4-Chloro-m-cresol, an activator of ryanodine receptors, inhibits voltage-gated K+ channels at the rat calyx of Held
Abstract
4-Chloro-m-cresol (4-CmC) is thought to be a specific activator of ryanodine receptors (RyRs). Using this compound, we examined whether the RyR-mediated Ca2+ release is involved in transmitter release at the rat calyx of Held synapse in brainstem slices. Bath application of 4-CmC caused a dramatic increase in the amplitude of excitatory postsynaptic currents (TIFCs) with the half-maximal effective concentration of 0.12 mm. By making direct patch-clamp whole-cell recordings from presynaptic terminals, we investigated the mechanism by which 4-CmC facilitates transmitter release. 4-CmC markedly prolonged the duration of action potentials, with little effect on their rise time kinetics. In voltage-clamp recordings, 4-CmC inhibited voltage-gated presynaptic K+ currents (IpK) by 53% (at +20 mV) but had no effect on voltage-gated presynaptic Ca2+ currents (IpCa). In simultaneous pre- and postsynaptic recordings, 4-CmC had no effect on the TIFC evoked by IpCa. Although immunocytochemical study of the calyceal terminals showed immunoreactivity to type 3 RyRs, ryanodine (0.02 mm) had no effect on the 4-CmC-induced TIFC potentiation. We conclude that the facilitatory effect of 4-CmC on nerve-evoked transmitter release is mediated by its inhibitory effect on IpK.
Introduction
Ryanodine-sensitive Ca2+ stores are involved in presynaptic modulation at some synapses, where ryanodine inhibits synchronous (Galante & Marty, 2003) as well as asynchronous (Smith & Cunnane, 1996; Narita et al., 1998, 2000; Emptage et al., 2001) neurotransmitter release. Ryanodine also attenuates Ca2+ transients evoked by nerve stimulation in bullfrog sympathetic ganglia, suggesting its modulatory role in hormonal secretion (Peng, 1996). Furthermore, ryanodine-sensitive Ca2+ stores are involved in the glutamate-induced release of γ-aminobutyric acid from rat retinal amacrine cells (Chávez et al., 2006) and spontaneous glutamate release from dissociated frog canal hair cells (Lelli et al., 2003).
The calyx of Held is a giant presynaptic terminal in the auditory brainstem, forming a glutamatergic synapse onto the postsynaptic principal cell in the medial nucleus of the trapezoid body (Forsythe & Barnes-Davies, 1993). Endoplasmic reticulum is frequently observed in the calyceal terminal (Lenn & Reese, 1966), suggesting that ryanodine receptors (RyRs) might be involved in synaptic modulation at this nerve terminal. To address this possibility, we tested the effect of 4-chloro-m-cresol (4-CmC) (Zorzato et al., 1993) on synaptic transmission. This compound activates RyRs by increasing their Ca2+ binding affinity (Jacobson et al., 2006). At the calyx of the Held synapse in rat brainstem slices, bath application of 4-CmC caused a dramatic increase in the amplitude of nerve-evoked excitatory postsynaptic currents (TIFCs). Taking advantage of its large structure, we performed direct whole-cell recordings from calyceal nerve terminals and investigated the mechanism underlying the 4-CmC-induced synaptic facilitation. Using type-specific antibodies, we also examined which types of RyRs were expressed at the calyceal terminals. Our results indicate that 4-CmC enhances nerve-evoked transmitter release by blocking voltage-gated K+ channels. Although calyceal terminals showed type 3 RyR immunoreactivity, ryanodine had no effect on the facilitatory effect of 4-CmC on evoked TIFCs.
Materials and methods
All experiments were performed in accordance with the guidelines of the Physiological Society of Japan.
Preparation and solutions
Transverse brainstem slices (175–250 µm thick) were prepared from Wistar rats (8–10 days old) killed by decapitation under halothane anesthesia. The slicing solution was the same as described previously (Forsythe & Barnes-Davies, 1993), except that equiosmolar concentrations of sucrose were substituted for NaCl and 6 mm MgCl2 was the only divalent ion in the solution. The artificial cerebrospinal fluid (aCSF) contained (in mm): 125 NaCl, 2.5 KCl, 10 glucose, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 0.5 ascorbic acid and 2 Na-pyruvate (pH 7.4, gassed with 95% O2/5% CO2). Soon after slicing, slices were incubated at 37 °C for 60 min in aCSF and thereafter stored at room temperature (24–28°C). For recording TIFCs evoked with a bipolar electrode, bicuculline methiodide (10 µm), strychnine hydrochloride (0.5 µm) and kynurenic acid (2 mm) were added to block inhibitory synaptic inputs and postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor saturation (Kaneko & Takahashi, 2004). Tetrodotoxin (1 µm) was added for recording presynaptic K+ currents (IpK). For recording presynaptic Ca2+ currents (IpCa) and TIFCs simultaneously, tetrodotoxin (1 µm), tetraethylammonium (TEA)-Cl (10 mm), equimolar replacement of NaCl, d-(–)-2-amino-5-phosphonopentanoic acid (50 µm) and kynurenic acid (1 or 2 mm) were added. 4-CmC was dissolved in dimethylsulphoxide to a final concentration of less than 0.1% and the same concentration of dimethylsulphoxide was included in control solutions. The postsynaptic patch pipette solution contained (in mm): 110 CsF, 30 CsCl, 10 HEPES, 5 EGTA, 1 MgCl2 and 5 QX-314 (pH 7.3, adjusted with CsOH). For recording action potentials (APs), the presynaptic patch pipette solution contained (in mm): 87.5 K-gluconate, 10 K-glutamate, 32.5 KCl, 10 HEPES, 0.5 EGTA, 12 Na2-phosphocreatine, 1 MgCl2, 2 Mg-ATP and 0.5 Na-GTP (pH 7.3, adjusted with KOH). For recording IpK, the pipette solution contained (in mm): 97.5 K-gluconate, 32.5 KCl, 10 HEPES, 5 EGTA, 12 Na2-phosphocreatine, 1 MgCl2, 2 Mg-ATP and 0.5 Na-GTP (pH 7.3, adjusted with KOH). For recording IpCa, the pipette solution contained (in mm): 110 Cs-gluconate, 10 Cs-glutamate, 30 CsCl, 10 HEPES, 0.5 EGTA, 12 Na2-phosphocreatine, 1 MgCl2, 3 Mg-ATP and 0.5 Na-GTP (pH 7.3, adjusted with CsOH) or 100 CsCl, 10 Cs-glutamate, 40 HEPES, 10 TEA-Cl, 0.5 EGTA, 12 Na2-phosphocreatine, 1 MgCl2, 2 Mg-ATP and 0.5 Na-GTP (pH 7.3, adjusted with CsOH).
Tetrodotoxin and ryanodine were obtained from Wako Pure Chemical (Osaka, Japan). Kynurenic acid and d-(–)-2-amino-5-phosphonopentanoic acid were obtained from Tocris Cookson (Bristol, UK). 4-CmC was obtained from Calbiochem (Darmstadt, Germany).
Recording
Whole-cell patch-clamp recordings were made from medial nucleus of the trapezoid body principal neurones, presynaptic calyces or simultaneously from both structures. When slices were ready for use, they were transferred to a recording chamber and continuously perfused with aCSF at a speed of 1.3–1.6 mL/min. Principal neurones and calyces were visually identified using a 60× water immersion lens (Olympus, Tokyo, Japan) attached to an upright microscope (Axioskop; Zeiss, Goettingen, Germany). Voltage-clamp and current-clamp recordings were performed using a patch-clamp amplifier (Axopatch 200B or Multiclamp 700A; Axon Instruments, Union City, CA, USA). Pipettes for pre- and postsynaptic recordings had resistances of 3–8 and 2–5 MΩ, respectively. Pre- and postsynaptic series resistances were 5–18 and 3–15 MΩ, respectively. The postsynaptic series resistance was not compensated for but data were discarded when the series resistance changed by >30%. The presynaptic series resistance was routinely compensated by 70–80%. Leak currents were subtracted for presynaptic currents by the scaled pulse (P/8) protocol. The liquid junction potential between the pipette solution and aCSF was not corrected. For recording single postsynaptic currents, TIFCs were evoked every 20 s by extracellular stimulation of presynaptic axons using a bipolar tungsten or platinum electrode. Only those TIFCs showing all-or-none responses to changes in stimulus intensity were selected. For recording IpK or IpCa, calyces were voltage-clamped at a holding potential of −70 or −80 mV and depolarizing voltage sttif were applied every 15–20 s. The presynaptic APs shown in Fig. 2A were elicited by a brief (1 ms) depolarizing pulse and those shown in Fig. 7A were evoked by afferent fibre stimulation. All experiments were performed at room temperature.
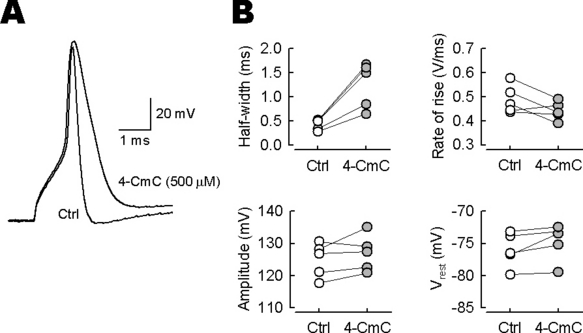
Broadening of presynaptic action potentials (APs) by 4-chloro-m-cresol (4-CmC). (A) Calyceal APs evoked by 1 ms depolarizing pulse were recorded and averaged before (Ctrl) and 4–5 min after 4-CmC (500 µm) application (superimposed). (B) In five presynaptic terminals, 4-CmC increased the half-width of presynaptic APs by 185 ± 18% (n = 5) on average (left upper panel, P < 0.01) but had no effect on their peak amplitude (left lower panel, P = 0.21), maximal rate of rise (right upper panel, P = 0.10) or resting potential (right lower panel, P = 0.07).
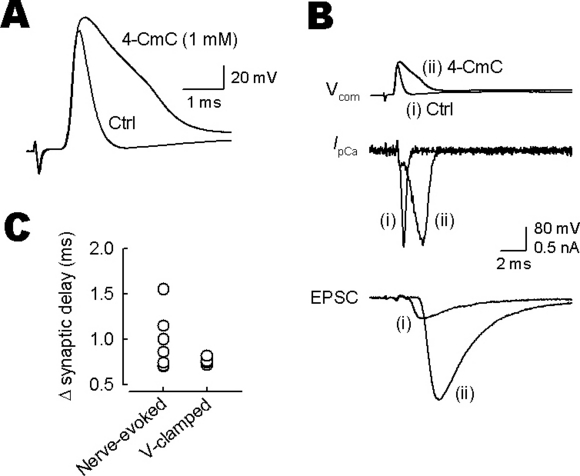
Mechanism underlying the effect of 4-chloro-m-cresol (4-CmC) on synaptic delay. (A) Calyceal action potentials (APs) evoked by input fibre stimulation were recorded and averaged before (Ctrl) and 4–5 min after 4-CmC (1 mm) application (superimposed). Note the lack of its effect on the axonal conduction velocity. (B) Paired voltage-clamp recordings of presynaptic Ca2+ currents (IpCa) and excitatory postsynaptic currents (TIFCs) evoked by AP waveform command pulses before (i) and after (ii) 4-CmC application (Vcom; superimposed). The artificial cerebrospinal fluid contained tetrodotoxin (1 µm), tetraethylammonium (10 mm, equimolar replacement of NaCl) and kynurenic acid (2 mm). (C) Summary data of the 4-CmC-induced prolongation of synaptic delay (Δ synaptic delay) of TIFCs evoked by afferent fibre stimulations (Nerve-evoked; n = 7) and by presynaptic AP command pulses (V-clamped; n = 4). There was no difference in the magnitude of 4-CmC-induced Δ synaptic delay (P = 0.55, Mann–Whitney test).
Data analysis
Records were low-pass filtered at 5 kHz and digitized at 20–100 kHz by a Digidata 1320A analogue/digital converter with pCLAMP9 software (Axon Instruments). Data were analysed using Clampfit (Axon Instruments). All values are given as means ± SEM. Statistical comparisons were made using Student's paired t-test, unless otherwise noted. A value of P < 0.05 was taken as the level of significance.
Immunocytochemistry
Immunocytochemical procedures were as previously described (Ishikawa et al., 2003). Briefly, Wistar rats (8 or 14 days old) were transcardially perfused with a fixative (4% paraformaldehyde and 0.1% picric acid in 0.1 m Na-phosphate buffer, pH 7.4). After fixation, rats were decapitated and tissue blocks were postfixed and cryoprotected. Transverse slices (30 µm thick) were cut using a cryostat (CM3050; Leica, Nussloch, Germany). The sections were then processed for blocking the tissues with phosphate-buffered saline containing 5% skimmed milk and 0.3% Triton X-100, followed by application of primary antibodies (Chemicon, Temecula, CA, USA). Presynaptic RyRs were detected with rabbit anti-type 1, type 2 or type 3 RyR antibody (diluted 1 : 500) and anti-synaptophysin antibody (diluted 1 : 200). Goat secondary antibodies (Invitrogen, Eugene, OR, USA) (diluted 1 : 200) conjugated with alexa fluor 488 and 568 were used to visualize RyR and synaptophysin signals, respectively. As neither the epitope peptides of RyRs nor their sequences were available, we treated slices only with anti-synaptophysin primary antibody (with no RyR antibody) and subsequently with the mixture of secondary antibodies as a control for background signals.
Stained sections were viewed using a 100 × oil-immersion objective (numerical aperture 1.35) attached to a confocal laser-scanning microscope (FLUOVIEW FV300; Olympus). Emission wavelengths were 510–550 nm for RyRs and > 610 nm for synaptophysin. For the RyR signal acquisition, the argon laser power (488 nm, 10 mW) was reduced to 0.18% (for type 1 RyR), 1.2% (for type 2 RyR and negative control) or 0.42% (for type 3 RyR) using an acousto-optic tunable filter module and neutral density filter. For imaging synaptophysin immunoreactivity, the krypton laser power (568 nm, 30 mW) was reduced to 2.4%. Single slice images (512 × 512 pixel, 95 nm/pixel) were averaged after passing a Kalman filter. The photomultiplier tube voltage was 800 V throughout.
Results
Presynaptic facilitatory effect of 4-chloro-m-cresol
In patch-clamp whole-cell recordings from medial nucleus of the trapezoid body principal neurones, TIFCs were evoked by stimulating input nerve fibers. Bath application of 4-CmC (10–1000 µm) dramatically enhanced the peak amplitude of TIFCs, in a concentration-dependent manner (Fig. 1). In these experiments, the low-affinity glutamate receptor antagonist kynurenic acid was included in the aCSF to minimize saturation and desensitization of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors by transmitter glutamate (Kaneko & Takahashi, 2004). The facilitatory effect of 4-CmC reached a maximum at around 500 µm and the half-maximal effective concentration was 116 µm (Fig. 1B). 4-CmC (500 µm) concomitantly decreased the paired-pulse ratio (the second amplitude relative to the first) of TIFCs (50 ms interstimulus interval) from 0.75 ± 0.07 to 0.34 ± 0.05 (n = 6; P < 0.01; Fig. 1C), suggesting that its site of action is presynaptic. In addition to its facilitatory effect, 4-CmC prolonged the synaptic delay (i.e. the time from the stimulus artifact to the TIFC onset; Fig. 1C, see also Fig. 7).
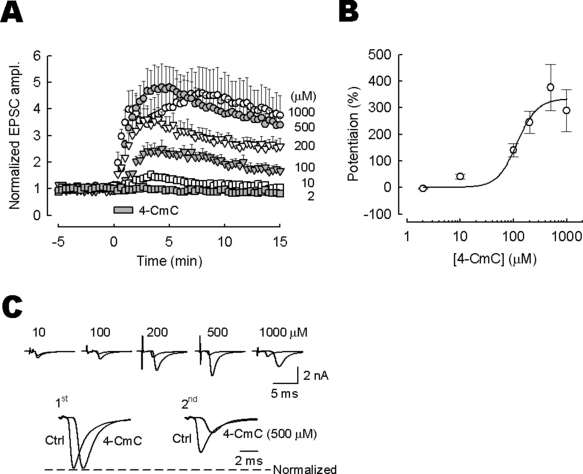
Potentiation of excitatory postsynaptic currents (TIFCs) by 4-chloro-m-cresol (4-CmC). (A) Time plots showing the facilitatory effect of 4-CmC on TIFC amplitude. Data derived from five to seven experiments at each dose (▪, 2 µm; □, 10 µm; , 100 µm; , 200 µm; •, 500 µm; ○, 1000 µm). The TIFC amplitude (ordinate) was normalized to the mean TIFC amplitude before 4-CmC application. 4-CmC was applied for 2 min as indicated by the bar. (B) Concentration dependence of the facilitatory effect of 4-CmC. Ordinate indicates the percentage increase of TIFCs measured 4–5 min after 4-CmC application. A curve fit to data points represents the equation: the magnitude of potentiation (%) = [maximal potentiation]/[1 + (EC50/[4-CmC])n], where the maximal potentiation is 334%, half-maximal effective concentration (EC50) is 116 µm and Hill coefficient (n) is 2.3. (C) Upper sample traces show averaged TIFCs before and 4–5 min after 4-CmC application (superimposed). Lower traces show averaged TIFCs evoked by a paired-pulse stimulation at a 50 ms interstimulus interval before (Ctrl) and 4–5 min after 4-CmC (500 µm) application (superimposed after normalizing to the first TIFC amplitude). Note the paired-pulse depression and delayed onset of TIFCs after 4-CmC application.
Effects of 4-chloro-m-cresol on presynaptic action potentials and K+ currents
To identify the presynaptic target of 4-CmC, we first recorded action potentials (APs) elicited by a brief (1 ms) depolarizing pulse in the calyceal nerve terminal. Bath application of 4-CmC (500 µm) markedly slowed the repolarizing phase of APs, with their half-widths (durations at 50% of peak amplitude) undergoing threefold prolongation from 0.43 ± 0.05 to 1.3 ± 0.2 ms (n = 5; P < 0.01; Fig. 2). However, 4-CmC had no effect on the peak amplitude of APs (from 125 ± 2.4 to 127 ± 2.5 mV; P = 0.21) or the resting membrane potential (from −76.0 ± 1.2 to −74.8 ± 1.3 mV; P = 0.073). It also had no significant effect on the AP rise time kinetics, with the maximum rate of rise remaining similar before (0.488 ± 0.024 V/ms) and after (0.440 ± 0.017 V/ms) 4-CmC applications (P = 0.098).
The dramatic prolongation of the AP duration by 4-CmC suggests that it might affect IpK. After blocking Na+ currents using tetrodotoxin (1 µm), we recorded IpK evoked by depolarizing square pulses (Ishikawa et al., 2003). 4-CmC (500 µm) attenuated IpK by 51–69%, between −40 and +20 mV, with the magnitude of inhibition being 52.6 ± 3.6% at +20 mV (n = 5; measured at 10 ms from the current onset; Fig. 3). Together these results suggest that 4-CmC prolongs presynaptic AP duration by inhibiting IpK, thereby facilitating nerve-evoked transmitter release.
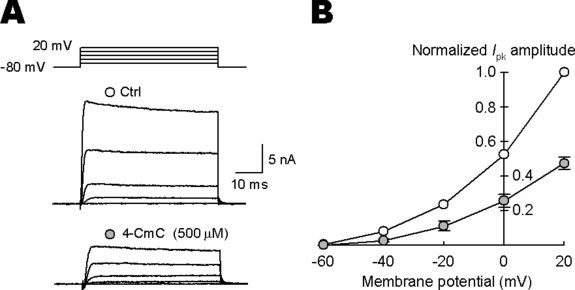
Bath-applied 4-chloro-m-cresol (4-CmC) attenuated presynaptic K+ currents (IpK). (A) IpK were evoked by 50 ms depolarizing pulses stepping from −80 mV holding potential to various potentials in the presence of tetrodotoxin (1 µm). Sample traces show IpK before (Ctrl) and 4–5 min after 4-CmC (500 µm) application and voltage commands (top traces). (B) ○ and •, data points before (Ctrl) and after 4-CmC application (n = 5). For the current/voltage relationship, the mean IpK amplitude was measured at 10 ms from the onset of the command pulse and normalized to the amplitude at +20 mV. After 4-CmC application, IpK became significantly smaller (between −40 and +20 mV, P < 0.01).
Effects of 4-chloro-m-cresol on presynaptic Ca2+ currents and excitatory postsynaptic currents
In addition to the inhibitory effect on IpK, 4-CmC might increase IpCa or stimulate exocytotic mechanisms downstream of Ca2+ entry. To examine these possibilities, we performed paired whole-cell recordings from a presynaptic terminal and its postsynaptic target cell, and evoked TIFCs by IpCa, after blocking Na+ and K+ conductance (Takahashi et al., 1996). As illustrated in Fig. 4, 4-CmC (500 µm) had little effect on the amplitude of IpCa (−6.6 ± 2.0%, n = 5) or TIFCs (−10 ± 10%). These results suggest that the presynaptic facilitatory effect of 4-CmC on TIFCs is predominantly mediated by its inhibitory effect on voltage-gated K+ channels.
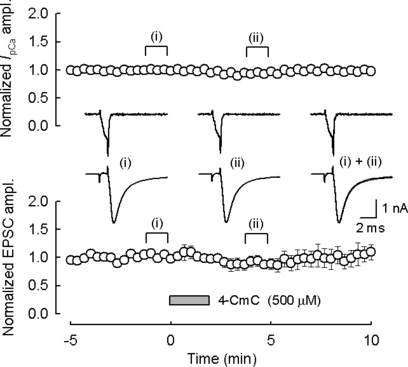
4-Chloro-m-cresol (4-CmC) had no effect on excitatory postsynaptic currents (TIFCs) evoked by presynaptic Ca2+ currents (IpCa). In simultaneous pre- and postsynaptic whole-cell voltage-clamp recording, the calyceal terminal was held at −70 mV and IpCa was evoked by 1 ms depolarizing pulse. This induced Ca2+ influx of 0.88 ± 0.09 pC (n = 5). Sample traces in inset show IpCa and TIFCs evoked by IpCa before (i) and 4–5 min after (ii) 4-CmC (500 µm) application. Data were normalized to the mean amplitude of IpCa and TIFCs before 4-CmC application. 4-CmC was applied for 2 min as indicated by the bar. The artificial cerebrospinal fluid contained tetrodotoxin (1 µm), tetraethylammonium (10 mm, equimolar replacement of NaCl), d-(–)-2-amino-5-phosphonopentanoic acid (50 µm) and kynurenic acid (1 mm).
Effect of ryanodine on the presynaptic facilitatory effect of 4-chloro-m-cresol
Type 2 RyR is expressed in chick cerebellar parallel fibre and mossy fibre terminals (Ouyang et al., 1997), and type 3 RyR is expressed at the frog motor nerve terminals (Kubota et al., 2005). However, it is unknown whether RyR is expressed at the calyx of Held presynaptic terminal. We investigated this issue using type-specific antibodies. The calyceal terminal region can be identified with the synaptophysin immunofluorescence. In both postnatal day (P)8 and P14 rats, calyceal terminals showed clear signals to type 3 RyR but no immunoreactivity was observed for type 1 or type 2 RyR (Fig. 5). No age difference was observed for the intensity of type 3 RyR immunofluorescence signals between P8 and P14.
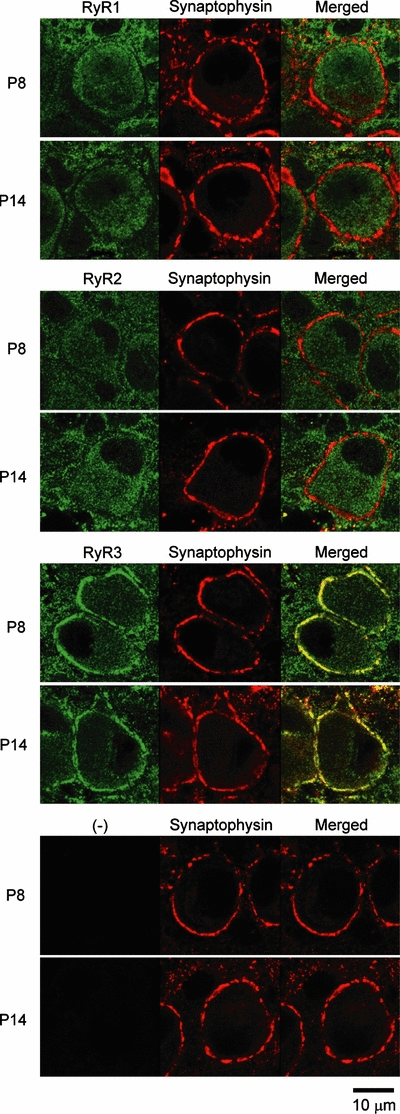
Ryanodine receptor (RyR) immunoreactivities at calyceal nerve terminals in 8-day-old (P8) and 14-day-old (P14) rats. RyR (green, left) and synaptophysin (red, centre) signals and their overlapped images (yellow, right) indicate that type 3 RyR (RyR3) is localized in the presynaptic compartment, and type 1 (RyR1) and type 2 (RyR2) RyR are absent at the calyx of Held. The bottom panel shows background noise in controls, where slices were treated only with anti-synaptophysin primary antibody (with no RyR antibody) and subsequently with the mixture of secondary antibodies.
Given that type 3 RyR is expressed at the calyceal terminal, ryanodine might attenuate the presynaptic facilitatory effect of 4-CmC. However, ryanodine (20 µm) by itself had no effect on the amplitude of TIFCs (−1.2 ± 1.8%, n = 3; data not shown) and preincubation of slices with ryanodine (20 µm, > 20 min) did not affect the facilitatory effect of 4-CmC on TIFCs (n = 6, P = 0.56; Fig. 6). These results suggest that RyR-mediated Ca2+ release is not involved in the nerve-evoked transmitter release and facilitatory effect of 4-CmC on the release at this synapse.
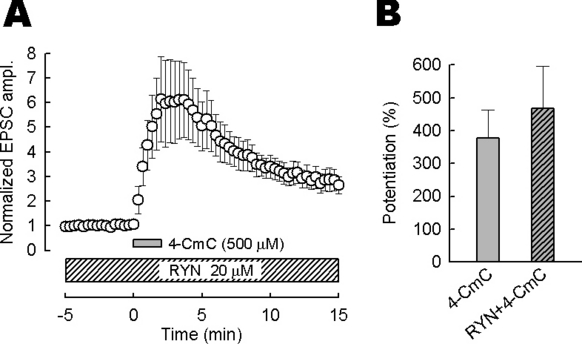
The effect of ryanodine (RYN) on the 4-chloro-m-cresol (4-CmC)-induced excitatory postsynaptic current (TIFC) potentiation. (A) Bath-applied 4-CmC (500 µm) potentiated TIFCs in the presence of RYN (20 µm). 4-CmC was applied more than 20 min after RYN application. The periods of application are indicated by bars. TIFC amplitudes (n = 6) were normalized to the mean values before 4-CmC application. (B) Summary data of 4-CmC-induced potentiation of TIFCs. RYN had no effect on the potentiation (P = 0.56, Mann–Whitney test). The mean magnitude of TIFC potentiation in the presence of RYN was 467 ± 130%.
Mechanism underlying prolonged synaptic delay by 4-chloro-m-cresol
As shown in Fig. 1C, 4-CmC prolongs synaptic delay. As 4-CmC blocks human muscle type Na+ channels at the half-maximal inhibitory concentration of 400 µm (Haeseler et al., 1999), the prolongation of synaptic delay by 4-CmC might result from slowing of axonal conduction velocity. To test this possibility, we made presynaptic recordings and evoked APs by presynaptic fibre stimulation. Bath-applied 4-CmC (1 mm) broadened presynaptic APs (see also Fig. 2A) without altering their onsets (Fig. 7A), indicating that 4-CmC has no effect on the axonal conduction velocity.
Expanding the duration of the presynaptic AP-like command pulse can delay the onset of TIFCs (Yang & Wang, 2006). To examine whether the AP broadening can explain the prolongation of synaptic delay, we made simultaneous pre- and postsynaptic voltage-clamp recordings and evoked IpCa by two kinds of AP waveforms, one recorded before (control) and the other recorded after 4-CmC application (Fig. 7B). The 4-CmC AP waveform command pulse produced biphasic IpCa, with a small step followed by a larger inward current. Its peak was significantly delayed from that of IpCa evoked by control command pulse. The initial small step must be caused by a reduced driving force for Ca2+ currents at the peak of the command pulse (Katz & Miledi, 1971). The peak amplitude of IpCa was similar between them. In parallel with the delayed peak of IpCa, the onset of TIFCs evoked by the 4-CmC command pulse was delayed from the control. The mean magnitude of prolongation of synaptic delay in these experiments was similar to, although less variable than, those observed for TIFCs evoked by extracellular stimulation (P = 0.55, Mann–Whitney test; Fig. 7C). The TIFC amplitude evoked by the 4-CmC command pulse was larger than that evoked by control pulse (potentiation by 382 ± 92%, n = 4). This magnitude is similar to the maximal potentiation of TIFCs induced by bath-applied 4-CmC (334%, Fig. 1B). Thus, the 4-CmC-induced facilitation of transmitter release can be fully explained by the broadening of presynaptic AP duration.
Discussion
At the rat calyx of Held, phorbol ester (Hori et al., 1999) and cyclic AMP (Kaneko & Takahashi, 2004) facilitate transmitter release by directly stimulating the exocytotic machinery downstream of Ca2+ influx. In the present study, we demonstrate that 4-CmC facilitates transmitter release by inhibiting voltage-gated K+ channels without affecting the exocytotic machinery in the calyceal terminal. This mechanism is similar to the presynaptic facilitatory effect of cyclothiazide (Ishikawa & Takahashi, 2001), which is, however, much weaker than that of 4-CmC.
In the nerve terminal, voltage-gated K+ channels play a regulatory role in Ca2+ influx by counteracting voltage-gated Ca2+ channels (Katz & Miledi, 1969). 4-CmC inhibited presynaptic voltage-gated K+ channels (Fig. 3) but it had no effect on voltage-gated Ca2+ channels (Fig. 4). As 4-CmC had no effect on the resting membrane potential (Fig. 2), it may not affect inwardly rectifying K+ channels, which regulate the resting membrane potential at the calyx of Held (Takahashi et al., 1998). Its lack of effect on the axonal conduction velocity (Fig. 7A) and on the rate of rise of APs further suggests that it has no effect on presynaptic Na+ channels. In these respects, the inhibitory effect of 4-CmC on voltage-gated K+ channels is highly specific.
At the P13–P15 rat calyx of Held terminal, IpK has two main components. One is the low-voltage-activated Kv1 current (20% of total K+ current at +40 mV) and the other is the high-voltage-activated Kv3 current (50%), both of which can be blocked by 4-aminopyridine (Ishikawa et al., 2003). As 4-CmC attenuated IpK between −40 and +20 mV (Fig. 3), 4-CmC may inhibit Kv1 and Kv3 currents like 4-aminopyridine. TEA (1 mm) blocks Kv3 channels and prolongs the presynaptic AP half-width by 68%. Kv1 channels normally do not take part in presynaptic AP repolarization but the Kv1 channel blocker margatoxin can further prolong AP half-width (by 36%) after blocking Kv3 channels by TEA (Ishikawa et al., 2003). Because 4-CmC prolonged the AP duration to a much greater extent (200% increase) than TEA plus margatoxin, the inhibitory effect of 4-CmC on voltage-gated K+ channels may be broad, inhibiting Kv3, Kv1 and probably 4-aminopyridine-resistant K+ channels.
Immunocytochemical study using type-specific RyR antibodies showed that the type 3 RyR was present at the calyceal terminal (Fig. 5). However, ryanodine (20 µm) had no effect on the basal TIFC amplitude or the 4-CmC-induced TIFC facilitation (Fig. 6), suggesting that RyR-mediated Ca2+ release is not involved in the nerve-evoked transmitter release or its modulation at the calyx of Held. Similar to our results, ryanodine (10–20 µm) has no effect on basal synaptic transmission at other central excitatory synapses (Reyes & Stanton, 1996; Emptage et al., 1999; Carter et al., 2002).
Calyceal terminals express type 3 RyRs, but not type 1 or type 2 RyRs, as at the frog motor nerve terminals (Kubota et al., 2005). Although type 3 RyR is relatively insensitive to 4-CmC compared with type 1 or type 2 RyR, 500 µm 4-CmC can activate RyR-mediated Ca2+ release in cell lines, which express type 3 RyRs (Fessenden et al., 2000). At the calyx of Held, 4-CmC increased the frequency of spontaneous miniature TIFCs and ryanodine (20 µm) attenuated this effect (D. Suzuki & T. Takahashi, unpublished observation), suggesting that activation of type 3 RyR by 4-CmC might increase spontaneous transmitter release.
4-Chloro-m-cresol has been regarded as a specific activator of RyRs. However, our results indicate that it has a strong side-effect inhibiting K+ channels. Thus, special care should be taken when using 4-CmC as a RyR activator. As a possible advantage of this side-effect, 4-CmC has a robust presynaptic facilitatory effect, causing up to a fourfold increase in the TIFC amplitude. Given that its mechanism of action is now clear at the calyx of Held, 4-CmC can be used as a tool to facilitate transmitter release, like 4-aminopyridine or TEA.
Acknowledgements
This study was supported by a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology. We thank Tatsuhito Yamauchi for his contribution to the early part of this study.
Abbreviations
-
- aCSF
-
- artificial cerebrospinal fluid
-
- AP
-
- action potential
-
- 4-CmC
-
- 4-chloro-m-cresol
-
- TIFC
-
- excitatory postsynaptic current
-
- I pCa
-
- presynaptic Ca2+ currents
-
- I pK
-
- presynaptic K+ currents
-
- P
-
- postnatal day
-
- RyR
-
- ryanodine receptor
-
- TEA
-
- tetraethylammonium.