose gel. RNA was retrotranscribed to cDNA using SuperScript III reverse transcriptase (Invitrogen) and random primers. The expression of CB2 mRNA was studied with primers that were designed to hybridize to exons 2 and 3 of the rat CB2 gene in order to differentiate between material amplified from cDNA or contaminating genomic DNA. The sequences of the primers used were 5′-AGACTGCCTGCTGCGGACATC-3′ and 5′-TATTAGGGAGGCTGATTGGTC-3′. We performed touch-down polymerase chain reaction with 1 µg of RNA using the Platinum Taq polymerase (Invitrogen). The first cycle of annealing was performed at 55 °C, whereas in the following cycles the temperature decreased by 0.3 °C/cycle. Denaturing steps were performed at 94 °C over 30 s and elongation was carried out at 72 °C for 45 s. The reaction products were resolved in 2% agarose gels containing SYBR green (Invitrogen) and the expected length of the fragment amplified from the cDNA was 142 bp, whereas that derived from genomic DNA would be 285 bp.
Immunohistochemistry
We used two different antibodies to localize CB2 in the post-natal SVZ, one generated against the N-terminal region and the other generated against the C-terminal region. Immunohistochemistry was performed following the protocol described above and control experiments were run in parallel to check for non-specific staining. These controls involved the omission of the primary antibody or pre-absorption of the CB2 antibody with 20-fold excess of the immunogenic peptide that includes the C-terminal residues of the CB2 protein (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Western blot analysis
The SVZ was dissected and lysed in Tris-buffered saline, pH 7.6, containing 10% glycerol, 1% Nonidet P-40, 1 mm EDTA, 1 mm EGTA, 1 mm sodium orthovanadate, 2 mm NaF, 5 mm dithiothreitol and a protease cocktail. Cell lysates were separated and transferred to membranes, and the blots probed with anti-CB2 (AB5642P, Chemicon, 1 : 750) at 4 °C as described by Molina-Holgado et al. (2002).
Statistical analysis
Student's t-test or anova followed by Student's t-test was performed with prism 3.0 software. P-values less than 0.05 were considered significant.
Results
Cannabinoid receptor 1 expression in cells of the subventricular zone
Post-natal day 7
The presence of CB1 in the embryonic and post-natal rat SVZ has been analysed previously by in-situ hybridization and through the binding of a radiolabelled ligand, without achieving cellular resolution (Berrendero et al., 1998; Buckley et al., 1998). Using specific antibodies, we found intense CB1 immunoreactivity in cells within both the ventricular zone and SVZ at P7. At this developmental stage the walls of the ventricles were mainly covered by radial glia, elongated cells with irregular nuclei and a long basal process. Dual immunohistochemistry for CB1 and the radial glia marker 3CB2 demonstrated that the vast majority of these radial glia express CB1 (Fig. 1A–C and Supplementary material, Tables S1 and S2). In contrast, very few cells coexpressed CB1 and PSA-NCAM, a marker of migrating cells (Fig. 1D–F and supplementary Tables S1 and S2). Cells that express PSA-NCAM are considered to be neuroblasts and these small cells form long chains that migrate towards the olfactory bulb in the rostral migratory stream. These chains of neuroblasts are surrounded by astrocyte processes or oligodendrocyte precursors that migrate individually without associating with other migratory cells or astrocytic feet (Doetsch et al., 1997; Menn et al., 2006). The ensheathing of PSA-NCAM cells by the thin processes of the radial glia/B-cells sometimes makes it difficult to clearly ascertain whether CB1 and PSA-NCAM are expressed by the same cell or whether CB1 is rather in the surrounding cellular processes. Accordingly, we performed electron microscopy studies to more accurately define the CB1-immunoreactive cells using the ultrastructural criteria of SVZ cell types described previously (Doetsch et al., 1997; Tramontin et al., 2003; Merkle et al., 2004). Briefly, type A cells, corresponding to the dark cells in toluidin stainings, show the smallest cross-sectional area and present an elongated cell body with few processes, a very thin cytoplasm and elongated nuclei with abundant lax chromatin. Type B cells have irregular contours that intermingle with the neighbouring cells, an irregular nucleus that frequently contains invaginations, light cytoplasm with few free ribosomes and abundant intermediate filaments and dense bodies. Type C cells are larger, less elongated and more electron-lucent than type A cells but more electron-dense than type B cells. Their nuclei contain deep invaginations and mostly lax chromatin. Type C cells have a large reticulated nucleolus and show in their cytoplasm a large Golgi apparatus and no apparent bundles of intermediate filaments. Ependymocytes have interdigitated processes, microvilli and cilia in the ventricular surface, spherical nuclei with non-clumped chromatin and electron-lucent cytoplasm with apical basal bodies. Radial glia have long basal processes and some anatomical features of cells in the astroglial lineage, including intermediate filaments and glycogen granules.
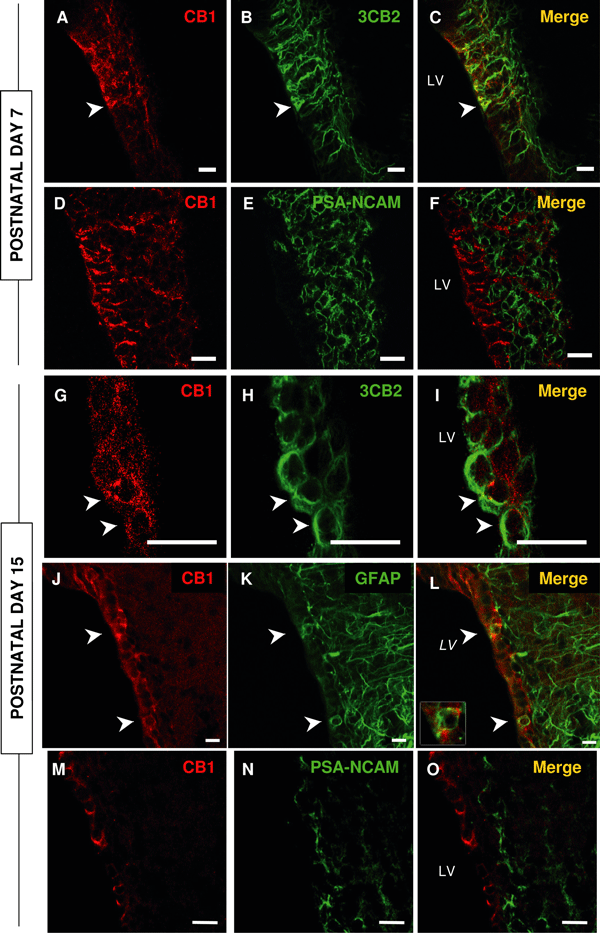
Cannabinoid receptor 1 (CB1) is expressed in the subventricular zone of post-natal day (P)7 and P15 rats. (A–C and G–I) At both ages, CB1 (red) colocalizes with 3CB2 (green), a marker of radial glia. (J–L) At P15, CB1 also colocalizes with glial fibrillary acidic protein (GFAP). (D–F and M–O) At either age, CB1 does not colocalize with a marker of migrating precursors, polysialylated neural cell adhesion molecule (PSA-NCAM). LV, lateral ventricle. Arrowheads, double-labelled cells. Bars: A–L, 12 µm; M–O, 25 µm.
We confirmed, in both semithin and ultrathin sections, that antibodies against CB1 did not stain any cells with the morphological properties of neuroblasts/A-cells. However, CB1 expression was found in many radial glia cells (Fig. 2A) and astrocyte-like cells, forming tubes around clusters of neuroblasts (Fig. 2B). In addition, CB1 is located in disperse cells at P7 that we could not clearly assign to the B-, radial glia- or even the C-cell type. Indeed, these cells displayed morphological characteristics that were apparently intermediate between these cell types. Although only a small number of these cells were observed, we performed double labelling for CB1 and aldolase C/zebrin II, a marker that does not stain C-cells (Marshall & Goldman, 2002). As expected, the cells that expressed CB1 largely overlapped with the cell population that expressed zebrin II (supplementary Fig. S1), confirming that CB1 is essentially absent from the populations of C- and A-cells.
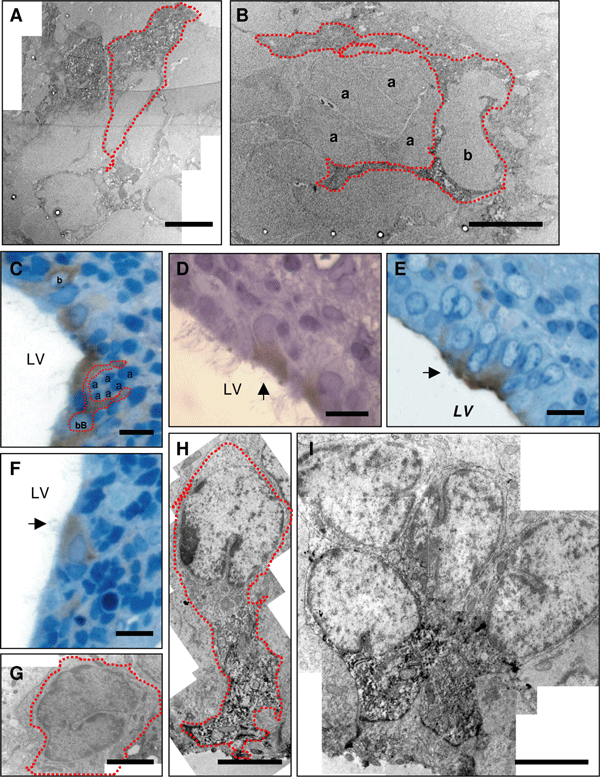
The cells expressing cannabinoid receptor 1 (CB1) in the subventricular zone are stem cells and ependymocytes. In post-natal day (P)7 rats, CB1 immunoreactivity (indicated by red dots) can be seen in radial glia (A) and astrocyte-like B-cells forming tubes around clusters of non-CB1-immunoreactive A-cells (B). By P15, some CB1-positive cells show B-cell-like morphological features (C) or resemble radial/immature glia (arrow) (D and H). These cells are also found in small clusters (E and I). CB1 immunoreactivity is also observed in multiciliated ependymocytes (arrow) (F). C-cells did not display any CB1 immunoreactivity (G). a, A-cell; b, B-cell; LV, lateral ventricle. Calibration bars: A, B, G and I, 5 µm; C, D, E and F, 10 µm; H, 3 µm.
Post-natal day 15
At P15, CB1 was detected in cells throughout the walls of the lateral ventricle and was expressed in cells that were also recognized by 3CB2 (Fig. 1G–I) or GFAP (Fig. 1J–L). In the SVZ, the CB1-positive/GFAP-positive cells were located adjacent to the ependymal layer and some of them appeared to send out a single cellular process to the ventricle (arrowheads in Fig. 1J–L). Indeed, about half of the population of radial glia cells (3CB2-positive) coexpressed CB1 at this stage. In addition, this receptor was detected at the apical surface of cells surrounding the ventricle that do not express GFAP or 3CB2. These cells were identified as ependymocytes in semithin sections due to the presence of multiple cilia in the surface exposed to the ventricle (Fig. 2F). These observations were confirmed by electron microscopy (Fig. 2G–I), which also revealed that CB1-positive cells have invaginated nuclei, intermediate filaments and a cellular process that makes contact with the lumen of the ventricle. These features are reminiscent of the morphological characteristics of B-cells in the SVZ. We also observed staining in the processes of these cells, which formed tubes around type A cells (Fig. 2C; see also Doetsch et al., 1997), as well as in other cells that had a more immature phenotype similar to radial glia (Fig. 2D, E, H and I). These data indicate that CB1 is expressed in cells with the ultrastructural and immunoreactive properties of SVZ neural stem cells. In contrast, but in accordance with the data from P7 rats, we observed very few cells that coexpressed PSA-NCAM and CB1 by confocal microscopy (Fig. 1M–O and supplementary Tables S1 and S2). No CB1-positive cell displayed the morphological characteristics of A-cells in electron microscopy. Similarly, we did not observe CB1 expression in C-cells (Fig. 2G) although, as at P7, a few disperse CB1-positive cells could not be clearly identified. Nevertheless, CB1 expression was largely restricted to the zebrin II population as observed at P7 (supplementary Fig. S1).
Finally, when we studied CB1 expression in the adult SVZ when radial glia were no longer present, we found that CB1 expression was restricted to the ependymocytes and B-cells (data not shown).
We estimated the proportion of the different cell types that expressed CB1 in the SVZ from confocal images (Table 2, supplementary Tables S1 and S2) and found that most of the CB1-positive cells were radial glia at P7, whereas most of the CB1-immunoreactive cells were stem cells or ependymocytes at P15 (either radial glia or B-cells). Only a very small proportion of cells seemed to express PSA-NCAM in confocal images.
Post-natal exposure to an agonist of cannabinoid receptor 1 modulates cell proliferation in the subventricular zone
The reduction in the number of proliferating cells in the CB1 knockout mice has implicated this cannabinoid receptor in the control of SVZ cell proliferation (Jin et al., 2004). When we estimated the cells immunostained for phospho-histone H3, a marker of all cells undergoing mitosis, exposure of post-natal rats to cannabinoids from P1 to P14 did not appear to affect the proliferation of cells in the SVZ at P15 (Fig. 3A and B). However, due to the slow cell cycle of the stem cells, any change in their proliferation may be masked by the larger number of transit amplifying cells that proliferate much more rapidly. Therefore, we attempted to specifically study the effect of cannabinoids on the proliferation of radial glia. In order to achieve this, we used the 4A4 antibody that specifically recognizes vimentin phosphorylated by the cdc2 kinase at Ser55 and that has been described as a marker of radial glial proliferation (Noctor et al., 2004). Chronic post-natal treatment with the selective CB1 agonist ACEA increased the number of 4A4-positive cells in the SVZ in P15 rats (Fig. 3C–E). We studied the whole population of radial glia by measuring the area occupied by the rat radial glia marker 3CB2 in the SVZ at P15. In rats treated with ACEA, we did not observe a significant increase in the presence of this marker when compared with their control littermates, although a trend towards an increase in 4A4 immunostaining was observed in the animals exposed to ACEA (P = 0.247, Fig. 3F–H).
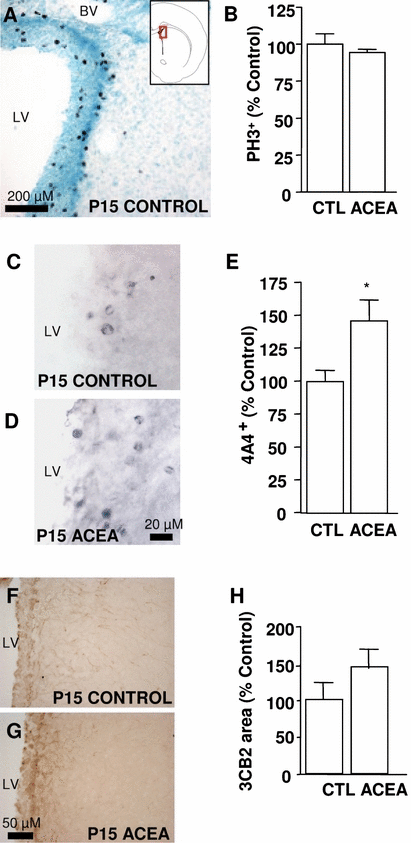
Chronic treatment with cannabinoid receptor 1 agonist arachidonyl-2-chloroethylamide (ACEA) enhances radial glial proliferation in the subventricular zone at post-natal day (P)15. (A–C) ACEA does not alter the total number of mitotic cells, i.e. phospho-histone H3-labelled cells (PH3). (C–E) ACEA increases the number of proliferating radial glia (4A4-positive cells). (F–H) Furthermore, exposure to ACEA does not increase the area occupied by the radial glia marker 3CB2. CTL, control; LV, lateral ventricle; BV, blood vessel. *P < 0.05 vs. control (Student-Newman-Keuls t-test).
Post-natal treatment with a cannabinoid receptor 1 agonist increases gliogenesis in the subventricular zone
Cannabinoid receptor 1 has recently been shown to be involved in gliogenesis within the subgranular zone (Aguado et al., 2006). Here, we studied the formation of gliogenic precursors in the post-natal rat SVZ by counting the number of Olig2-positive cells and the capacity of the neural precursor cells to migrate analysed by measuring the expression of PSA-NCAM.
Olig2 is a basic helix-loop-helix transcription factor (Lu et al., 2000) that is expressed early in glial precursors. It has recently been shown to both prevent neuronal differentiation and to drive neural precursor cells towards oligodendroglial fates in the post-natal and adult SVZ (Hack et al., 2005; Marshall et al., 2005). Post-natal treatment of rats with ACEA increased the number of Olig2-positive cells in the SVZ at P15 (Fig. 4A–C). Glial progenitors of the SVZ migrate through the dorsolateral SVZ to the white matter, where they differentiate into oligodendroglial cells (Levison & Goldman, 1993). PSA-NCAM is expressed by these cells (Menn et al., 2006) and is necessary for the migration of glial progenitors in the normal and injured brain (Wang et al., 1994; Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002). Unlike the effects observed on Olig2, we did not observe changes in the expression of PSA-NCAM in the dorsolateral SVZ after treatment with ACEA (Fig. 4D–F).
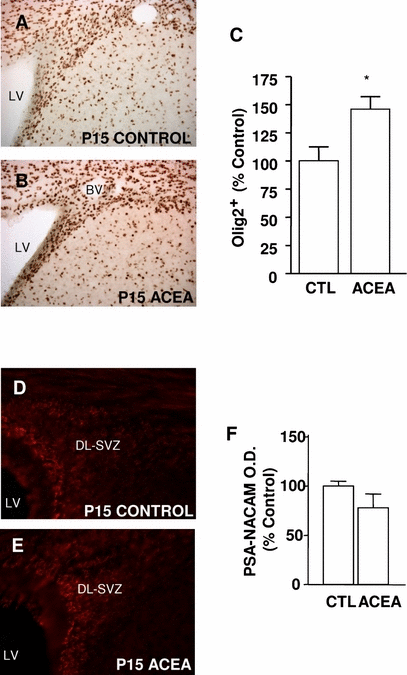
Chronic activation of cannabinoid receptor 1 with arachidonyl-2-chloroethylamide (ACEA) increases gliogenesis in the post-natal subventricular zone (SVZ). (A–C) Exposure to ACEA increases the number of Olig2-positive cells in the SVZ. (D–F) In contrast, ACEA administration does not modify the intensity of polysialylated neural cell adhesion molecule (PSA-NCAM) staining in the SVZ when compared with control littermates. CTL, control; DL-SVZ, dorsolateral SVZ; LV, lateral ventricle; BV, blood vessel; P, post-natal day. *P < 0.05 vs. control (Student-Newman-Keuls t-test).
Cannabinoid receptor 2 is expressed in the post-natal subventricular zone
There is increasing evidence that CB1 is not the only receptor that mediates the effects of cannabinoids in the central nervous system. Thus, we wondered whether CB2 might also be present in the post-natal rat SVZ. Hence, we analysed the expression of CB2 mRNA at P7 and P15 in the dissected SVZ by reverse transcriptase-polymerase chain reaction with primers designed to hybridize in two separate exons. CB2 transcripts were clearly identified in the isolated SVZ at both ages, indicating that this receptor may be present in this tissue (Fig. 5A and B).
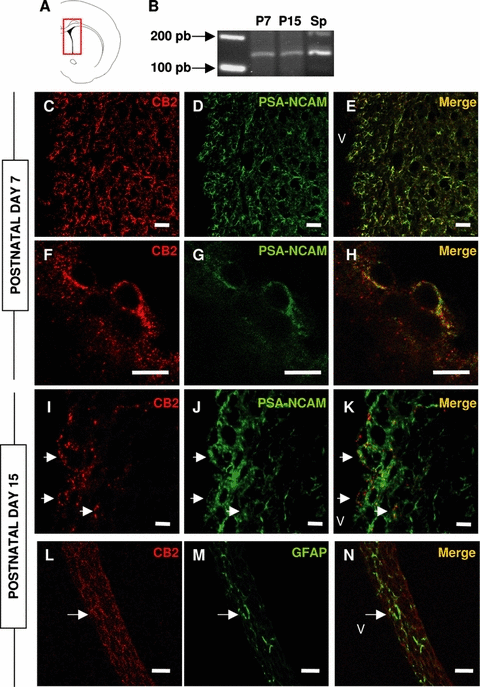
Cannabinoid receptor 2 (CB2) is expressed in the subventricular zone (SVZ) of post-natal rats. (A) Scheme showing the area from which RNA was isolated for reverse transcriptase-polymerase chain reaction (RT-PCR). (B) RT-PCR showing CB2 expression in the SVZ of post-natal day (P)7 and P15 rats, in a band equivalent to that found in the positive control [Spleen (Sp)]. (C–E) In the SVZ of P7 rats, CB2 (red) was mostly found in polysialylated neural cell adhesion molecule (PSA-NCAM)-expressing cells (green). (F–H) Detail showing colocalization of both markers in the same cell. (I–K) In the SVZ of P15 rats, CB2 is still predominantly colocalized with PSA-NCAM. (L- N) Only a few glial fibrillary acidic protein (GFAP)-positive cells (green) also express CB2 (arrows, green). v, ventricle. Bars: 12 µm except G–I, 6 µm.
Subsequently, we used two different antibodies to localize CB2 in the SVZ, one generated against the N-terminal region (1–33 amino acids, Affinity Bioreagents) and the other raised against the C-terminal domain of the rat CB2 (18 amino acids, Chemicon). Both antibodies stained equivalent populations in the SVZ, both at P7 and P15, and these antibodies recognized cells with a location distinct from that obtained with the CB1 antibodies. Most cells recognized by the CB2 antibodies were isolated from the ventricle lumen and they were also labelled with PSA-NCAM antibodies (Fig. 5C–K). In addition, we found some GFAP-positive cells that displayed CB2 immunoreactivity at P15 (Fig. 5L–N). Omission of the primary antibodies abolished this staining and pre-absorption of C-terminal antibody with its immunogenic peptide led to a strong decrease in immunogenicity (supplementary Fig. S2). In addition, in western blots of post-natal SVZ, this antibody recognized bands corresponding to the molecular weights previously described for CB2 (supplementary Fig. S2) (Van Sickle et al., 2005; Gong et al., 2006). This was also demonstrated using the antibody raised against the N-terminal region of CB2 (supplementary Fig. S2).
Post-natal exposure to a cannabinoid receptor 2 agonist increases polysialylated neural cell adhesion molecule expression in the subventricular zone
In contrast to what we had observed following ACEA treatment, the activation of CB2 with the selective agonist JWH did not significantly affect the number of Olig2-positive cells in the SVZ (Fig. 6A–C). Indeed, JWH increased the expression of PSA-NCAM by more than two-fold in this region (Fig. 6D–F).

Chronic activation of cannabinoid receptor 2 with JWH-056 (JWH) increases polysialylated neural cell adhesion molecule (PSA-NCAM) expression in the post-natal subventricular zone (SVZ). (A–C) Exposure to JWH does not modify the number of Olig2-positive cells in the SVZ. (D–F) However, JWH administration does increase the intensity of PSA-NCAM staining in the SVZ. CTL, control; DL-SVZ, dorsolateral SVZ; LV, lateral ventricle; BV, blood vessel; P, post-natal day. *P < 0.05 vs. control (Student-Newman-Keuls t-test).
Post-natal cannabinoid treatment modulates myelin basic protein expression in the external capsule
Olig2 is a transcription factor that is necessary for oligodendrocyte formation and the posterior myelination of the white matter (Lu et al., 2000; Hack et al., 2005; Marshall et al., 2005; Yue et al., 2006). In contrast, PSA-NCAM is a molecule that facilitates the migration of oligodendrocyte precursors (Wang et al., 1994; Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002). As the first weeks of post-natal development represent an intense period of myelination (Bjelke & Seiger, 1989; Hamano et al., 1996), we looked for a possible correlation between the changes in the expression of these factors and the modification of MBP expression, which can be considered as a good index of myelin synthesis (Cohen & Guarnieri, 1976). We focused on the external capsule, which is one of the first regions inside the subcortical white matter to be myelinated (Fig. 7). In spite of their effects on glial precursors (Olig2 population) or the migrating phenotype of the cells (PSA-NCAM expression), the activation of CB1 (ACEA treatment) or CB2 (JWH) alone did not exert a significant influence on MBP expression, although JWH treatment did induce a trend towards its up-regulation (P = 0.095).
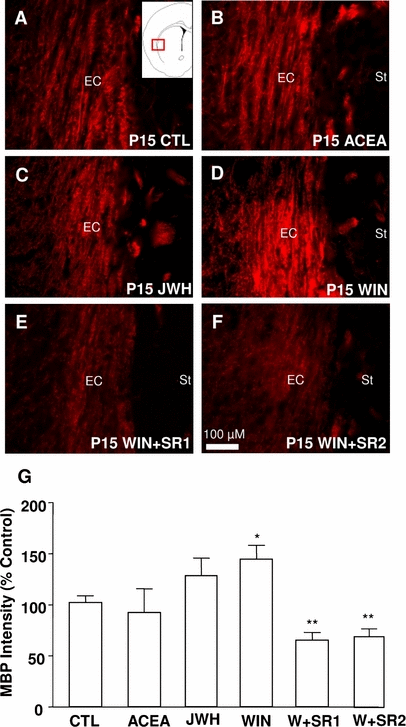
The activation of both cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) enhances myelin basic protein (MBP) expression in the external capsule. A representative micrograph of MBP expression (red staining) is shown for animals treated with vehicle (A), the CB1 agonist arachidonyl-2-chloroethylamide (ACEA) (B), the CB2 agonist JWH-056 (JWH) (C), the non-selective CB1/CB2 agonist (+)WIN 55,212-2 (WIN) (D), WIN plus the CB1 antagonist SR141716A (SR1) (E) or WIN plus the CB2 antagonist SR144528 (SR2) (F). (G) Histogram showing the quantification of MBP expression after different treatments. EC, external capsule; St, striatum; CTL, control; P, post-natal day. *P < 0.05 vs. control; **P < 0.01 vs. control (Student-Newman-Keuls t-test following significant anova).
We then explored the effect of a post-natal exposure to WIN, a synthetic cannabinoid that activates both CB1 and CB2. We found that exposure to WIN increased MBP immunostaining in the external capsule of P15 rats when compared with control littermates (Fig. 7A, D and G). When the rats were treated simultaneously with WIN and either a specific CB1 (SR1) or specific CB2 (SR2) antagonist, the increase in MBP induced by WIN was not only inhibited but, moreover, MBP expression decreased to levels below those of control littermates (Fig. 7E–G).
Discussion
In this study, we demonstrate that CB1 and CB2 are present in the rat post-natal SVZ and that the exogenous administration of cannabinoid receptor agonists to newborn animals affects the development of this zone. Moreover, we clearly show that CB1 is expressed by stem cells (radial glia and B-cells) and that its activation by the selective CB1 agonist ACEA augments their proliferation. Both of these observations are in accordance with previous reports describing the expression of CB1 in the SVZ and the effect of cannabinoids on the proliferation of neural precursors (Berrendero et al., 1999; Jin et al., 2004). In addition, we found that ACEA administration induces an increase in the number of glial precursors in the SVZ (Olig2-positive cells), consistent with cannabinoids exerting a CB1-dependent gliogenic effect on neural precursors/stem cells in the subgranular zone (Aguado et al., 2006). It was recently demonstrated that stem cells of the SVZ are the origin of Olig2-expressing cells that give rise to mature oligodendrocytes after migrating into the white matter (Menn et al., 2006). Although we show that stem cells express CB1 and that this could account for the aforementioned effects, we cannot exclude an indirect influence on this process exerted by CB1-positive ependymocytes.
Cannabinoid receptor 2 was originally considered to be a peripheral cannabinoid receptor, restricted to cells of the immune lineage in the nervous system (Correa et al., 2005). Nevertheless, there is increasing evidence that CB2 is also expressed in neural cell types in the mammalian brain (Van Sickle et al., 2005; Ashton et al., 2006; Gong et al., 2006). We confirmed that CB2 is expressed in the rat SVZ using polymerase chain reaction, western blotting and immunohistochemistry. This expression is somewhat complementary to that of CB1, as CB2 is predominantly expressed in PSA-NCAM-positive precursors, although there are a small number of stem cells that are immunoreactive for CB2. To our knowledge, this is the first description of CB2 expression in a neurogenic region of the brain. Furthermore, we show that CB2 affects SVZ function, as treatment with a selective agonist (JWH) increases the optical density of PSA-NCAM. PSA-NCAM-expressing cells in the SVZ have been identified as migratory neuroblasts that enter the rostral stream, as well as oligodendrocyte precursors that migrate towards the adjacent areas of white matter (Doetsch et al., 1999; Menn et al., 2006). It has been shown that PSA-NCAM is necessary for the migration of oligodendrocyte precursors and that it is up-regulated in the SVZ after injury to the corpus callosum (Wang et al., 1994; Nait-Oumesmar et al., 1999; Picard-Riera et al., 2002; Zhang et al., 2004). In this regard, the increased expression of PSA-NCAM could be related to the enhanced ability of cells in the SVZ to migrate (Ono et al., 1994; Hu et al., 1996; Chazal et al., 2000).
Thus, it appears that the activation of CB1 increases the number of Olig2-positive cells in the post-natal SVZ, whereas the activation of CB2 induces the expression of PSA-NCAM. As the initial weeks after birth are a period of intense oligodendrogenesis and myelination and the SVZ is a source of oligodendrocytes, we studied the expression of MBP in the external capsule after cannabinoid treatment. Neither ACEA nor JWH alone promoted the expression of MBP, whereas the CB1 and CB2 agonist WIN enhanced its expression in a CB1- and CB2-dependent manner. Indeed, the blockage of either receptor with SR1 or SR2 abolishes the effects of WIN. This effect on MBP expression could be related to the increased gliogenesis and migratory capacity of the SVZ precursors as measured by the number of Olig2-positive cells and PSA-NCAM expression, respectively. Nevertheless, we cannot exclude an effect on white matter oligodendrocyte precursors.
The signals controlling the physiology of post-natal SVZ development remain unknown, despite the fact that some candidates for the control of these processes have been already identified (Machold et al., 2003; Palma et al., 2005; Jackson et al., 2006; Ramirez-Castillejo et al., 2006). Our results indicate that cannabinoids may provide one such signal and they suggest that both CB1 and CB2 may be implicated in post-natal oligodendrogenesis.
Acknowledgements
This research was financed by grants from the Fondo de Investigaciones Sanitarias, Spain (04/2120) and the Consejería de Sanidad de la Junta de Comunidades de Castilla-La Mancha (04061-00). A.R.-A. holds a fellowship from the Consejería de Educación y Ciencia de la Junta de Comunidades de Castilla-La Mancha. We are indebted to Dr Rowitch and the laboratory of Dr Stiles for generously providing us with the anti-Olig2 antibody, Dr Hawkes for the anti-zebrin II antibody, Dr de la Rosa for the 3CB2 antibody and Dr Martínez-Cerdeño for the 4A4 antibody. We are grateful to C. Sanchez-Caro and M.Q. Uyen Le for their excellent technical assistance. We also thank Dr Guaza for her helpful discussion and support, Dr García-Segura, Dr Azcoitia, Dr Veiga and J.A. Maldonado for their advice and technical support in the electron microscopy experiments, and Dr García-Verdugo for his helpful opinions on the transmission electron microscopy images. We would also like to thank Dr Mark Sefton for critical reading of the manuscript and grammatical assistance.
Abbreviations
-
- ACEA
-
- arachidonyl-2-chloroethylamide
-
- CB1
-
- cannabinoid receptor 1
-
- CB2
-
- cannabinoid receptor 2
-
- GFAP
-
- glial fibrillary acidic protein
-
- JWH
-
- JWH-056
-
- MBP
-
- myelin basic protein
-
- P
-
- post-natal day
-
- PSA-NCAM
-
- polysialylated neural cell adhesion molecule
-
- SR1
-
- SR141716A
-
- SR2
-
- SR144528
-
- SVZ
-
- subventricular zone
-
- WIN
-
- (+)WIN 55,212-2.