Recovery of two independent sweet taste systems during regeneration of the mouse chorda tympani nerve after nerve crush
Abstract
In rodents, section of the taste nerve results in degeneration of the taste buds. Following regeneration of the cut taste nerve, however, the taste buds reappear. This phenomenon can be used to study the functional reformation of the peripheral neural system responsible for sweet taste. In this study we examined the recovery of sweet responses by the chorda tympani (CT) nerve after nerve crush as well as inhibition of these responses by gurmarin (Gur), a sweet response inhibitor. After about 2 weeks of CT nerve regeneration, no significant response to any taste stimuli could be observed. At 3 weeks, responses to sweet stimuli reappeared but were not significantly inhibited by Gur. At 4 weeks, Gur inhibition of sweet responses reached statistically significant levels. Thus, the Gur-sensitive (GS) component of the sweet response reappeared about 1 week later than the Gur-insensitive (GI) component. Moreover, single CT fibers responsive to sucrose could be classified into distinct GS and GI groups at 4 weeks. After 5 weeks or more, responses to sweet compounds before and after treatment with Gur became indistinguishable from responses in the intact group. During regeneration, the GS and GI components of the sucrose response could be distinguished based on their concentration-dependent responses to sucrose. These results suggest that mice have two different sweet-reception systems, distinguishable by their sensitivity to Gur (the GS and GI systems). These two sweet-reception systems may be reconstituted independently during regeneration of the mouse CT nerve.
Introduction
Selective inhibitors of the taste response have been used to characterize a variety of taste receptor cell types and the axons that innervate them. For example, amiloride, which blocks the epithelial Na+ channel (ENaC), specifically inhibits the taste response to NaCl in rodents but does not suppress the responses to sweet, sour or bitter substances. Electrophysiological studies of the rodent chorda tympani (CT) nerve have shown that NaCl-responsive fibers could be classified into two types, amiloride-sensitive (AS) and amiloride-insensitive (AI). AS-type fibers showed a selective response to NaCl (AS- or N-type), whereas AI-type fibers showed broad responsiveness to several electrolytes (AI-, E- or H-type; Ninomiya & Funakoshi, 1988; Ninomiya et al., 1989; Hettinger & Frank, 1990). The CT nerve, which innervates taste buds on the anterior tongue, exhibits both AS and AI responses to NaCl. In contrast, the glossopharyngeal nerve, which innervates taste buds on the posterior tongue, shows only the AI response (Ninomiya et al., 1989; Formaker & Hill, 1991; Doolin & Gilbertson, 1996; Ninomiya, 1998).
The peptide gurmarin (Gur) inhibits CT nerve responses to sweet compounds by ∼50% but does not affect responses to salty, sour or bitter substances (Imoto et al., 1991; Miyasaka & Imoto, 1995; Ninomiya & Imoto, 1995; Ninomiya et al., 1997, 1998). As observed for amiloride suppression of the NaCl response, Gur only slightly suppresses the sweet response of the glossopharyngeal nerve, if at all (Ninomiya et al., 1997). In C57BL mice, sweet-responsive CT fibers could be classified into two distinct groups, Gur-sensitive (GS) and Gur-insensitive (GI), suggesting that there may be corresponding receptor subtypes for mouse sweet responses (Ninomiya et al., 1998). Unlike the AS and AI systems, however, the GS- and GI-type CT fibers are both sweet-best responsive, and their response profiles for various compounds do not significantly differ (Ninomiya et al., 1998).
In rodents, section of the taste nerve results in degeneration of the taste buds. Following regeneration of the cut taste nerve, however, the taste buds reappear (Guth, 1958; Zalewski, 1969). By using this phenomenon to study the functional relationship between particular taste fibers and receptor cells, we previously examined reformation of the AS and AI NaCl-responsive systems in the regenerated CT nerve (Ninomiya, 1998; Yasumatsu et al., 2003). We found that AI-E-type fibers reappeared earlier (after 3 weeks of regeneration) than AS-N-type fibers (at 4 weeks). During the course of recovery, N- and E-type fibers could be clearly distinguished on the basis of their amiloride sensitivities, their KCl/NaCl response ratios and their concentration-dependent responses to NaCl, suggesting that the two salt-responsive systems were reconstituted independently (Yasumatsu et al., 2003).
If the GS and GI sweet taste systems are based on specific subtypes of sweet taste receptors, cells and fibers, these two receptor-neural systems might be differentially reformed during regeneration of the CT nerve, analogous to our observations for the AI and AS salt taste systems. In the present study we examined Gur inhibition of sweet taste responses during regeneration of the mouse CT nerve. We also examined recovery of expression of T1R3 mRNA, a functional sweet-receptor subunit (Li et al., 2002), in fungiform taste bud cells to investigate its potential link with that for GS and GI sweet taste systems.
Materials and methods
Experimental manipulation
All experimental procedures were approved by the committee for Laboratory Animal Care and Use at Kyushu University (Fukuoka, Japan) and were in accordance with the Animal Care Guidelines of Kyushu University. Subjects were adult male and female C57BL/6NCrj mice (Charles River Japan, Tokyo, Japan) that were 8–20 weeks of age, ranging in weight from 20 to 32 g. At 8–10 weeks of age, mice were divided into five groups, one intact control group and four nerve-crush groups, which were later examined at 2, 3, 4 and ≥ 5 weeks after their bilateral CT nerves were crushed. Animals of the four nerve-crush groups were used to obtain nerve recordings at the time-points of 14–16, 21–23, 28–30 and ≥ 35 days (< 50 days) after the CT nerve crush and regeneration. The procedure used to crush the CT nerve crush was described previously (Yasumatsu et al., 2003). Animals were anesthetized with pentobarbital sodium (50–60 mg/kg, i.p.; Somnopentyl, Schering-Plough Co., Kenilworth, NJ, USA). The bilateral CT nerves were exposed at ∼5 mm rostrally apart from their entry to the bulla and repeatedly crushed at a single point with number 5 forceps until only a thin strand of nerve sheath remained (5–10 crushes).
Recordings of responses from intact and regenerated chorda tympani nerves
At selected times after the nerve crush, the experimental groups were reoperated under pentobarbital anesthesia (again 50–60 mg/kg) to expose the regenerated nerve and to dissect a single fiber or a few fibers from the nerve for electrophysiological recording. The procedures for dissection and recording of responses from the regenerated nerve and fibers were the same as those used previously (Ninomiya, 1998; Yasumatsu et al., 2003). Under pentobarbital anesthesia, the trachea of each mouse was cannulated and the mouse was then fixed in the supine position with a head holder to allow dissection of the CT nerve. The right CT nerve was dissected free from surrounding tissues after removal of the pterygoid muscle and cut at the point of its entry to the bulla. For whole-nerve recording, the entire nerve was placed onto an Ag/AgCl electrode. For single-fiber recording, a single fiber or a few fibers of the nerve were teased apart with a pair of needles and lifted onto the electrode. An indifferent electrode was placed in nearby tissue. Neural activities resulting from chemical and electrical stimulations (ESs) of the tongue were fed into an amplifier (K-1; Iyodenshikogaku, Nagoya, Japan), and monitored on an oscilloscope and audiomonitor. Whole-nerve responses were integrated with a time constant of 1.0 s and recorded on a computer for later analysis using a PowerLab system (PowerLab/sp4; AD Instruments, Australia).
Chemical and electrical stimulations of the tongue
The anterior half of the tongue was enclosed in a flow chamber made of silicone rubber (Ninomiya & Funakoshi, 1981). Solutions were delivered into the chamber by gravity flow and flowed over the tongue for a controlled period. Solutions used as chemical stimuli were: 0.1 m NH4Cl, 0.1 m NaCl, 0.01 m HCl, 0.02 m quinine HCl, 0.01–1 m sucrose (Suc), 0.1–8 mm SC45647 (SC), 0.01–1 m glucose (Glc), 0.5 m maltose (Mal), 0.5 m fructose (Fru), 1 m sorbitol, 20 mm saccharin Na (Sac), 0.3 m l-alanine and 0.5 m l-proline (Wako Pure Chemicals Industries, Osaka, Japan). These chemicals were dissolved in distilled water and used at ∼24 °C. For whole-nerve recording in the second week, the response of the CT to ES applied to the tongue was tested because the 2-week group responded to chemical stimuli only slightly or not at all. For ES, an Ag/AgCl electrode was placed on the inside wall of the flow chamber or was placed directly on the tongue when required. The Ag/AgCl indifferent electrode was positioned in nearby tissue. Anodal current was passed through the tongue from a ramp current generator (Densi-Sekkei, Nagoya, Japan). The bathing medium used during the current stimulation was 1 mm NaCl (Ninomiya & Funakoshi, 1981). As found in our previous study (Yasumatsu et al., 2003), anodal current with an intensity of 20 µA (rate of raise, 100 µA/s; duration, ∼20 s) provoked robust responses in the CT at 2 weeks after nerve crush. Therefore, we used the response to anodal current as the standard for calculating relative magnitudes of response to chemical stimuli at each experimental time-point. During chemical stimulation of the tongue, the test solution flowed for ∼30 s at the same flow rate as the distilled water used for rinsing the tongue (∼0.1 mL/s). The tongue was rinsed with distilled water for an interval of ∼1 min between successive stimulations. The order of chemical stimulation for whole-nerve recording was: 0.1 m NH4Cl, 0.01–1 m Suc, 0.1–8 mm SC, 0.1 m NH4Cl, 0.01–1 m Glc, 0.5 m Mal, 0.5 m Fru, 1 m sorbitol, 20 mm Sac, 0.3 m l-alanine and 0.5 m l-proline. To examine Gur inhibition of the CT responses, the tongue was treated with 30 µg/mL (∼7.13 µm) Gur dissolved in 5 mm phosphate buffer (pH 6.8; made with Na2HPO4.12H2O and NaH2PO4.2H2O) for 5 min as described in our previous studies (Ninomiya & Imoto, 1995; Ninomiya et al., 1999). After Gur treatment, responses to chemical and electrical stimulation were recorded. The stability of each preparation was monitored by periodic application of ES or 0.1 m NH4Cl. A recording was considered to be stable when ES or NH4Cl response magnitudes at the beginning and end of each stimulation series deviated by no more than 15%. Only responses from stable recordings were used in the data analysis. The order for single-fiber recording was 0.5 m Suc, 0.1 m NaCl, 0.01 m HCl and 0.02 m quinine HCl. When a single fiber responding to 0.5 m Suc was found, responses of the fiber to Suc before and after the Gur treatment were examined. In both whole-nerve and single-fiber recording experiments, Gur was applied to the tongue only once and therefore data from only one preparation were obtained from each animal.
Data analysis
In the analysis of whole-nerve responses, the magnitude of the integrated responses from 5 to 25 s (every 5 s) after stimulus onset was measured and averaged. The averaged response magnitude for each test stimulus was normalized to the response magnitude for ES (20 µA anodal current), which was taken as unity (1.0), and this relative response was used for statistical analysis. Repeated anova and Student's t-test were performed to evaluate the effect of Gur on taste responses statistically. In the analysis of single-fiber responses, single fibers were identified by their uniform spike height, singular wave form and intervals between contiguous spikes (Ninomiya, 1998; Kawai et al., 2000). Frequency/time histograms of impulse discharges before, during and after chemical stimulation of the tongue were calculated by means of a spike-analysis system (SAS-1; Iyodensikogaku). For data analysis, we used the net average frequency for the first 10 s after the stimulus onset, which was obtained by subtracting the spontaneous frequency for the 10-s period before stimulation. We calculated the percentage of Gur inhibition of the response to 0.5 m Suc for each fiber and used the 60% control level to classify the fibers as either GS-type (< 60%) or GI-type (≥ 60%). Our previous study showed that the 60% level was the most appropriate boundary for classifying the two groups of fibers with different Gur sensitivity in mice (Ninomiya et al., 1997).
CRNA probes for in-situ hybridization
Each mouse was anesthetized with pentobarbital sodium (50–60 mg/kg, i.p.; Somnopentyl, Schering-Plough Co.), killed by cervical dislocation and the tongue rapidly removed. After washing the tongues with a normal extracellular solution (140 mm NaCl, 5 mm KCl, 1 mm MgCl2, 1 mm CaCl2, 5 mm Glc, 1 mm sodium pyruvate, 10 mm HEPES-NaOH, pH 7.4), 0.2 mL of normal extracellular solution containing 1.0 mg/mL elastase (Roche, Indianapolis, IN, USA) was injected between the epithelium and muscle layers of the tongue. After incubation in normal extracellular solution continuously bubbled with 95% O2 and 5% CO2 for 15–20 min at 26 °C, the epithelial sheet containing fungiform papillae (FP) was peeled off from the underlying muscle. The epithelium was washed with normal extracellular solution, pinned serosal side up in a silicone dish and incubated in divalent cation-free extracellular solution containing 2 mm EDTA for 20 min at room temperature (26°C). Taste buds were individually removed from fungiform papillae (FP) by sucking with a 100 µm transfer pipette. One hundred taste buds from the taste papillae were transferred to an Eppendorf tube containing 100 µL (1 volume) of lysis buffer (4 m guanidine thiocyanate, 25 mm trisodium citrate, pH 7.0, 0.5% sodium N-lauroyl-sarcosine, 0.1 m 2-mercaptoethanol) and homogenized. Yeast transfer RNA (1.0 µg) was used as a carrier. Sequentially, 0.1 volume of 2 m Na-acetate (pH 4.0), 1 volume of acidic phenol and 0.2 volume of chloroform : isoamyl alcohol (49 : 1) were added, the mixture being vortexed after each addition. After incubation at 4 °C for 15 min and centrifugation at high speed, the aqueous phase was recovered and RNA was precipitated with 1 volume of isopropanol. After centrifugation, the pellet was resuspended in 1 volume of lysis buffer and RNA was reprecipitated with isopropanol. After two washings with 75% ethanol, the pellet was dried, dissolved in water. A cDNA was generated by reverse transcription [oligo (dT)12−18 primer] with the superscript preamplification system (Gibco/BRL, Gaithersburg, MD, USA). Polymerase chain reactions (PCRs) were carried out with an equivalent of 10 taste buds per reaction. Genomic DNA did not contribute to the signal as suggested by two protocols. In the first, RNA was treated in parallel in the presence and absence of reverse transcriptase, and the material was then used for PCR. In the absence of reverse transcriptase, there was no amplification of fragments of the expected size. Primers were chosen to span one or more introns in order to exclude confusion with amplified fragments from genomic DNA. PCR reactions led in this case to the amplification of two bands, either specific for the genomic DNA (characterized by a longer size owing to the presence of at least one intron) or for the reverse-transcribed complementary DNA. The primers used for DNA amplification were as follows: T1r3, 5′-TGCTGCTATGACTGCGTGGAC-3′ and 5′- AAGAAGCACATAGCACTTGGG-3′ (NM_031872, 910 bp); and α-gustducin, 5′-AGATGGGAAGTGGAATTAGTTCAGA-3′ and 5′-GCTCAGAAGAGCCCACAGTCTTTGA-3′ (X65747, 1069 bp). PCR was performed on PE9700 with the following conditions: 95 °C for 5 min (one cycle); 94 °C for 30 s, 58 °C for 30 s, 72 °C for 120 s (40 cycles); and 72 °C for 5 min (one cycle). The PCR solution contained 10 mm Tris/HCl, 50 mm KCl, 1.5 mm MgCl2, 0.5 µm of each primer, 200 µm deoxyribonucleoside 5′-triphosphate and 0.05 unit/mL of Ex Taq polymerase (Takara Bio, Otsu, Japan). These DNA fragments were purified and cloned into the pGEM T-Easy vector (Promega, Madison, WI, USA), confirmed by direct sequencing and digested with appropriate restriction enzymes. Biotin-labeled antisense RNA probes were generated by in-vitro transcription using a digoxigenin-RNA labeling mix and SP6 or T7 RNA polymerase (Roche).
in-situ hybridization
Frozen blocks of the dissected anterior and posterior parts of the tongue embedded in the OCT compound (Sakura Finetechnical, Tokyo, Japan) were sectioned into 5–7 µm thick slices, which were mounted on silane-coated glass slides. The cryosections were fixed in 4% paraformaldehyde in phosphate-buffered saline for 10 min at room temperature, treated twice with 0.1% diethyl pyrocarbonate in phosphate-buffered saline for 15 min, washed with 5 × SSC (standard saline citrate) for 15 min at room temperature and then prehybridized in hybridization buffer consisting of 50% formamide, 5 × SSC, 5 × Denhardt's solution, 500 µg/mL denatured salmon testis DNA, 250 µg/mL denatured baker's yeast tRNA and 1 mm dithiothreitol for 1 h at room temperature. Hybridization was carried out in a hybridization buffer to which was added 200 ng/mL antisense riboprobe for 18 h at 58 °C. After hybridization, sections were washed twice in 5 × SSC for 5 min each and twice in 0.2 × SSC for 30 min each at 65 °C. Subsequently, the sections were immersed in Tris-buffered saline consisting of 50 mm Tris/HCl (pH 7.5) and 150 mm NaCl for 5 min at room temperature, put in the blocking solution containing 0.5% blocking reagent (Roche) in Tris-buffered saline for 30 min and incubated with anti-digoxigenin Fab fragments conjugated with alkaline phosphatase (1 : 400 dilution in the blocking solution) for 60 min at room temperature. After three washes of 5 min each in Tris buffer, consisting of 50 mm Tris/HCl (pH 7.5), 150 mm NaCl and 0.05% Tween 20, sections were immersed in alkaline phosphatase buffer, consisting of 100 mm Tris/HCl (pH 9.5), 100 mm NaCl and 50 mm MgCl2, for 5 min. The signals were developed using nitroblue-tetrazolium chloride and 5-bromo-4-chloro-3-indolylphosphate as chromogenic substrates. The reaction was then stopped by rinsing the slides in Tris-EDTA (TE) buffer, after which they were mounted. The signal specificities of mRNA for each gene in the taste tissues were tested by using a sense probe as a negative control.
Results
Reappearance of gurmarin sensitivity during chorda tympani nerve regeneration
At 2 weeks after the CT nerve crush, the regenerated nerve responded to anodal current but showed no significant response to 0.5 m Suc (Fig. 1). After 3 weeks of regeneration, the response to Suc reappeared but was not significantly inhibited by lingual treatment with Gur. At 4 weeks, the response to Suc increased and was significantly inhibited by Gur. Concentration-dependent responses for Suc before and after Gur are shown in Fig. 2. Statistically significant inhibition of the Suc response by Gur was not observed at 3 weeks (repeated anova; F1,50 = 0.5; P > 0.05) but was clearly evident 4 weeks after the nerve crush (F1,60 = 24.0; P < 0.001; t-test; P < 0.05 for 30 mm−1 m Suc). At ≥ 5 weeks, the concentration-dependent responses for Suc before and after Gur became similar to those in the intact control group (before Gur, F1,60 = 1.23, P > 0.05; after Gur, F1,60 = 0.23, P > 0.05) and the inhibition of responses to Suc by Gur was significant (F1,60 = 14.5; P < 0.01 for the ≥ 5 week group; F1,60 = 21.5; P < 0.001 for the control group).

Sample recordings of integrated whole-nerve responses of regenerated chorda tympani nerves to an anodal current of 20 µA [electrical stimulation (ES)] or to 0.5 m sucrose (Suc) before (left trace) and after (right trace) lingual treatment with 30 µg/mL gurmarin for 10 min in experimental mice at 2, 3, 4 and ≥ 5 weeks after nerve crush.
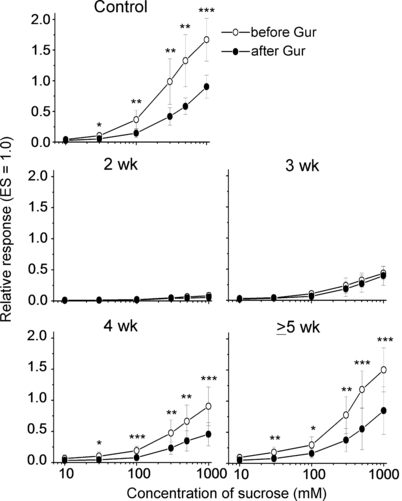
Concentration-dependent responses to sucrose before (○) and after (•) lingual treatment with 30 µg/mL gurmarin (Gur) for 10 min in intact control mice (n = 7) and experimental mice at 2 (n = 5), 3 (n = 6), 4 (n = 7) and ≥ 5 weeks (n = 7) after nerve crush. The response to an anodal current of 20 µA applied to the tongue was used as unit (1.0). Values indicated are means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 (t-test). ES, electrical stimulation.
Recovery of gurmarin-sensitive responses to various sweet compounds
Figure 3 shows the concentration-dependent responses for SC and Glc. As compared with the responses to Suc, the responses to SC were more strongly inhibited by Gur (by ∼80% of control), whereas the responses to Glc were more weakly inhibited (by ∼30% of control). Thus, the magnitude of Gur inhibition of CT responses varied among sweet compounds. In contrast, the recovery of Gur sensitivity in the responses to these compounds after the nerve crush appeared very similar to the recovery of Gur sensitivity in the response to Suc. Gur inhibition of the SC and Glc responses was not observed at 3 weeks (F1,32 = 2.27, P > 0.05 for SC; F1,24 = 0.79, P > 0.05 for Glc) but it became statistically significant at 4 weeks after the nerve crush (F1,32 = 9.35, P < 0.05 for SC; F1,24 = 5.60, P < 0.05 for Glc). At ≥ 5 weeks, the concentration-dependent responses for SC and Glc before and after Gur became similar to those in the intact control group (before Gur, F1,32 = 0.12, P > 0.05 for SC; F1,24 = 1.48, P > 0.05 for Glc; after Gur, F1,32 = 0.55, P > 0.05 for SC; F1,24 = 0.55, P > 0.05 for Glc), with significant inhibition of the responses to SC and Glc by Gur (≥ 5 week group, F1,32 = 17.74, P < 0.01 for SC; F1,24 = 10.72, P < 0.05 for Glc; control group, F1,48 = 15.74, P < 0.01 for SC; F1,36 = 27.48, P < 0.001 for Glc).
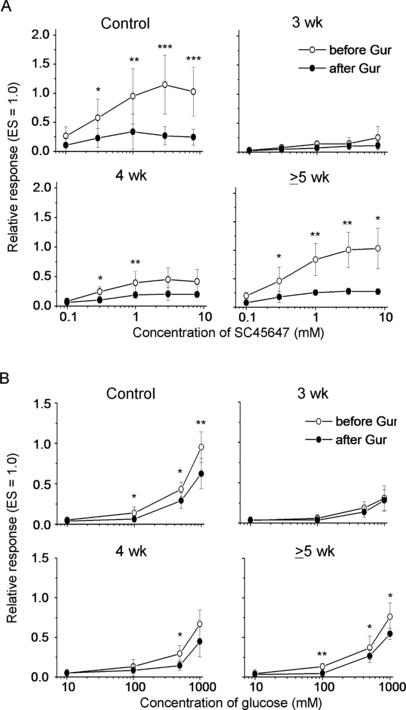
Concentration-dependent response relationships to SC45647 (A) and glucose (B) before (○) and after (•) lingual treatment with 30 µg/mL gurmarin (Gur) for 10 min in intact control mice (n = 7) and experimental mice (n = 5 each) at 3, 4 and ≥ 5 weeks after nerve crush. The response to an anodal current of 20 µA applied to the tongue was used as unit (1.0). Values indicated are means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 (t-test). ES, electrical stimulation.
Figure 4 shows the responses to six sweet compounds before and after Gur treatment in intact and experimental mice at 3, 4 and ≥ 5 weeks after the nerve crush. As observed for Suc, SC and Glc, the responses to all six compounds were not significantly inhibited by Gur at 3 weeks (t-test, Ps > 0.05). At 3 weeks the magnitude of this GI taste response had already reached more than 50% of the GI response in intact mice. At 4 weeks, significant inhibition by Gur was observed in the responses to Mal, Sac, l-alanine and l-proline (Ps < 0.05), whereas inhibition for Fru and sorbitol was not yet significant (Ps > 0.05). At ≥ 5 weeks, the responses to all six compounds became indistinguishable from those in the control group (Ps > 0.05) and all showed clear inhibition by Gur (Ps < 0.05–0.01).
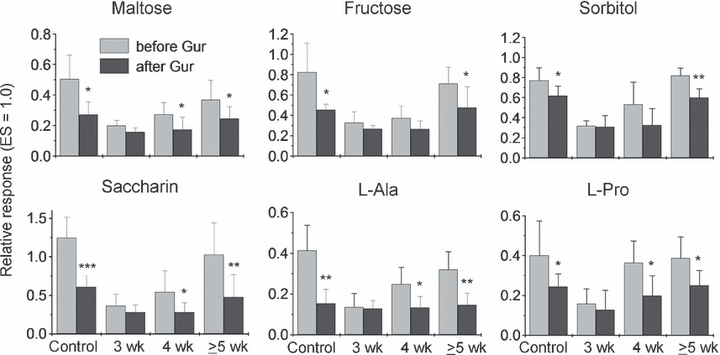
Responses to six sweeteners before and after lingual treatment with 30 µg/mL gurmarin (Gur) in intact control and experimental mice at 3, 4 and ≥ 5 weeks after bilateral chorda tympani (CT) nerve crush. Taste stimuli were 0.5 m maltose, 0.5 m fructose, 1 m sorbitol, 20 mm saccharin Na (Saccharin), 0.3 m l-alanine (L-Ala) and 0.5 m l-proline (L-Pro). The response to an anodal current of 20 µA applied to the tongue was used as unit (1.0). Values indicated are means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001 (t-test). ES, electrical stimulation.
Kinetic analyses of concentration-dependent responses for the gurmarin-sensitive and gurmarin-insensitive components of the sucrose response during recovery after the nerve crush
We calculated the dissociation constant and maximum response (Lineweaver & Burk, 1934) on the basis of the concentration-dependent responses for the GS and GI components of the Suc response at four different concentrations (e.g. 0.1, 0.3, 0.5 and 1.0 m) from 3 to ≥ 5 weeks after the nerve crush. As shown in Table 1, for both the GS and GI components, the maximum response values gradually recovered to the control levels during this period. In contrast, during regeneration, the dissociation constant values of the GI component ranged from 0.84 to 0.89 and those of the GS component ranged from 0.42 to 0.51. In both the GI and GS components, the dissociation constant values did not substantially change (t-test, P > 0.05).
| Group | GI component(% of control) | GS component (% of control) |
|---|---|---|
| Control | ||
| Kd | 0.82 ± 0.36 m (100) | 0.45 ± 0.19 m* (100) |
| Vmax | 1.52 ± 0.20 (100) | 1.40 ± 0.24 (100) |
| 3 weeks | ||
| Kd | 0.84 ± 0.07 m (102.4) | – |
| Vmax | 0.78 ± 0.05++ (51.3) | – |
| 4 weeks | ||
| Kd | 0.87 ± 0.20 m (106.1) | 0.42 ± 0.09 m** (93.3) |
| Vmax | 1.01 ± 0.20++ (66.4) | 0.57 ± 0.08**,+++ (40.7) |
| ≥ 5 weeks | ||
| Kd | 0.89 ± 0.36 m (108.5) | 0.51 ± 0.11 m* (113.3) |
| Vmax | 1.32 ± 0.37 (86.8) | 0.99 ± 0.27+ (74.3) |
- Values are means ± SD. Number of subjects in each group = 5–7. t-test between GI and GS components: *P < 0.05, **P < 0.01. t-test vs. control values: +P < 0.05, ++P < 0.01, +++P < 0.001.
Single-fiber response analyses on gurmarin sensitivity at 4 weeks after the nerve crush
We examined the single-fiber responses of the regenerated CT to various taste stimuli at 4 weeks after the nerve crush. In total, we successfully recorded spike activities from 80 regenerated single fibers. Sixty-five of the 80 responded to some of the taste stimuli (0.1 m NaCl, 0.1 m KCl, 0.01 m HCl, 0.02 m quinine HCl and 0.5 m Suc), whereas the remaining 15 fibers did not respond to any of these taste stimuli. Of 65 taste-responsive fibers, 23 responded predominantly to Suc. Of these 23 fibers, we found that six fibers (GS-type) showed clear inhibition of the Suc response by Gur (to 0–32% of control), whereas the remaining 17 fibers (GI-type) did not (ranging from 69 to 114% of control) (Fig. 5). Therefore, at 4 weeks, the GS and GI response components were clearly segregated based on both whole-nerve integrated response and single-fiber response analyses.
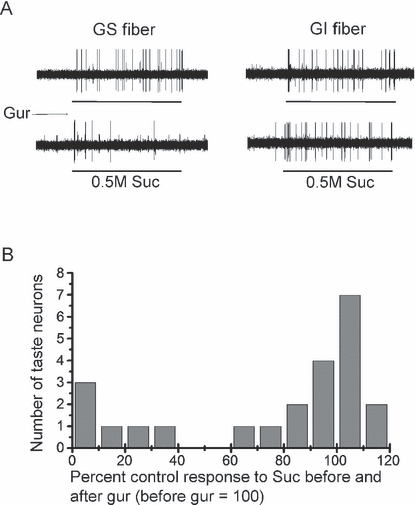
(A) Sample recordings of single chorda tympani (CT) fibers showing two types of sweet response. Gurmarin (Gur)-sensitive (GS) and Gur-insensitive (GI) fibers were treated with 0.5 m sucrose (Suc) before or after lingual treatment with Gur at 4 weeks after bilateral CT nerve crush. (B) Distribution of mouse CT fibers according to their percent control responses to 0.5 m Suc after 30 µg/mL Gur (control, before Gur = 100%) at 4 weeks after bilateral CT nerve crush.
Expression of T1R3 and gustducin during regeneration
Antisense riboprobes specific for T1R3 and gustducin mRNAs were applied to horizontal sections of the mouse fungiform papillae after the CT nerve crush. T1R3 and α-gustducin mRNAs were detected in some cells in taste buds of the fungiform papillae throughout the period tested (Fig. 6). T1R3 and α-gustducin mRNAs were clearly detected in circumvallate papillae. As reported in our previous studies (Shigemura et al., 2005; Yasumatsu et al., 2005b), taste buds in fungiform papillae were reduced in size and number at 2 weeks after the CT nerve crush. Some shrunken fungiform papillae or dermal indentations into the epithelium without taste buds were also detected. Although the size of taste buds was reduced, a few positive taste cells for T1R3 and α-gustducin were detected at this period. The intensity of the signals for T1R3 and α-gustducin was weaker than that of intact control mice. At 3 weeks, the size of taste buds increased (Shigemura et al., 2005) and hybridization signal for T1R3 and α-gustducin were more clearly detected. At 4 and 5 weeks, the expression patterns for T1R3 and α-gustducin were almost the same as those at 3 weeks. Staining using a sense probe was negative in control fungiform and circumvallate papillae, and regenerating fungiform papillae.
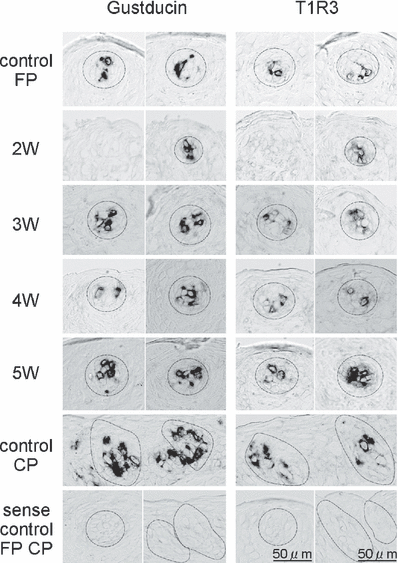
In-situ hybridization analysis of T1R3 and α-gustducin mRNAs in fungiform (FP) and circumvallate (CP) papillae of intact control mice and FP of experimental mice at 2, 3, 4 and 5 weeks after bilateral chorda tympani nerve crush. Taste bud cells at an early stage (2 weeks after nerve crush) already express T1R3 or α-gustducin. The size of FP taste buds expressing each gene reached the control level at 3 weeks postsurgery. The dotted lines indicate the outlines of sample taste buds.
Discussion
In the present study, we first examined whether it was possible to distinguish between the GS and GI components of the sweet response during regeneration of the CT nerve. To do this, we used Gur at a concentration [30 µg/mL (∼7.13 µm)]that was 50% higher than the concentration (20 µg/mL) sufficient to produce maximum suppression of the Suc response in C57BL/6 mice (Ninomiya & Imoto, 1995). Under these conditions, we expected that any GS responses would be maximally inhibited. Therefore, the residual response after Gur treatment at any particular stage of regeneration would represent the GI component; similarly, the difference between the residual GI component and the control response would represent the GS component. We found that the response to sweet compounds in the regenerating CT nerve reappeared at ∼3 weeks after the nerve crush. However, lingual treatment with Gur did not inhibit these sweet responses at 3 weeks, indicating that these sweet responses were GI. From ∼4 weeks onwards, a portion of the Suc response became clearly GS 1-3). Thus, reformation of the GS component of the sweet response began ∼1 week later than reformation of the GI component. This delay in recovery of the GS component was observed for all nine sweet compounds tested. Full recovery of both GI and GS sweet-responsive components took at least 5 weeks 1-3).
This differential recovery is similar to the previously described recovery of the AS and AI components of the NaCl response during regeneration of the mouse CT nerve (Yasumatsu et al., 2003). In that study, NaCl responses began to recover from ∼3 weeks after the nerve crush, whereas amiloride sensitivity reappeared from ∼4 weeks onwards. By means of single-fiber recordings, we found that nearly all fibers responding to NaCl were AI at 3 weeks postcrush. At 4 weeks some AS fibers appeared that were clearly different from AI fibers based on three criteria: their amiloride sensitivity, their KCl : NaCl response ratios and their NaCl concentration-dependent responses. Therefore, we concluded that the AI and AS salt-responsive systems were reconstituted independently. Similarly, based on the analysis of whole-nerve responses, we found that the dissociation constant values of the GI and GS responses to Suc were distinct and separable in both intact and regenerated CT nerves (Table 1). Thus, the binding affinity of GS receptors for Suc in the intact CT nerve was different from that of GI receptors and this difference was maintained during the course of nerve regeneration. Furthermore, when Gur sensitivity became evident at 4 weeks after the nerve crush, Suc-responsive single fibers could be identified whose response to Suc was clearly inhibited by Gur. At 4 weeks after the nerve crush, regenerated CT fibers were distributed between two distinct groups based on the level of inhibition in the presence of Gur, i.e. ≤ 32% of control for the GS-type vs. ≥ 69% of control for the GI-type (Fig. 4). This distribution is the same as shown previously in intact mice (Ninomiya et al., 1998). Thus, these two sweet-responsive systems, with corresponding types of receptor cells and fibers, may be separately reconstituted in the same manner as the two salt-responsive systems.
In addition to the inhibition by Gur shown in this and previous studies, incomplete suppression of whole-nerve responses to Suc has been reported in several systems, i.e. by zizyphin in rats and hamsters (Yamada & Imoto, 1987), by Zn and/or Cu ions in rats (Yamamoto & Kawamura, 1971), hamsters (Myers et al., 1993) and mice (Iwasaki & Sato, 1984), and by gymnemic acid in chimpanzees. However, the existence of residual responses to Suc after treatment with such inhibitors does not necessarily indicate the existence of two different Suc receptor sites; the degree of response suppression might be dependent on the potency of the inhibitors or on other possible factors (i.e. concentration dependencies, competitive or non-competitive). For example, Hellekant et al. (1998) examined chimpanzee CT fiber responses and demonstrated that gymnemic acid exclusively inhibited the responses to sweet compounds in the S-type (Suc-best) fibers (classified by a hierarchical cluster analysis) but not the responses in other types of fibers. The residual responses after gymnemic acid could have been derived from those non-S-type fibers, which might have been activated by other taste stimulants. Our previous study first demonstrated that two groups of Suc-best CT fibers could be classified according to their Gur sensitivity (Ninomiya et al., 1998). The current results on taste responses of the regenerated CT nerve provide additional evidence for the independent reformation of two distinct peripheral sweet perception systems in mice, GS-type and GI-type, with different receptor sites, taste cells and fiber types.
Based on current knowledge, however, it is very difficult to explain the existence of two different receptor types or corresponding fiber types for sweet taste. Recent molecular studies have shown that the heterodimer T1R2/T1R3 functions as a sweet taste receptor, responding to all classes of sweet compounds in cell-based assays (Li et al., 2002). Moreover, mice lacking this broadly tuned sweet receptor, T1R2/T1R3, showed full abolition of behavioral and neural sweet taste responses (Zhao et al., 2003). Damak et al. (2003) demonstrated that C57BL/6 mice with a T1R3 knockout mutation exhibited dramatically reduced CT responses to 0.02 m Sac or 0.3 m Suc (to ∼12 or ∼20% of wild type, respectively). Thus, most of the GS and GI components of responses to Suc and Sac in C57BL/6 mice are likely to occur through activation of a T1R3-containing receptor(s). If receptors for sweet taste are exclusively composed of T1R2 and T1R3 molecules, differential receptor systems may be produced only by possible combinations of their monomers and dimers.
The previous and present results indicate that the GS and GI systems vary in their responsiveness to sweet compounds. For example, the GS system appears to respond more broadly to non-sugar sweet compounds than does the GI system; the CT response to d-phenylalanine and its enhancement by Sac were totally abolished by Gur (Ninomiya & Imoto, 1995; Ninomiya et al., 1998). Moreover, the present study reveals that the GS proportion of the sweet response (the GS component) varied among the compounds tested, in the following order: SC (∼80% of the control) > Sac, l-alanine, Suc, Fru, Mal and l-proline (∼50% of control) > Glc and sorbitol (∼30% of control). In general, the GS component is more responsive to non-sugar sweeteners than to sugars. Conversely, the GI component is more responsive to sugars than to non-sugar sweeteners. It is noted that the order of sweet compounds according to degree of Gur inhibition in intact mice is similar to the order of diminished responses in mice lacking T1R3. The diminution of CT responses in T1R3-knockout mice were larger in the following order: SC (≥ 95% of control) > Sac, d-tryptophan (∼88%), Suc (∼80%), Fru (∼70%), Mal (∼50%) > Glc and sorbitol (∼30%) (Damak et al., 2003). The T1R3 knockout reduced the responses to Glc and sorbitol to a similar extent as did Gur treatment of intact mice. Furthermore, T1R3-knockout mice exhibited no significant reduction in responses to Suc by the GI glossopharyngeal nerve, except at a concentration of 1.0 m (Damak et al., 2003). Moreover, our recent study showed that, in T1R3-knockout mice, residual responses of the CT nerve to Glc and sorbitol were not significantly inhibited by Gur, although responses to Suc were weakly but significantly suppressed by Gur (Yasumatsu et al., 2005a; our unpublished results). This evidence suggests that a T1R3-containing receptor(s) is important for the GS component of the sweet response but not for the GI component. The GI component, especially in response to Glc and sorbitol, seems likely to occur through the activation of receptors that do not contain T1R3.
In the present study, we examined the recovery of expression of T1R3 mRNA in taste cells during CT nerve regeneration. We found that T1R3 was expressed in a subset of taste cells from an early stage (2 weeks) before the reappearance of the response of the regenerated CT nerve to taste stimuli. This is consistent with the case of the reappearance of epitherial Na+ channel (ENaC) subunits (1–2 weeks) for salt taste after the CT nerve crush (Shigemura et al., 2005). The fact that recovery of expression of taste receptor molecules in taste cells precedes that of taste nerve responses indicates the difficulty of examining the potential linkage between molecular events occurring in the taste buds and recovery of each of the GS and GI sweet-reception and neural systems. Future studies are thus needed to clarify molecular mechanisms for the two sweet-reception systems.
Gurmarin inhibition of sweet responses has also been studied in the rodent central nervous system. Neurons in the nucleus of the solitary tract, the second order taste relay, have been classified into three classes (classes S, N and H) according to a hierarchical cluster analysis. Among these three classes, neurons of classes S and N exhibited significant inhibition of Suc responses by Gur (Lemon et al., 2003). In nucleus of the solitary tract neurons, the degree of inhibition of Suc responses by Gur ranged from > 90% of control to ∼0%, with no clear segregation of distinctly GS or GI components. Both GS and GI CT fibers may project into the more rostral part of the nucleus, whereas GI glossopharyngeal fibers may input to the more caudal part, with some overlaps. The existence of intermediate types of neurons is clearly different from the present data from mouse peripheral CT fibers. If the peripheral taste system in rats is similar to that in mice, information from both GS and GI receptor processes may converge onto the intermediate type of nucleus of the solitary tract neurons in rats. In rat nucleus of the solitary tract neurons, responses to different sweet compounds in single neurons were also differentially affected by Gur (Lemon et al., 2003). This may also result from the convergence of different combinations of GS and GI CT fibers with different responsiveness to sweet compounds.
As shown in our previous study, robust responses to anodal current were observed at 2 weeks after crushing of the CT nerve, when significant responses to chemical stimuli were not yet observed. Although the number of taste buds with a taste pore had decreased to 17.9% of the control at this stage, we did observe fungiform papillae with a taste bud primordium or with regenerating nerve fibers penetrating into the epithelium of the papillae (Yasumatsu et al., 2005b). We also observed the expression of epitherial Na+ channel (ENaC) subunits in taste bud cells by in-situ hybridization analysis at 2 weeks after crushing (Shigemura et al., 2005). It is likely that the anodal current may force small cations to pass through the paracellular pathways (Elliott & Simon, 1990; Ye et al., 1991, 1993) or the epithelium to activate the nerve fibers directly or via crude taste receptor cells (Ninomiya & Funakoshi, 1981, 1988).
In summary, the present study recorded the responses of the regenerated mouse CT nerve to various sweet compounds after the nerve crush and examined both the recovery of these responses and their inhibition by a sweet response inhibitor, Gur. The results indicate that the mouse CT response to sweet compounds can be segregated into GS and GI components, and that recovery of the GI component (at 3 weeks after the nerve crush) preceded recovery of the GS component (at 4 weeks) by about 1 week. Single CT fibers responsive to Suc could be clearly classified into GS and GI groups at 4 weeks. During the course of CT regeneration, the GS and GI components of the Suc response could also be distinguished on the basis of their concentration-dependent responses to Suc. The two sweet-reception systems may be reconstituted independently during regeneration of the CT nerve.
Acknowledgements
We thank Dr Sami Damak for his valuable comments and suggestions on the manuscript. This work was supported by Grant-in-Aids 18109013 and 18077004 (Y.N.) for Scientific Research from the Japan Society for the Promotion of Science.
Abbreviations
-
- AI
-
- amiloride-insensitive
-
- AS
-
- amiloride-sensitive
-
- CT
-
- chorda tympani
-
- ES
-
- electrical stimulation
-
- Fru
-
- fructose
-
- GI
-
- gurmarin-insensitive
-
- Glc
-
- glucose
-
- GS
-
- gurmarin-sensitive
-
- Gur
-
- gurmarin
-
- Mal
-
- maltose
-
- PCR
-
- polymerase chain reaction
-
- Sac
-
- saccharin Na
-
- SC
-
- SC45647
-
- SSC
-
- standard saline citrate
-
- Suc
-
- sucrose.